Abstract
Objective
To comprehensively assess recent data on the effects of orthodontic forces on the dental pulp and to critically evaluate, whether any of the changes are permanent.
Materials and methods
Articles published between 2/2009 and 2/2022 were searched electronically on the PubMed, EMBASE and SCOPUS databases. The initial search retrieved 780 publications and, applying the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, 33 relevant articles were identified. Twenty articles fulfilled the requirements for high (n = 1) or moderate (n = 19) methodological quality and were included. All assessments were made independently by three researchers.
Results
Orthodontic forces appeared to cause a reduction in pulpal blood flow and a reduction in tooth sensibility, as indicated by increased response thresholds and increased amounts of negative responses to tooth sensibility tests. In addition, there were increases in the expression or activity levels of enzymes and neuropeptides associated with hypoxia and inflammation. Fibrotic tissue formation in the pulp was also reported.
Conclusions
Except for some histological and morphological alterations, the observed pulpal changes were in most cases only temporary, appearing within days of initiating the treatment and usually lasting for weeks. There were no clear signs of permanent damage.
Introduction
In recent years, a considerable number of new studies have focussed on the possible changes that occur in the dental pulp as a result of the application of orthodontic force. Various aspects have been studied, for example, pulpal blood flow, responses to tooth sensibility tests, the expression or activity levels of different enzymes and neuropeptides as well as histological and morphological changes [Citation1,Citation2].
The pulpal blood flow is an indicator of pulpal health. A decreased pulpal blood flow or transient ischaemia may cause a decrease in pulpal sensory responses and the sensibility of teeth. Since the pulp tissue is encased in calcified tissues, only indirect tests can be used to assess pulp vitality. Vitality tests assess pulpal circulation, which can be evaluated by using Laser Doppler Flowmetry (LDF). LDF is a non-invasive method of measurement that utilises the frequency change (Doppler shift) of light reflecting back from moving red blood cells [Citation3]. Pulp sensibility tests in turn extrapolate pulpal health from responses to electric current (electric pulp testing, EPT) and thermal changes (cold and heat). Higher response thresholds or failure to respond to EPT or thermal tests are indications of reduced sensibility. Sensibility tests can also be used to reproduce the symptoms reported by the patient. However, a positive reaction to sensibility tests indicates only that pulpal nerves are functioning but does not give any direct information about the pulpal blood flow [Citation4].
Both immunohistochemistry and biochemical assays have been used to measure the intrapulpal activity levels of proteins such as aspartate aminotransferase (AST), neuropeptides, and endogenous opioids. AST is an enzyme involved in amino acid metabolism [Citation5]. Increased AST activity can be an indication of metabolic changes occurring inside the pulp, and it has been previously associated with reversible pulpitis [Citation6]. Calcitonin gene-related peptide (CGRP) and substance P (SP) are neuropeptides that have important functions in the dental pulp. The release of these neuropeptides from the afferent nerve endings leads to pro-inflammatory effects (neurogenic inflammation), and with their vasodilatory function, they play an important role in the local control of pulpal blood flow [Citation7,Citation8]. Numerous studies in the past have indicated that there is an elevated expression of neuropeptides such as CGRP and SP or their receptors in an inflamed dental pulp, compared to a healthy pulp [Citation9,Citation10], and in teeth with pain symptoms compared to teeth without pain [Citation11,Citation12].
Several reviews on pulpal reactions to orthodontic treatment have been published in the last ten years [Citation1,Citation2,Citation13–15], but the latest reviews [Citation13–15] have focussed on restricted points of view, for example, clinical and radiographic outcomes. Therefore, a comprehensive synthesis of recent data from an orthodontic point of view was considered clinically informative. This review had three aims: (1) to provide an updated synthesis of data concerning various effects of orthodontic treatment on the dental pulp, (2) to critically assess any signs of permanent damage to the pulp and (3) to compare the results with regard to the type of tooth movement.
