Abstract
Objective
This cross-sectional study compared tooth and dental arch dimensions of individuals with Osteogenesis imperfecta (OI) and healthy controls.
Material and Methods
The 37 OI patients and 37 controls were aged 10 to 74 years. Mesio-distal tooth size, dental arch dimensions, and palatal height were measured from dental models. The differences between the patient and control groups were analysed statistically with a t-test, chi-square test, and Mann–Whitney U test.
Results
The average mesio-distal tooth size of individuals with OI was smaller by 0.1 to 0.8 mm, corresponding to 1.4 to 7.3% of the size of the tooth. The patients and controls showed similar anterior-posterior lengths of maxillary and mandibular arches. The OI patient group exhibited increasingly wider maxillary dental arches posterior to the canines and a shallow palate.
Conclusions
Reduced tooth size is a developmental feature of OI and a shallow palate a characteristic possibly associated with previously documented imparity of vertical jaw development. Observed posterior widening of the dental arches may follow from altered tongue position. Smaller tooth size can be favourable from orthodontic point of view in alleviating crowding, but it might further predispose to fracturing of teeth which is a considerable risk associated with dentine abnormality. The shallow jawbones may initiate development of posterior open bite, rare in general population but relatively often encountered in OI.
Introduction
Osteogenesis imperfecta (OI) is a group of rare hereditary connective tissue disorders, where majority of cases arise from faulty production of collagen type I [Citation1]. Typical clinical characteristics of OI include recurring bone fractures, bone deformities, short stature, as well as abnormalities of craniofacial morphology and teeth [Citation2–5]. The phenotypic classification of OI into types I–V is based on the clinical severity of the disorder, and on the typical radiographic findings and mutation analysis [Citation6–8]. The prevalence of OI types I, III, and IV has been estimated to be 7.4/100 000 [Citation9].
Oral manifestations of OI include the classic finding of dentinal abnormality (‘dentinogenesis imperfecta, DI’), impaction and missing of teeth, abnormal eruption development, and relative mandibular prognathism [Citation2,Citation3,Citation10,Citation11]. The latter seems to be related to vertically small jaws and consequent anterior growth rotation of the lower jaw [Citation10]. The quality of life of patients with OI may be impaired by aesthetic and, especially, functional concerns related to both DI and malocclusions, typically reverse overjet, posterior crossbite and open bite [Citation12,Citation13]. The aetiological sequences behind these occlusal features are only partly understood.
Prevalence of tooth agenesis in OI is higher than in general population, with 17–25% of individuals with OI congenitally lacking one to several teeth [Citation4,Citation14–16]. Missing teeth increase an inherent risk for malocclusion. Adults with OI frequently display endodontically treated and restored teeth [Citation14,Citation15], and are in presence of DI in increased risk for acquired tooth loss due to root fractures. In addition to structural and numeric alterations in teeth, cranial skeletal features, muscle function and variations in dental morphology contribute to function of occlusion and mastication ability.
Most studies on occlusal characteristics of individuals with OI have been based on descriptive clinical findings and on radiographic findings in dental panoramic and lateral cephalometric views, whereas little is known regarding the dimensions of the teeth, dental arches, and the palate.
In order to further clarify the development of the various types of malocclusions in OI, our study assessed from plaster models whether the teeth and dental arches of individuals with OI differ in size from those of matched controls without OI.
Materials and methods
Study material
The study material comprised plaster-cast dental models of 37 OI patients, examined at Department of Oral and Maxillofacial Diseases, University of Helsinki. As controls, a random plaster model sample of 37 healthy subjects, group matched according to age and gender, were collected from among participants and participant parents of Helsinki Longitudinal Growth Study [Citation17]. The studies had been approved by Ethics Committee of the Institute of Dentistry, University of Helsinki.
