Abstract
Background Cancellous bone can act as a delivery vehicle for vancomycin without impairment of graft incorporation. However, local and systemic antibiotic levels, biological activity of vancomycin, interaction with antibiotic-loaded cement, and also nephrotoxicity of these composites have not yet been studied clinically.
Material and methods Blood, drainage and urine samples of 20 consecutive patients undergoing revision total hip arthroplasties with impaction grafting technique utilizing 1 g of vancomycin per femoral head were studied. Plain PMMA cement was used in 10 cases, while PMMA with gentamycin was used in 5 cases and tobramycin was used in the remaining 5 cases. Biological activity of vancomycin was studied using kinetic killing curves in three ATCC organisms (methicillin-sensitive Staphylococcus aureus,methicillin-resistant Staphylo-coccus aureusand Pseudomonas aeruginosas).Quantification was done with fluorescent polarized immunoassay. Renal function was evaluated with preoperative and postoperative urea and creatinine.
Results Local active bactericidal levels of vancomycin reached 1 400 μg/mL (average 5-point level = 367 μg/mL) without nephrotoxicity. Vancomycin was present in urine until the fifteenth day. Both aminoglycosides in the cement had activity against Pseudomonas aeruginosas.
Interpretation Local levels of vancomycin were 35 times greater than the highest levels reported with vancomycin-loaded PMMA. A synergistic effect was observed between vancomycin released from impacted allografts and aminoglycoside-loaded PMMA.
Cancellous bone can act as a delivery vehicle for vancomycin in vitro and in vivo. High peak concentrations of vancomycin can be reached in the surrounding medium initially, followed by steady levels for longer periods (Witsø et al. Citation1999, Citation2000, Winkler et al. Citation2000). The addition of vancomycin to morsellized bone allografts has been shown not to impair graft incorporation (Buttaro et al. Citation2003). However, local and systemic antibiotic levels, biological activity of vancomycin, interaction with antibiotic-loaded cement, and also the nephrotoxicity of these composites have not yet been studied clinically.
We analyzed blood, drainage and urine samples in 20 consecutive reconstructive hip procedures using vancomycin-supplemented impacted bone allografts.
Patients and methods
From July to November 2002, we performed a prospective study involving 20 consecutive patients (20 hips) who underwent THA revisions, including reconstruction with cancellous impacted allografts supplemented with vancomycin. The procedures were carried out in accordance with the ethical standards of the committee responsible for human experimentation, with those of the local ethics review board, and in accordance with the Helsinki Declaration. There were 9 women and 11 men with a mean age of 65 (44–78) years. None of the patients received vancomycin, tobramycin or gentamycin intravenously during or after surgery.
Allografts were obtained from fresh frozen femoral heads from our own bank and were not treated with irradiation. 56 femoral heads were used (3 heads on average for each revision). Morsellized bone graft was fragmented manually in 0.4–0.6 cm bits and mixed for 15 min with 1 g of powdered vancomycin(Lilly Indianapolis, IN) per femoral head. Impaction grafting was performed by a routine technique as described by Slooff et al. (Citation1984) for the acetabulum and by Gie et al. (Citation1993) for the femur. Graft packing was made with two specific instrumentation sets (Primary Impaction Grafting Instruments, De Puy Int., Leeds, UK, and X-Change Revision Instruments System, Howmedica, Rutherford, NJ).
The components were fixed with Simplex-P radiopaque bone cement (Howmedica, Rutherford, NJ) in 5 patients, CMW1 (De Puy, Warsaw, IN) in 5 patients, Simplex with tobramycin cement (Howmedica) in 5 patients and CMW1 with gentamycin (De Puy) in 5 patients.
After implantation, 10 mL samples of the wound drainage were collected under sterile conditions at 1, 6, 12, 24 and 48 h, and immediately centrifuged and frozen at −20°. Serum samples were obtained at 1, 6, 12, 24, 48 and 72 h and urine samples on days 1, 2, 3, 4, 5, 15 and 45 after surgery.
Biological activity of vancomycin was studied from the drain fluid using kinetic killing curves (Metodology for the serum bactericidal test: approved guideline. NCCLS document M21-A, 1999) in three American Type Culture Collection (ATCC) organisms at 5 time points (1, 6, 12, 24 and 48 h). The organisms used were methicillin-sensitive Staphylococcus aureus (MSSA-ATCC 29213), methicillin-resistant Staphylococcus aureus (MRSA-ATCC 43300) and Pseudomonas aeruginosas (PA-ATCC 27853). Tests were performed in tubes containing equal volumes of the drain sample and the microorganisms to be tested in Müller Hinton broth incubated at 35°C. Starting inocula were 4 ×105 to 6 × 105 colony-forming units (CFU)/mL. Aliquots were removed after 0, 4, 8 and 24 h and then plated quantitatively onto CLDE agar. Colonies were counted after overnight incubation. Incubation was prolonged 48 h if the colonies were small. A graph of log10CFU against time was made. Bactericidal effect was defined as a 3-point drop in log10CFU/mL compared to the initial inoculum after 6–8 h of incubation.