Materials and methods
Eligibility criteria
The focus question ‘What kind of harmful effects are created by orthodontic forces in human dental pulp’ was formulated according to the PICO (Patient; Intervention; Control and Outcomes) model to aid the selection of eligible studies. The inclusion criteria were as follows:
P: Human subjects with healthy teeth
I: Orthodontic force applied to the teeth that were studied
C: A control group or baseline values or contralateral teeth with no orthodontic treatment
O: The measured change between baseline and follow-up values. All changes had to be observed inside the pulp. In addition, original studies only were included.
Excluded: Studies where the force was applied to other structures than teeth (e.g. sutures), studies reporting root resorption or changes in tissues other than the pulp (e.g. periodontal tissues), studies which included traumatised teeth and studies with poor quality or inconsistencies were excluded as well as review articles and case reports.
Information sources and search strategy
Initial searches were made in PubMed using the search term ‘Dental Pulp’[Mesh] AND (Orthodontics [Mesh] OR ‘orthodontic force*’ OR ‘tooth movement*’ OR intrusion OR extrusion OR rotation) and, with slight modification, in SCOPUS and EMBASE. In order to analyse the consequences of current orthodontic practices and for example, flexible and tissue friendly materials, the search period was limited to 2/2009–2/2022.
Based on the initial searches, additional subject-specific search terms were designed and 23 supplementary searches were made in all three databases. The subject-specific search terms are listed in Supplementary Table S1 [Supplementary Appendix 1]. Any articles that were found through the searches or references in other articles were added if they met the inclusion criteria.
Study selection
All retrieved studies were assessed step by step by title and abstract according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. All assessments were made independently by three researchers. The flow-chart describing the inclusion-exclusion process is shown in (Edited from: [Citation16]). The full texts of all approved studies were read and categorised according to their methodological quality using the modified method by the Swedish Council of Technology Assessment in Health Care [Citation17,Citation18]. The studies were graded with a score of A to C according to the criteria shown in . Disagreements were resolved by consensus-based discussions.
Figure 1. PRISMA flow diagram presenting the identification and screening of eligible articles for this systematic review. Edited from: Page et al., 2021 [Citation16].
![Figure 1. PRISMA flow diagram presenting the identification and screening of eligible articles for this systematic review. Edited from: Page et al., 2021 [Citation16].](/cms/asset/54654c87-2901-4161-806e-92237d217a83/iode_a_2137232_f0001_c.jpg)
Table 1. Criteria for assessment of the methodological quality of the studies.
Results
The final material consisted of 20 articles, of which one was categorised as Grade A (High Quality) and 19 Grade B (Moderate Quality). The excluded 13 articles were categorized as Grade C (Low Quality), primarily because of insufficient descriptions of the tests for reliability and reproducibility.
The effects of orthodontic force on dental pulp were analysed during the first and second stages of orthodontic treatment, that is, alignment and levelling and space closure, and more specifically during three types of tooth movement (intrusion, extrusion and tipping). The changes were studied in pulpal blood flow (PBF), tooth sensibility, the levels of certain enzymes and other proteins, and in histology and morphology. One of the studies [Citation19] covered two different topics (AST and tooth sensibility). A summary of the results is shown in .
Table 2. Studies related to the changes in pulpal blood flow (PBF), tooth sensibility, expression of inflammation-related proteins and histological and morphological changes as a consequence of orthodontic force.
Changes in pulpal blood flow
In all the included 10 studies, there was a significant reduction in PBF as a result of orthodontic treatment [Citation20–24,Citation27,Citation29–32]. This reduction was seen during the first few days or weeks after applying the force, and it was followed by a recovery back towards the baseline PBF values. In all studies, LDF was used as the PBF measuring method. A comparison of the PBF progressions between the different studies is presented in . The respective data are given in Supplementary Tables S2 & S3 [Supplementary Appendix 2].
Figure 2. Progression of mean pulpal blood flow (PBF) values relative to the baseline value of each study or group (PBF at timepoint X/Baseline PBF of the same group). *Average between tooth types, **average between tooth types and treatment methods, ***baseline value not presented in numerical form in the source study, interpreted from a graph to be 13.0 AU (arbitrary units).