The patients represented OI types I (15 individuals), III (7 individuals), and IV (14 individuals). OI type of one patient was unknown. Of the patients 11 were males and 26 females, and the mean age was 34 years (range 10 to 75 years). Data on the severity of OI and presence of DI were drawn from clinical patient records, and the number of permanent teeth missing for any reason, were recorded from the cast models. The status of missing teeth was not verified radiologically, or from patient records. presents basic data of the patients and controls.
Table 1. Basic data and occlusal characteristics of Osteogenesis imperfecta (OI) patient sample and healthy age- and gender-matched controls.
Occlusal characteristics and dentoalveolar measurements
The plaster models were first inspected in occlusion. Overjet and overbite were measured manually to the closest 0.5 mm. Occlusion was inspected for common deviations: crossbite, scissors bite, and open bite.
Measurements regarding tooth size, dental arch dimensions and palatal height were performed manually and repeated digitally to determine the reliability of the method. For manual measurements, a calliper with 0.1 mm accuracy was applied (Schwert Instruments, Germany). The plaster models were scanned with ROMER Absolute Arm 7525 Si laser scanner (Hexagon Manufacturing Intelligence, UK), and the digitized models were analyzed with Polyworks 2017 (64-bit) software (InnovMetric, Canada).
Tooth size was measured in mesio-distal direction from all maxillary and mandibular permanent teeth on the person’s left, third molars excluded. If a tooth had undergone dental treatment, it was excluded and the contralateral one was measured. Dental arch measurements were carried out in both jaws as indicated in . Maxillary and mandibular arch lengths were measured from the extreme labial surfaces of the incisors perpendicular to a line indicating the extreme mesial point of the first permanent molars. Maxillary and mandibular arch widths were each evaluated at four sites: as distances between the crown tips of the canines, lingual cusps of the premolars, and mesiolingual cusps of the first permanent molars, after a modified protocol of Pirilä-Parkkinen [Citation18]. The four palatal height measurements included the perpendicular distances from the palatal midline to the level of gingival margins of first premolars and first permanent molars, as well as to lines connecting the palatal and mesiopalatal cusps of those teeth ().
Figure 1. Dental arch measurements. 1. Upper arch length from the extreme labial surfaces of the incisors perpendicular to a line connecting the extreme mesial points of the first permanent molars. 2. Upper inter-canine width, between the crown tips. 3. Upper inter-premolar widths, between the palatal cusp tips of both first and second premolars. 4. Upper inter-molar width, between the mesiopalatal cusp tips of first permanent molars. Similarly: 5. Lower arch length, 6. Lower inter-canine width, 7. Lower inter-premolar widths, 8. Lower inter-molar width.
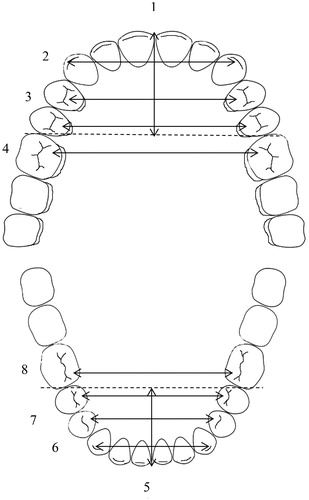
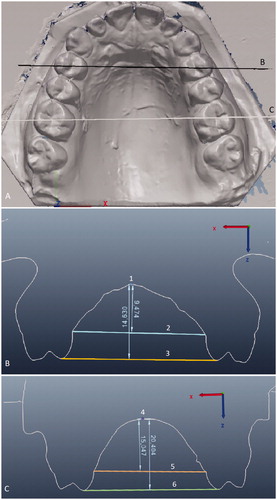
Figure 2. Palatal height measurements. (A) The black line marked B indicates the cross-section level in (B), and the white line marked C indicates the cross-section level in (C). B) Anterior palatal height measured from the mid-palate (1) to two reference lines: a line (2) drawn at the gingival margin level of the first premolars, and a line (3) on their palatal cusp tips. C) Posterior palatal height measured from the mid-palate (4) to two reference lines: a line (5) drawn at the gingival margin level of the first permanent molars, and a line (6) on their mesiopalatal cusp tips.