Quantification of vancomycin in every sample was performed by means of fluorescent polarized immunoassay (FPIA) (Axsym System, Abbott Laboratories, Abbott Park, IL) (Jolley Citation1981, Jolley et al. Citation1981). The assay characteristics are: total standard deviation and coefficient of variation (%), 0.32 and 4.3, respectively, at 7 μg/mL; 1.04 and 2.9 at 35 μg/mL, and 2.97 and 4.2 at 75 μg/mL. Finally, the lower limit of detection was 2 μg/mL.
Renal function was evaluated with tests for blood urea and creatinine preoperatively and on days 1, 2, 3, 4 and 5. Nephrotoxicity was defined as an increase in serum creatinine of 0.5 mg/dL above pretreatment levels (Zaske Citation1992).
Statistics
Correlation analysis was performed by ANOVA, comparing preoperative and postoperative urea and creatinine. Pearson coefficient was used for comparison between vancomycin doses and local and urinary levels of vancomycin each day.
Results
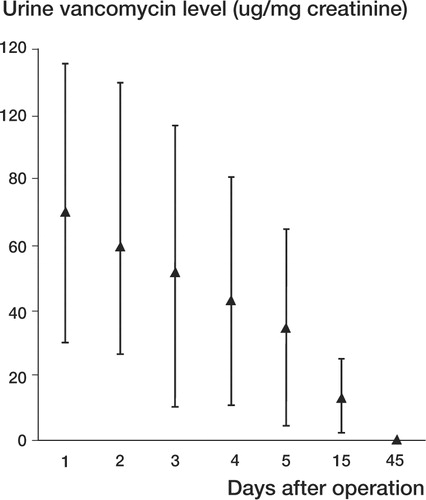
The average vancomycin drainage level obtained by FPIA was 408 μg/mL during the first hour and 265 μg/mL after 48 h (Table, ). Average urine vancomycin concentration was 70 μg/mg of urine creatinine on the first day, and was undetectable at 45 days ().
Figure 1 Average local vancomycin concentration (μg/mL) at 5 different time points. Bars represent SD.
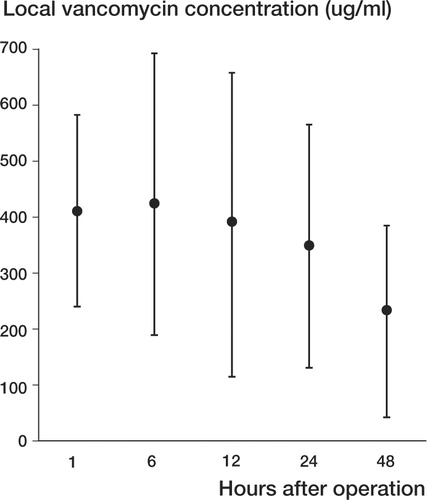
Figure 2 Average urinary vancomycin levels (vancomycin μg/mg creatinine) at 1, 2, 3, 4, 5, 15 and 45 days after implantation. Bars represent SD.
Methicillin-sensitive Staphylococcus aureus (MSSA-ATCC 29213) killing curves achieved bactericidal effect after 8 h of incubation when plain cement was used, whereas the same fall was achieved after 4 hours with antibiotic-loaded cement. Methicillin-resistant Staphylococcus aureus (MRSA-ATCC 43300) showed no changes with different cements and the bactericidal effect occurred at 8 h. As expected, vancomycin alone did not have any effect on Pseudomonas aeruginosas (ATCC 27853), while both tobramycin and gentamycin samples proved to be effective after 8 hours of exposure.
Postoperatively, average urea (mg/dL) was 34 on the first day, 35 on the second day, 30 on the third day, 29 on the fourth day, and 31 on the fifth day (overall range 10–74). Postoperatively, average creatinine (mg/dL) was 0.85 on the first day, 0.88 on the second day, 0.85 on the third day, 0.86 on the fourth day, and 0.85 on the fifth day (overall range 0.3–1.6).