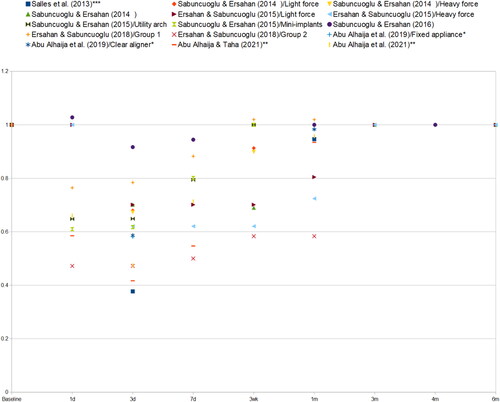
A greater intrusive force (250 g) caused a greater reduction in PBF when compared to a lower level of intrusive force (125 g) in the study on maxillary first molars by Ersahan & Sabuncuogly [Citation32]. However, another study on maxillary incisors [Citation29] found no significant difference between the different force groups (40 and 120 g of intrusive force).
Changes in tooth sensibility
Pulp sensibility tests included EPT and thermal tests (cold or heat). In all of the included 3 studies [Citation19,Citation25,Citation38], an increase in EPT readings or an increase in the number of negative responses to EPT was found as a result of the use of orthodontic force. Cold testing was done in only one study. Alomari et al. [Citation38] found an increase in the number of negative responses to the test. No signs of permanent damage were found in any of the studies.
Changes in the levels of proteins associated with inflammatory changes and hypoxia
Five studies on AST, neuropeptides, and Vascular endothelial growth factor (VEGF) were included in this review [Citation19,Citation26,Citation33,Citation34,Citation37]. Two studies focussed on AST activity or expression [Citation19,Citation26]. Veberiene et al. [Citation19] found an increase in AST activity after 7 days, but with a much longer treatment period (6 months), no difference in AST levels was seen [Citation26].
In the included three studies concerning neuropeptides [Citation33,Citation34,Citation37], a common finding was an increase in the expression of substance P (SP). For CGRP, two studies [Citation34,Citation37] showed a significant increase and one study [Citation33] an increasing trend, which was not statistically significant. Increased VEGF expression in pulp was measured after treatment of 24 h [Citation37]. The changes for the endogenous opioids β-endorphin and methionine-encephalin were not statistically significant, but they, too, showed increasing trends both after 24 h and 7 days of treatment [Citation33,Citation34].
Histological and morphological changes
Two studies reported findings of histological changes related to orthodontic treatment [Citation35,Citation36]. Both studies reported fibrotic tissue formation. Vacuolisation and disruption of the odontoblastic layer, as well as vascular changes (blood vessel dilation or congestion), were also observed. In addition, occasional pulpal nodules and odontoblastic aspiration were reported in extracted orthodontically treated teeth [Citation35]. However, the cause for odontoblastic aspiration was suggested to be a result of the tooth extraction process rather than orthodontic treatment.
In a recent study, Hatrom et al. [Citation28] used Cone Beam Computed Tomography (CBCT) to show reduced pulp volume after conventional and piezocision-assisted orthodontic tooth movement, but there was no difference between the two treatment methods.
Comparisons between the main types of tooth movement
Of the included 20 studies, 7 analysed alignment and levelling of dental arches [Citation20–26] and 9 intrusion [Citation19,Citation29–36]. A closer inspection revealed that dental arches were aligned and levelled using round, 0.014–0.016 superelastic arch wires with a shape memory. These arch wires exert light, continuous forces to the teeth and the induced tooth movement is mainly tipping.
In tipping, the loading is exerted near the root apex on the side of the force and near the alveolar crest on the opposite side [Citation39]. The compression depends on the force magnitude. In studies included in this review, forces from 70 g to 100 g were applied to maxillary incisors [Citation20,Citation21] and from 150 g to 200 g to maxillary premolars [Citation26]. All studies evaluating changes in blood flow had the last follow-up at 1 month after placement of first arch wires [Citation20–24].