Normality of the data distribution was tested with Kolmogorov–Smirnov test. The differences between the patient and control groups were tested statistically with a t-test and Mann–Whitney U test. A chi-square test of independence was performed to examine the relation between DI, missing teeth, crossbite, and study groups.
Results
Comparison of the study and control groups
As the study group displayed a wide age-range, the available controls were grouped to better match to patients. Equal number (n = 5) of OI patients and controls were in mixed-dentition phase and their age ranged between 9.8 and 12.8 years. Median age difference between the study subjects and controls in this matched group was 0.7 years and maximum difference 0.9 years. Similarly, equal number (n = 32) of study patients and controls were in permanent dentition phase (aged between 18.8 and 74.7 years), and the median difference in age was 1.7 years (maximum difference 16.3 years). This difference in age was not expected to have an impact on the dental measurements as the approximal wear of teeth and alterations in palatal size are minimal. Loss of permanent teeth would be the only significant factor affecting dental arch measurements in later age years. The OI and the control groups did not differ in terms of number of permanent teeth missing for any reason, third molars excluded from the analysis (p > .64).
Dentinal abnormality
According to patient records, 46% of the OI-patients displayed DI as clinically diagnosed by abnormal colour and translucency of teeth (). DI was present in two individuals (13%) with type I OI, in six (86%) with type III OI, and in nine (64%) with type IV OI. Within the OI sample, the presence of DI and incompleteness of the permanent dentition did not correlate (p > .62).
Occlusion
Overjet median in the OI group was 1.4 mm (range −5 to 12 mm) and in the control group 2.8 mm (range 0.5 to 7 mm). On average, the overjet was 1.4 mm smaller in OI-patients than in controls, with a statistically significant difference (p = .014). In eight individuals with OI and none of the controls, the overjet was negative, indicating anterior crossbite. The association between OI and anterior crossbite was statistically significant (p = .002).
Overbite median in the OI group was 1.8 mm (range −3.5 to 7.0 mm) and in the controls 3.3 mm (range 0 to 11 mm). On average, the overbite was 1.5 mm smaller in OI-patients than in controls, with a statistically significant difference (p = .006). Either anterior or posterior open bite was seen in one patient with type I OI and in two patients with OI type III, but not in any of the controls. Scissors bite was observed in one patient with OI type III and in four controls, unilaterally. Lateral crossbite was evident in 22 patients with OI, of whom 12 showed crossbite bilaterally, whereas in controls, unilateral crossbite was present in six individuals and bilateral in none. The association between OI and posterior crossbite was statistically significant (p = .000) ().
Tooth size
The measurements carried out manually on plaster models and by computer from scanned models showed good concordance for all the measures (p = .24-.99) verifying reliability of the manual measurement method, the results of which were used in the analysis. In 11 out of the 14 teeth measured, the average mesio-distal tooth size of patients with OI was smaller, in two it was identical, and in one tooth 0.4 mm larger than in the controls (). In four of the 14 teeth measured (29%) the smaller tooth size, as compared to the controls, reached statistical significance (p-value range .000 to .03). The average actual difference between the smaller tooth size of OI-patients and controls ranged between 0.1 and 0.8 mm being 1.4 to 7.3% of the size of the tooth and hence also clinically significant.
Table 2. Mesio-distal widths of permanent teeth, third molars excluded, in patients with Osteogenesis imperfecta (OI) and in controls.
Dental arch size
The anterior-posterior lengths of both maxillary and mandibular arches were similar in the OI and control groups. The OI patient group in comparison to controls exhibited increasingly wider maxillary dental arches posterior to the canines, and the difference of 2.0 mm reached statistical significance in the molar region (p = .04). Similarly, in the mandible, the arch was wider in the OI group, but already from the level of the canines, and gradually expanded in width more extensively than in the controls. Even the greatest difference of 1.9 mm in the molar region remained, however, below the level of statistical significance (p = .08) ().