Local vancomycin levels (μg/mL) in the 20 cases determined by FPIA at 1 h, 6 h, 12 h, 24 h and 48 h postoperatively. Number of femoral heads used in each patient (A) and grams of vancomycin (B)
Nephrotoxicity, defined according the criteria described above, was not observed in any of these patients. No statistically significant differences were noted concerning serum laboratory nephro-toxicity parameters measured preoperatively and on days 1, 2, 3, 4 and 5 after implantation (p < 0.05). No statistical correlation was found between the number of femoral heads per g vancomycin used and local levels of vancomycin. We found a statistically significant correlation between grams of local vancomycin and urine levels on days 2, 3, 4, 5 and 15 (Pearson coefficient).
Discussion
The capacity of cancellous bone allografts to act as a vehicle of vancomycin has been confirmed both in vitro and in vivo by means of bioassay (Witsø et al. Citation1999, Citation2000, Winkler et al. Citation2000). This makes it possible to reach local concentrations of this antibiotic that are higher than the 90% MIC forS. aureus. 1 gram of this antibiotic per femoral head causes a 400 times higher concentration than that necessary to achieve antimicrobial activity against Staphylococcus aureus and Staphylococcus epidermidis. Vancomycin is also effective against methicillin-resistant Staphylococcus aureus (MRSA).
In this clinical study, levels of vancomycin sufficient to inhibit multiplication of S. aureus were achieved without impairing renal function. Bactericidal effect occurred in both gram-positive organisms, and this was achieved in half the time when confronted to methicillin-sensitive Staphylococcus aureus (MSSA). Vancomycin proved to be more effective when combined with aminoglycosides in the cement.
We observed high local levels of vancomycin during the first 48 h after implantation, and in the local bone antibiotic levels were most likely toxic to even the most resistant organisms. Some micro-organisms are sensitive to vancomycin at minimum serum levels of of 4 μg/mL (Brien et al. Citation1993). Later, these levels should fall but could provide a toxic bactericide environment for the remaining sensitive organisms. This fact could be explained by the finding of vancomycin urine levels 15 days after surgery. The urinary levels were related to the original amounts of antibiotic administered. Other authors have observed this same phenomenon using other local antibiotic delivery systems (Yu et al. Citation1992, Laurencin et al. Citation1993).
Local antibiotic levels of vancomycin reached peak values as high as 1 400 μg/mL in our patients (average 5-point level: 359 μg/mL). Different authors have found local vancomycin levels eluted from methylmethacrylate ranging from 0.76 to 11 μg/mL (Brien et al. Citation1993, Klekamp et al. Citation1999, Gonzalez Della Valle et al. Citation2001). In a recent study, the highest reported peak value of vancomycin eluted from cement (measured by FPIA) was 19 μg/mL (Gonzalez Della Valle et al. Citation2001). Brien et al. (Citation1993) found undetectable local vancomycin levels in 30% of the cases when impregnated cement was used. The lowest vancomycin level observed in our study was 42 μg/mL at 48 h. According to these observations, adding vancomycin to the bone allograft instead of the cement would make it possible to achieve an average of 35 (2–70) times higher levels of this antibiotic. On the other hand, to reach such levels, it would be necessary to mix 35 g of vancomycin into every 40 mg of PMMA, causing deleterious mechanical effects in the cement as well as systemic toxicity (Askew et al. Citation1990, Klekamp et al. Citation1999).
Vancomycin and an aminoglycoside are often combined in the PMMA to achieve a potentially synergistic effect in the treatment of severe infections caused by methicillin-resistant Staphylococcus aureus (Masri et al. Citation1998, Klekamp et al. Citation1999). Although controversies on its use exist, vancomycin constitutes 18% of all antibiotics added to cement by hip reconstructive surgeons in the United States (Heck et al. Citation1995).
Nephrotoxicity has been defined as an increase in serum creatinine of 0.5 mg/dL above pretreatment levels (Zaske Citation1992). We found no patient with renal toxicity in this series. This can be explained because our sample size was not large enough to address the true effect of vancomycin on the kidneys. Lack of statistical significance in the differences between creatinine measurements may also be explained by small study power. However, the risk of vancomycin toxicity in combination with allografts is very low, since undetectable blood levels were observed in all our patients. Levels in the order of 60 mg/L are commonly achieved during intravenous therapy (Chohfi et al. 1998).
Gradual development of vancomycin resistance as a result of wide use, prolonged release in subtherapeutic doses, or the employment of large amounts is one of the current concerns. We consider that vancomycin-supplemented bone allografts should be used only for revisions of infected arthroplasties, or if there is a potential risk of infection.
In summary, vancomycin-supplemented bone allografts reached local concentrations that were 20–300 times higher than the 90% MIC for S. aureusat the 5 different time points measured, and without impairing renal function. These levels were highly superior to those reported for vancomycin-impregnated methylmethacrylate. The bactericidal effect occurred in both gram-positive organisms. Tobramycin and gentamycin samples proved to be effective against Pseudomonas aeruginosas. When confronted to MSSA there were synergistic effects between the biological activity of vancomycin and aminoglycosides in the cement. Bactericidal effects could be seen after 4 h instead of 8 h.