In intrusion, the applied cantilever whole arch wires were stiffer, from 0.016ʺ × 0.022ʺ to looped 0.018ʺ × 0.025ʺ stainless steel (SS) [Citation31–34] exerting an intermittent force to the teeth. The intrusive forces were generated by a utility arch [Citation30], a looped whole arch [Citation33,Citation34], a NiTi closed coil spring [Citation29,Citation30] or elastics [Citation31,Citation32] with Mini Implants as anchorage. Other methods were a NiTi open coil spring with a removable appliance [Citation35] and cantilever SS spring with a rigid unit connecting the first molars as anchorage [Citation19,Citation36].
In intrusion, the loading is concentrated over a small area at the root apex [Citation39]. In included studies, the reported forces varied from 40 to 120 g for incisors [Citation29,Citation30], from 25 g (with a removable appliance) to 200 g for premolars [Citation19,Citation33–36] and from 100 g to 250 g for molars [Citation31,Citation32]. The follow-ups in evaluating changes in blood flow varied from 3 weeks [Citation29,Citation30] to 6 months [Citation31,Citation32]. Within the periods from 3 to 4 weeks, the results in blood flow and sensibility tests were similar in both movement types.
Discussion
In elective orthodontic treatments, it is of vital importance that the teeth and especially the pulps, are not damaged by the orthodontic forces. In several studies, the focus has been on the possible changes in PBF. However, orthodontic forces can cause many other responses and changes in the pulp. It can be speculated that the reduction seen in PBF is an original direct effect of the orthodontic force, and many of the other observed changes would be subsequent secondary effects caused by the reduction in blood flow.
In the recent studies [Citation20–24,Citation27,Citation29–32], a common finding was a significant reduction in pulpal blood flow during the first few days or weeks of the orthodontic force application. This is in line with previous findings where the application of short-term intrusive force caused a reduction in PBF [Citation40,Citation41]. Only the study by Barwick & Ramsay [Citation42] found no significant changes in PBF after a short duration (4 min) of intrusive force on maxillary central incisors, but the small sample size may have prevented small changes in PBF from showing as statistically significant.
Under normal conditions, pulpal blood flow is controlled both remotely, by the autonomous nervous system, and locally, through the local release of vasoactive substances and stimulation of the local sensory nerves [Citation8]. The blood supply to the dental pulp is provided by blood vessels entering through the narrow apex area. The reduction seen in PBF during orthodontic treatment has been considered to be a consequence of the direct mechanical compression of these blood vessels [Citation29,Citation31,Citation41,Citation42], as the orthodontic force creates a movement of the apical root area relative to the surrounding bone.
As different types of force create different forms of movement, their effects on PBF may differ. Intrusion has often been considered the type of movement most likely to cause changes in PBF, but in recent studies, other movement types caused reductions in PBF as well. It has also been suggested [Citation27] that developing teeth with a more open apex area could be more resistant to the blood flow effects of orthodontic force, as larger blood vessels can better enter the pulp. With ageing, deposition of secondary dentine may further narrow the apex area. In the 2018 study by Ersahan & Sabuncuogly [Citation21], significant differences in pulpal blood flow changes were found between 18–25-year-olds and 42–55-year-olds. The older group had a more severe reduction in PBF as a result of the treatment, and PBF in the older group did not recover back to baseline as quickly as in the younger group.
In the study by Sabuncuogly & Ersahan [Citation30], a very small initial increase in PBF was found after 24 h, before a subsequent larger decrease. As suggested by the authors, such small initial increases could be attributable to an acute inflammatory reaction involving blood vessel dilation and increased circulation.
EPT responses seem to be affected by the temporary disturbances of orthodontic forces. Recent studies [Citation19,Citation25,Citation38] showed higher threshold values and an increase in the number of negative responses to EPT. The findings concerning thermal testing (cold or heat) are less clear. The actual mechanism through which the orthodontic force causes the changes in tooth sensibility is not clear, but nerve fibres may be affected by the hypoxic conditions caused by a reduction in blood flow [Citation43,Citation44].