Table 3. Dental arch dimensions of patients with Osteogenesis imperfecta (OI) and healthy controls; means (and standard deviation) in millimetres.
Palatal height
All measures regarding the height of the palate were less in the patients with OI than in the controls, reaching statistical significance posteriorly at the level of first molar teeth. There, the palate was on average 1.6 mm shallower at the gingival margin level (p = .006) and 2.0 mm shallower measured from cusps (p = .011) ().
Table 4. Palatal height of patients with Osteogenesis imperfecta (OI) and healthy controls; means (and standard deviation) in millimetres.
Discussion
Dental and craniofacial issues related to OI affect quality of living of majority of individuals with the disorder [Citation19]. These issues include the dentinal abnormality, DI, with an aesthetic and functional burden, hypodontia and various malocclusions. To date, this is the first report quantifying the combined changes in dental and dentoalveolar dimensions in patients with OI.
Genetic defects, such as OI, that are associated with disturbance of mesenchymal matrix deposition can be speculated to also affect tooth morphogenesis, which is regulated by reciprocal interaction between dental epithelium and the underlying tooth mesenchyme. Dentine is of ectomesenchymal origin and rich in type I collagen. During tooth morphogenesis, formation of a layer of type I collagen-containing predentin precludes final differentiation of epithelium-derived ameloblasts and deposition of enamel. Disturbances in tooth morphogenesis due to mutations in several regulatory signalling genes are known to cause hypodontia and oligodontia, but quantitative factors may also be involved [Citation20]. In OI patients with the DI phenotype, the abnormal dentine displays significantly less than normal dentinal tubules with varying shape and size [Citation2,Citation21,Citation22]. DI is present in approximately one fourth of OI patients [Citation15,Citation16]. However, normally coloured teeth and absence of radiographic signs of DI do not necessarily indicate absence of dentine anomalies [Citation4,Citation22,Citation23]. Individuals with OI have twice as many missing teeth as the general population [Citation14]. Tooth agenesis has been noted to be slightly more common in OI patients with a mutation leading to qualitative defect in collagen type I and the severe disorder type than in those with quantitative mutations [Citation24]. Presence of dentine abnormality has been shown to increase the risk of hypodontia in individuals with OI [Citation21], and the diminished amount or structural abnormality of predentin might have an effect on tooth size [Citation2,Citation22]. This study supports evidence from a previous observation reporting smaller than normal tooth size in several teeth of patients with OI [Citation25]. The exact mechanism of hypodontia in OI is thus far unknown, but the diminished tooth size and hypodontia may be of shared aetiology as in population in general [Citation26].
A variety of malocclusion has been found to be associated with OI. Posterior crossbite has been previously documented in 35 to 47% of patients with OI, and class III occlusal relationship in more than 60% [Citation3,Citation12,Citation27,Citation28]. Anterior or posterior open bite has been reported in 19 to 46% of OI patients [Citation3,Citation12]. In our OI patient sample, open bite (posterior or anterior) was seen in only 8%, whereas anterior crossbite was present in 22% and posterior crossbite in 59%. Our finding on the prevalent posterior crossbite was interesting as the distance between the first molars was larger in OI patients compared to the healthy controls, and therefore the crossbite is most likely attributed to the relative mandibular prognathism commonly encountered in patients with OI [Citation3,Citation10].
Isshiki examined dental casts of 10 children with OI and compared the measures to previously published age norms [Citation22] of different ethnic background. The study found a tendency towards shorter and wider upper dental arch and a normal-sized lower dental arch [Citation29]. A shorter than normal maxillary dental arch, also based on observations on frequent impaction of the maxillary posterior molars [Citation27], might have been observed in our study, too, if the arch length was measured from more posterior teeth than the first permanent molars. Since young patients were included in the sample, the second molars had not erupted in all study individuals, and therefore the first permanent molars were chosen for the present anterior-posterior measurement.