No competing interests declared.
We thank the EpidemiologyUnit, Department of Internal Medicine at the Hospital Italiano de Buenos Aires, for help with the statistics. We thank Miss Bettina Gollo for preparation of the manuscript. The source of funding was the Department of Orthopedics at the Hospital Italiano de Buenos Aires.
- Askew M J, Kufel M F, Fleissner P R, Jr, Gradisar I A, Salstrom S J, Tan J S. Effect of vaccum mixing on the mechanical properties of antibiotic impregnated poly-methylmethacrylate bone cement. J Biomed Mater Res 1990; 24: 573–80
- Brien W W, Salvati E A, Klein R, Brause B, Stern S. Antibiotic impregnated bone cement in total hip arthroplasty. An in vivo comparison of the elution properties of tobramycin and vancomycin. Clin Orthop 1993; 296: 242–8
- Buttaro M A, González Della Valle A, Piñeiro L, Mocetti E, Morandi A, Piccaluga F. Vancomycin-supplemented bone allografts incorporation. Radiological, histopathological and immunohistochemical study in pigs. Acta Orthop Scand 2003; 74(5)505–13
- Chohfi M, Langlais F, Fourastier J, Minet J, Thomazeu H, Cormier M. Pharmacokinetics, uses, and limitations of vancomycin-loaded bone cement. Int Orthop 1998; 22: 171–7
- Gie G A, Linder L, Ling R S, Simon J P, Slooff T J, Timperley A J. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg (Br) 1993; 75(1)14–21
- Gonzalez Della Valle A, Bostrom M, Brause B, Harney C, Salvati E. Effective bactericidal activity of tobramycin and vancomycin eluted from acrylic bone cement. Acta Orthop Scand 2001; 72(3)237–40
- Heck D, Rosenberg A, Schink-Ascani M, Garbus S, Kiewitt T. Use of antibiotic-impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty 1995; 10(4)470–5
- Jolley M E. Fluorescence polarization immunoassay for the determination of therapeutic drug levels in human plasma. J Anal Toxicol 1981; 5(5)236–40
- Jolley M E, Stroupe S D, Schwenzer K S, Wang C J, Lu-Steffes M, Hill H D, Popelka S R, Holen J T, Kelso D M. Fluorescence polarization immunoassay III. An automated system for therapeutic drug determination. Clin Chem 1981; 27(9)1575–9
- Klekamp J, Dawson J M, Haas D W, DeBoer D, Christie M. The use of vancomycin and tobramycin in acrylic bone cement: Biomechanical effects and elution kinetics for use in joint arthroplasty. J Arthroplasty 1999; 14: 339–46
- Laurencin C T, Gerhart T, Witschger P, Satcher R, Domb A, Rosenberg A E, Hanff P, Edsberg L, Hayes W, Langer R. Bioerodible polyanhydrides for antibiotic drug delivery: in vivo osteomyelitis treatment in a rat model system. J Orthop Res 1993; 11(2)256–62
- Masri B A, Duncan C P, Beauchamp C P. Long-term elution of antibiotics from bonecement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROS-TALAC) system. J Arthroplasty 1998; 13(3)331–8
- Methodology for the serum bactericidal test: approved guideline. NCCLS document M21-A. 1999
- Slooff T J, Huiskes R, van Horn J, Lemmens A J. Bone grafting in total hip replacement for acetabular protrusion. Acta Orthop Scand 1984; 55(6)593–6
- Winkler H, Janata O, Berger C, Wein W, Geotgopoulos A. In vitro release of Vancomycin and tobramycin from impregnated human and bovine bone grafts. J Antimicrob Chemoter 2000; 46(3)423–8
- Witsø E, Persen L, Loseth K, Bergh K. Adsorption and release of antibiotics from morselized cancellous bone. In vitro studies of 8 antibiotics. Acta Orthop Scand 1999; 70(3)298–304
- Witsø E, Persen L, Loseth K, Bergh K. Cancellous bone as an antibiotic carrier. Acta Orthop Scand 2000; 71(3)80–4
- Yu D, Wong J, Matsuda Y, Fox J L, Higuchi W I, Otsuka M. Self-setting hydroxyapatite cement: a novel skeletal drug-delivery system for antibiotics. J Pharm Sci 1992; 81(6)529–31
- Zaske D E. Amynoglycosides. E E Evans, J J Schentag, W Jusko, V A Alexandria. Applied Therapeutics Inc. 1992; 7–31, Applied Therapeutics ed 3