Recent studies suggested that there was a transient increase in AST activity inside the dental pulp during the first weeks following the application of orthodontic force [Citation19,Citation45]. This is supported by the earlier study by Perinetti et al. [Citation46], which found significantly increased AST activity after 7 days of treatment with a fixed appliance. Also, samples taken from gingival crevicular fluid (GCF) around orthodontically moved teeth have shown increased activity of AST [Citation46,Citation47].
The expression of neuropeptides CGRP, and SP as well as endothelial growth factor VEGF also appears to increase as a result of orthodontic treatment [Citation33,Citation34,Citation37]. Similar results have been observed previously in animal studies. In a study with rats by Kvinnsland & Kvinnsland [Citation48], an increase in the staining intensity and a number of CGRP-immunoreactive nerve fibres was observed in the pulps after 5 days of the orthodontic force application. In a study with cats, Nicolay et al. [Citation49] found an increased intensity of SP immunoreactivity in teeth subjected to orthodontic force.
Increased levels of AST, neuropeptides and VEGF have been associated with inflammatory conditions and hypoxia of the pulp, which is in line with the detected reduced pulpal blood flow discussed above. Furthermore, increased levels of these proteins following orthodontic treatment could suggest that the orthodontic force might cause a low-level inflammatory reaction inside the pulp.
In the recent studies reporting findings of histological changes in the pulp related to orthodontic treatment [Citation35,Citation36,Citation50–52], the most common finding was fibrotic tissue formation. Additionally, disruption of the odontoblastic layer and vascular changes as well as pulp stone formation and reduction in pulp volume were reported. Similar changes have been reported previously. Mostafa et al. [Citation53] looked at the histological changes resulting from 1–4 weeks of extrusion of maxillary premolars. They found vacuolisation, congestion and dilation of the blood vessels, degeneration of odontoblasts, and oedema. Also pulp fibrosis was found after 4 weeks. Kayhan et al. [Citation54] observed increases in blood vessel diameter as a result of 1–3 months of rapid palatal expansion. Additionally, some fibrosis formation was observed after 3 months, but no inflammatory cell infiltration was found. Taspinar et al. [Citation55] observed significant increases in blood vessel diameter, haemorrhage, congestion and inflammatory cell infiltration after 3 months of rapid palatal expansion. Sübay et al. [Citation56] found no signs of inflammatory responses following the application of extrusive forces but did find pulp stone formation and odontoblast aspiration into the dentine tubules.
Hatrom et al. [Citation28] reported a reduction in pulp volume after orthodontic treatment. Popp et al. [Citation57] studied changes in pulp volume related to orthodontic treatment using periapical radiographs. During the 5-year study period, narrowing of the pulp was observed both in the experimental group and the control group, and was interpreted to be caused by the secondary dentine formation related to the natural ageing process. On the other hand, CBCT applied by Venkatesh et al. [Citation58] indicated slight decrease in pulp volume after 17–18 months of the orthodontic force application. The decrease was interpreted to be caused by tertiary dentine deposition. While secondary dentine is formed continuously throughout life, tertiary dentine is formed in response to an external stimulus [Citation59]. More studies are needed to find out whether orthodontic treatment truly causes narrowing of the pulp chamber through the formation of tertiary dentine, or whether the observed slight narrowing results from secondary dentine formation.
The recently published systematic reviews [Citation1,Citation2,Citation13–15] have been limited in their conclusions, despite noted changes in blood flow [Citation1,Citation15], pulp sensibility [Citation14], pulp morphology [Citation14,Citation15] and in the expression of certain tissue factors [Citation14]. Although the current review sheds light on changes in dental pulp during various types of tooth movement, there are limitations as well: Some studies did not describe the full details of applied appliances and most of the follow-ups were scheduled up to 1 month, not allowing for long-term analyses. Only the study by Alomari et al. [Citation38] followed changes in EPT from the beginning of orthodontic treatment until one year of retention. Only one study analysed changes during bodily movement [Citation28] and one during torque [Citation38], that is, during those types of tooth movement having the largest areas of compression, high magnitudes of force and a long duration. Furthermore, the number of studies related, for example, to tooth sensibility and morphological changes was small as well. These factors limit the level of certainty with which any conclusions can be drawn and call for more higher quality studies.