The size and form of the dental arches depends largely on the size and position of the tongue that moulds the growth of the alveolar bone and effects the positions of teeth, the cheeks forming a counteracting force. This study observed, looking at the transversal dimensions, that in the patients with OI, the maxillary arch was more V-shaped than that of the controls, being narrower in the canine region and expanding more strongly towards the posterior region. In the mandible, there was a similar, posteriorly flaring arch form except that an increase in the width started already in the canine region. The discrepancy in the inter-canine widths may be associated with anterior cross-bite.
Waltimo-Sirén and co-workers [Citation10] found a lower than normal alveolar bone height in both jaws, with a 9.4 to 14% vertical growth reduction. This is likely reflected as a shallow palate. The current study documents that the palate is shallower in the OI patient group compared to the controls, and the difference is more pronounced in the posterior region. The difference in palatal height between the patients and controls was larger measured to the level of the cusps as compared to the gingival margin level, likely due to vertically smaller teeth in the OI patients or their lateral tipping, or both. This means that the tongue, being of normal size, has particularly in the posterior regions little space because of compromised vertical development of the alveolar arches [Citation10]. This leads to increased muscular pressure against the posterior alveolar walls in both jaws. The arches widen and teeth may tilt buccally, but eventually, posterior, or anterior, open bite may develop.
Limitations
Major limitation of this study was the small sample size due to rarity of OI. Quality of the plaster models was not uniform which might have affected the measurements.
Conclusions
Based on the findings of this study, individuals with OI appear to have significantly smaller-sized teeth, compared to controls, possibly due to the lack of normal type I collagen production by odontoblasts. Smaller tooth size can be favourable from orthodontic point of view since it alleviates crowding and probably also tendency for upper second-molar impaction. On the other hand, small tooth size might further predispose to fracturing of teeth which is a considerable risk faced by individuals with OI and dentine abnormality. Furthermore, smaller tooth size and palatal height contribute to a smaller oral cavity volume creating a problem to fit the tongue and predisposing to open bite development. Posterior widening of the dental arches is a nature’s way to compensate for the vertical lack. Posterior widening of the maxillary arch may not be sufficient in the presence of sagittal skeletal jaw discrepancy and posterior cross-bite develops, nevertheless.
Acknowledgements
The authors wish to thank Teollisuuden Mittaus- ja Kalibrointipalvelu Oy for providing the digital measuring and calibration equipment, and Mr Mikko Ahola for technical assistance.
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article. Raw data were generated at University of Helsinki and is available from the corresponding author HA on request.
References
- Forlino A, Marini JC. Osteogenesis imperfecta. Lancet Lond Engl. 2016;387(10028):1657–1671.
- Lukinmaa PL, Ranta H, Ranta K, et al. Dental findings in osteogenesis imperfecta: I. Occurrence and expression of type I dentinogenesis imperfecta. J Craniofac Genet Dev Biol. 1987;7(2):115–125.
- O’Connell AC, Marini JC. Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(2):189–196.
- Malmgren B, Norgren S. Dental aberrations in children and adolescents wweith osteogenesis imperfecta. Acta Odontol Scand. 2002;60(2):65–71.
- Kovero O, Pynnönen S, Kuurila-Svahn K, et al. Skull base abnormalities in osteogenesis imperfecta: a cephalometric evaluation of 54 patients and 108 control volunteers. J Neurosurg. 2006;105(3):361–370.
- Sillence DO, Rimoin DL, Danks DM. Clinical variability in osteogenesis imperfecta-variable expressivity or genetic heterogeneity. Birth Defects Orig Artic Ser. 1979;15(5B):113–129.
- Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16(2):101–116.
- Glorieux FH, Rauch F, Plotkin H, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000;15(9):1650–1658.
- Lindahl K, Åström E, Rubin C-J, et al. Genetic epidemiology, prevalence, and genotype-phenotype correlations in the Swedish population with osteogenesis imperfecta. Eur J Hum Genet. 2015;23(8):1112.