In conclusion, orthodontic forces appear to cause multiple detectable changes in the dental pulp. These changes include a reduction in pulpal blood flow, reduction in tooth sensibility, and a likely increase in the expression or activity levels of certain enzymes and neuropeptides associated with inflammation. Common histological changes seem to include fibrotic tissue formation. For the most part, these changes appear to be only temporary and to present during the first few days or weeks after applying orthodontic force. Only few effects appear to be detectable after several months of treatment; instead, recovery and reversal back to normal can be seen in most cases even during an ongoing treatment. In the included studies, there were no clear signs of permanent destructive effects following treatment.
Supplemental Material
Download MS Word (24.8 KB)Appendix_1.docx
Download MS Word (37.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- von Böhl M, Ren Y, Fudalej PS, et al. Pulpal reactions to orthodontic force application in humans: a systematic review. J Endod. 2012;38(11):1463–1469.
- Javed F, Al-Kheraif AA, Romanos EB, et al. Influence of orthodontic forces on human dental pulp: a systematic review. Arch Oral Biol. 2015;60(2):347–356.
- Micheels J, Alsbjorn B, Sorensen B. Laser Doppler flowmetry. A new non-invasive measurement of microcirculation in intensive care? Resuscitation. 1984;12(1):31–39.
- Butt K, Harris I. Making sense of sensibility: part 1. Br Dent J. 2022;232(5):307–310.
- Huang XJ, Choi YK, Im HS, et al. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors. 2006;6(7):756–782.
- Spoto G, Fioroni M, Rubini C, et al. Aspartate aminotransferase activity in human healthy and inflamed dental pulps. J Endod. 2001;27(6):394–395.
- Caviedes-Bucheli J, Muñoz HR, Azuero-Holguín MM, et al. Neuropeptides in dental pulp: the silent protagonists. J Endod. 2008;34(7):773–788.
- Olgart L. Neural control of pulpal blood flow. Crit Rev Oral Biol Med. 1996;7(2):159–171.
- Bowles WR, Withrow JC, Lepinski AM, et al. Tissue levels of immunoreactive substance P are increased in patients with irreversible pulpitis. J Endod. 2003;29(4):265–267.
- Sattari M, Mozayeni MA, Matloob A, et al. Substance P and CGRP expression in dental pulps with irreversible pulpitis. Aust Endod J. 2010;36(2):59–63.
- Awawdeh L, Lundy FT, Shaw C, et al. Quantitative analysis of substance P, neurokinin A and calcitonin gene-related peptide in pulp tissue from painful and healthy human teeth. Int Endod J. 2002;35(1):30–36.
- Rodd HD, Boissonade FM. Substance P expression in human tooth pulp in relation to caries and pain experience. Eur J Oral Sci. 2000;108(6):467–474.
- Weissheimer T, Silva EJNL, Pinto KP, et al. Do orthodontic tooth movements induce pulp necrosis? A systematic review. Int Endod J. 2021;54(8):1246–1262.
- Vitali FC, Cardoso IV, Mello FW, et al. Effect of orthodontic force on dental pulp histomorphology and tissue factor expression. Angle Orthod. 2021;91(6):830–842.
- Vitali FC, Cardoso IV, Mello FW, et al. Association between orthodontic force and dental pulp changes: a systematic review of clinical and radiographic outcomes. J Endod. 2022;48(3):298–311.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Bondemark L, Holm AK, Hansen K, et al. Long-term stability of orthodontic treatment and patient satisfaction. A systematic review. Angle Orthod. 2007;77(1):181–191.
- Reddy RR, Gosla Reddy S, Vaidhyanathan A, et al. Maxillofacial growth and speech outcome after one-stage or two-stage palatoplasty in unilateral cleft lip and palate. A systematic review. J Craniomaxillofac Surg. 2017;45(6):995–1003.
- Veberiene R, Smailiene D, Danielyte J, et al. Effects of intrusive force on selected determinants of pulp vitality. Angle Orthod. 2009;79(6):1114–1118.
- Salles AWR, Salles AMC, Nogueira GEC. Laser Doppler blood-flow signals from human teeth during an alignment and leveling movement using a superelastic archwire. ISRN Dent. 2013;2013:102816.
- Ersahan S, Sabuncuoglu FA. Effect of age on pulpal blood flow in human teeth during orthodontic movement. J Oral Sci. 2018;60(3):446–452.
- Abu Alhaija ESJ, Al-Abdallah SY, Taha NA. A comparative study of initial changes in pulpal blood flow between clear aligners and fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2019;156(5):603–610.
- Abu Alhaija ESA, Shahin AY, Badran SA, et al. Pulpal blood flow changes and pain scores related to using superelastic 0.018-inch nickel titanium as the first orthodontic alignment archwire: a prospective clinical trial. J Appl Oral Sci. 2021;29:e20210089.
- Abu Alhaija ES, Taha NA. A comparative study of initial changes in pulpal blood flow between conventional and self-ligating fixed orthodontic brackets during leveling and alignment stage. Clin Oral Investig. 2021;25(3):971–981.
- Modaresi J, Aghili H, Dianat O, et al. The effect of orthodontic forces on tooth response to electric pulp test. Iran Endod J. 2015;10(4):244–247.
- Veberiene R, Latkauskiene D, Racinskaite V, et al. Aspartate aminotransferase activity in the pulp of teeth treated for 6 months with fixed orthodontic appliances. Korean J Orthod. 2015;45(5):261–267.
- Sabuncuoglu FA, Ersahan S. Changes in human pulp blood flow during canine retraction. Acta Odontol Scand. 2016;74(6):436–442.
- Hatrom AA, Howait MS, Zawawi KH, et al. Pulp volume changes after piezocision-assisted tooth movement: a randomized clinical trial. BMC Oral Health. 2021;21(1):28.
- Sabuncuoglu FA, Ersahan S. Changes in maxillary incisor dental pulp blood flow during intrusion by mini-implants. Acta Odontol Scand. 2014;72(7):489–496.
- Sabuncuoglu FA, Ersahan S. Comparative evaluation of pulpal blood flow during incisor intrusion. Aust Orthod J. 2015;31(2):171–177.
- Sabuncuoglu FA, Ersahan S. Changes in maxillary molar pulp blood flow during orthodontic intrusion. Aust Orthod J. 2014;30(2):152–160.
- Ersahan S, Sabuncuoglu FA. Effects of magnitude of intrusive force on pulpal blood flow in maxillary molars. Am J Orthod Dentofacial Orthop. 2015;148(1):83–89.
- Chavarría-Bolaños D, Martinez-Zumaran A, Lombana N, et al. Expression of substance P, calcitonin gene-related peptide, β-endorphin and methionine-enkephalin in human dental pulp tissue after orthodontic intrusion: a pilot study. Angle Orthod. 2014;84(3):521–526.
- Chavarria-Bolaños D, Flores-Reyes H, Lombana-Sanchez N, et al. Sensory neuropeptides and endogenous opioids expression in human dental pulp with asymptomatic inflammation: In vivo study. Mediators Inflamm. 2015;2015:879126.
- Ramazanzadeh BA, Sahhafian AA, Mohtasham N, et al. Histological changes in human dental pulp following application of intrusive and extrusive orthodontic forces. J Oral Sci. 2009;51(1):109–115.
- Lazzaretti DN, Bortoluzzi GS, Torres Fernandes LF, et al. Histologic evaluation of human pulp tissue after orthodontic intrusion. J Endod. 2014;40(10):1537–1540.
- Caviedes-Bucheli J, Lopez-Moncayo LF, Muñoz-Alvear HD, et al. Expression of substance P, calcitonin gene-related peptide and vascular endothelial growth factor in human dental pulp under different clinical stimuli. BMC Oral Health. 2021;21(1):152.
- Alomari FA, Al-Habahbeh R, Alsakarna BK. Responses of pulp sensibility tests during orthodontic treatment and retention. Int Endod J. 2011;44(7):635–643.
- Proffit WR, Fields HW, Larson BE, et al. Contemporary orthodontics. 6th ed., Philadelphia (PA), Elsevier; 2018.