- Waltimo-Sirén J, Kolkka M, Pynnönen S, et al. Craniofacial features in osteogenesis imperfecta: a cephalometric study. Am J Med Genet A. 2005;133A(2):142–150.
- Vuorimies I, Arponen H, Valta H, et al. Timing of dental development in osteogenesis imperfecta patients with and without bisphosphonate treatment. Bone. 2017;94:29–33.
- Nguyen MS, Binh HD, Nguyen KM, et al. Occlusal features and need for orthodontic treatment in persons with osteogenesis imperfecta. Clin Exp Dent Res. 2017;3(1):19–24.
- Najirad M, Ma MS, Rauch F, Members of the BBD, et al. Oral health-related quality of life in children and adolescents with osteogenesis imperfecta: cross-sectional study. Orphanet J Rare Dis. 2018;13(1):187.
- Saeves R, Lande Wekre L, Ambjørnsen E, et al. Oral findings in adults with osteogenesis imperfecta. Spec Care Dentist. 2009;29(2):102–108.
- Thuesen KJ, Gjørup H, Hald JD, et al. The dental perspective on osteogenesis imperfecta in a Danish adult population. BMC Oral Health. 2018;18(1):175.
- Hald JD, Folkestad L, Swan CZ, et al. Osteogenesis imperfecta and the teeth, eyes, and ears-a study of non-skeletal phenotypes in adults. Osteoporos Int. 2018;29(12):2781–2789.
- Evalahti M. Craniofacial growth and development of Finnish children: a longitudinal study [Thesis]. Helsinki: University of Helsinki; 2020.
- Pirilä-Parkkinen K, Pirttiniemi P, Nieminen P, et al. Dental arch morphology in children with sleep-disordered breathing. Eur J Orthod. 2009;31(2):160–167.
- Tauer JT, Robinson M-E, Rauch F. Osteogenesis imperfecta: new perspectives from clinical and translational research. JBMR Plus. 2019;3(8):e10174.
- Nieminen P. Genetic basis of tooth agenesis. J Exp Zool B Mol Dev Evol. 2009;312B(4):320–342.
- Lukinmaa PL. Developmental dental aberrations in osteogenesis imperfecta. A clinical, radiographic, histological and immunohistochemical study. Proc Finn Dent Soc Suom Hammaslaakariseuran Toim. 1988;84(Suppl 6-7):1–97.
- Waltimo J, Ojanotko-Harri A, Lukinmaa PL. Mild forms of dentinogenesis imperfecta in association with osteogenesis imperfecta as characterized by light and transmission electron microscopy. J Oral Pathol Med. 1996;25(5):256–264.
- Rao S, Witkop CJ. Inherited defects in tooth structure. Birth Defects Orig Artic Ser. 1971;7(7):153–184.
- Malmgren B, Andersson K, Lindahl K, et al. Tooth agenesis in osteogenesis imperfecta related to mutations in the collagen type I genes. Oral Dis. 2017;23(1):42–49.
- Larsen LS, Thuesen KJ, Gjørup H, et al. Reduced mesiodistal tooth dimension in individuals with osteogenesis imperfecta: a cross-sectional study. Acta Odontol Scand. 2020; 1–6.
- Brook AH, Jernvall J, Smith RN, et al. The dentition: the outcomes of morphogenesis leading to variations of tooth number, size and shape. Aust Dent J. 2014;59(Suppl 1):131–142.
- Schwartz S, Tsipouras P. Oral findings in osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol. 1984;57(2):161–167.
- Chang P-C, Lin S-Y, Hsu K-H. The craniofacial characteristics of osteogenesis imperfecta patients. Eur J Orthod. 2007;29(3):232–237.
- Isshiki Y. Morphological studies on osteogenesis imperfecta, especially in teeth, dental arch and facial cranium. Bull Tokyo Dent Coll. 1966;7(1):31–49.