- Ikawa M, Fujiwara M, Horiuchi H, et al. The effect of short-term tooth intrusion on human pulpal blood flow measured by laser Doppler flowmetry. Arch Oral Biol. 2001;46(9):781–787.
- Sano Y, Ikawa M, Sugawara J, et al. The effect of continuous intrusive force on human pulpal blood flow. Eur J Orthod. 2002;24(2):159–166.
- Barwick PJ, Ramsay DS. Effect of brief intrusive force on human pulpal blood flow. Am J Orthod Dentofacial Orthop. 1996;110(3):273–279.
- Hall CJ, Freer TJ. The effects of early orthodontic force application on pulp test responses. Aust Dent J. 1998;43(5):359–361.
- Rowe AH, Pitt Ford TR. The assessment of pulpal vitality. Int Endod J. 1990;23(2):77–83.
- Wei FL, Geng J, Guo J, et al. Metabolic changes of human dental pulp after rapid palatal expansion. Orthod Craniofac Res. 2013;16(3):185–192.
- Perinetti G, Paolantonio M, Serra E, et al. Longitudinal monitoring of subgingival colonization by Actinobacillus actinomycetemcomitans, and crevicular alkaline phosphatase and aspartate aminotransferase activities around orthodontically treated teeth. J Clin Periodontol. 2004;31(1):60–67.
- Perinetti G, Paolantonio M, D'Attilio M, et al. Aspartate aminotransferase activity in gingival crevicular fluid during orthodontic treatment. A controlled short-term longitudinal study. J Periodontol. 2003;74(2):145–152.
- Kvinnsland I, Kvinnsland S. Changes in CGRP-immunoreactive nerve fibres during experimental tooth movement in rats. Eur J Orthod. 1990;12(3):320–329.
- Nicolay O, Shanfeld J, Davidovitch Z, et al. SP immunoreactivity in the dental pulp and periodontium during tooth movement. Ann N Y Acad Sci. 1991;632:452–454.
- D ’ Attilio M, De Angelis F, Vadini M, et al. Endodontic-orthodontic relationships: expression of no synthase in human dental pulp during orthodontic tooth movement. J Biol Regul Homeost Agents. 2012;26(2 Suppl):35–43.
- Leone A, Angelova Volponi A, Campanella C, et al. Human dental pulp cell apoptosis: immunohistochemical study after applying orthodontic traction. J Biol Regul Homeost Agents. 2012;26(4):713–720.
- Han G, Hu M, Zhang Y, et al. Pulp vitality and histologic changes in human dental pulp after the application of moderate and severe intrusive orthodontic forces. Am J Orthod Dentofacial Orthop. 2013;144(4):518–522.
- Mostafa YA, Iskander KG, El-Mangoury NH. Iatrogenic pulpal reactions to orthodontic extrusion. Am J Orthod Dentofacial Orthop. 1991;99(1):30–34.
- Kayhan F, Küçükkeleş N, Demirel D. A histologic and histomorphometric evaluation of pulpal reactions following rapid palatal expansion. Am J Orthod Dentofacial Orthop. 2000;117(4):465–473.
- Taspinar F, Akgul N, Simsek G. The histopathological investigation of pulpal tissue following heavy orthopaedic forces produced by rapid maxillary expansion. J Int Med Res. 2003;31(3):197–201.
- Subay RK, Kaya H, Tarim B, et al. Response of human pulpal tissue to orthodontic extrusive applications. J Endod. 2001;27(8):508–511.
- Popp TW, Artun J, Linge L. Pulpal response to orthodontic tooth movement in adolescents: a radiographic study. Am J Orthod Dentofacial Orthop. 1992;101(3):228–233.
- Venkatesh S, Ajmera S, Ganeshkar SV. Volumetric pulp changes after orthodontic treatment determined by cone-beam computed tomography. J Endod. 2014;40(11):1758–1763.
- Mjör IA. Dentin permeability: the basis for understanding pulp reactions and adhesive technology. Braz Dent J. 2009;20(1):3–16.
