Abstract
Background The question whether the tibial component of a total knee arthroplasty should be fixed to bone with or without bone cement has not yet been definitely answered. We studied movements between the tibial component and bone by radiostereometry (RSA) in total knee replacement (TKR) for 3 different types of fixation: cemented fixation (C-F), uncemented porous fixation (UC-F) and uncemented porous hydroxyapatite fixation (UCHA-F).
Patients 116 patients with osteoarthrosis, who had 146 TKRs, were included in 2 randomized series. The first series included 86 unilateral TKRs stratified into 1 of the 3 types of fixation. The second series included 30 patients who had simultaneous bilateral TKR surgery, and who were stratified into 3 subgroups of pairwise comparisons of the 3 types of fixation.
Results After 5 years 2 knees had been revised, neither of which were due to loosening. 1 UCHA-F knee in the unilateral series showed a large and continuous migration and a poor clinical result, and is a pending failure. The C-F knees rotated and migrated less than UC-F and UCHA-F knees over 5 years. UCHA-F migrated less than UC-F after 1 year.
Interpretation Cementing of the tibial component offers more stable bone-implant contact for 5 years compared to uncemented fixation. When using uncemented components, however, there is evidence that augmenting a porous surface with hydroxyapatite may mean less motion between implant and bone after the initial postoperative year.
We used RSA to evaluate the effect of augmenting a porous undersurface of the tibial component in TKR with hydroxyapatite, and to compare the performance of hydroxyapatite-porous-coated implants with that of porous-coating alone and of cemented implants. We used a single-blind, randomized protocol designed to include both patients who underwent unilateral TKR and also simultaneous bilateral TKR. We have already reported the results after 2 years (Önsten et al. Citation1998). That report was incomplete regarding the patients who underwent bilateral, simultaneous TKR. The editor requested that this patient group should be represented by only 1 knee per patient. We now report the 5-year results for all patients included in the study. Patients who underwent unilateral TKR and simultaneous bilateral TKR are presented as separate series to avoid any misunderstanding relating to the bilaterally operated patients.
Patients and methods
From 1992 to 1995, 120 patients with knee osteoarthrosis who had 150 TKRs were stratified into 1 of 2 series. The exclusion criteria are listed in . Series I comprised 90 patients undergoing unilateral TKR and series II comprised 30 patients who had bilateral, simultaneous TKR. Within each series, the knees were randomized with respect to the fixation of the tibial component: cemented fixation (C-F), uncemented porous fixation (UC-F) and uncemented hydroxyapatite-augmented fixation (UCHA-F). The study protocol was designed as a single-blind, randomized study. All patients gave written consent and the Research Ethics Committee of Lund University approved the study.
Table 1. Exclusion criteria for series I and II
In series I (), there were 4 errors related to the randomizing procedure: in 2 cases it was later found that the patients did not fulfill the inclusion criteria and in another 2 cases the implant was not in accordance with the protocol.
Table 2. Demographic data from series I, stratified with respect to the type of fixation
In series II (), the knee first operated on was randomly assigned to one of the three types of fixation, and the second knee to one of the two remaining fixations. No randomization errors occurred in this series. The 30 patients were thus stratified into one of each of the 3 subgroups with 10 patients in each group: C-F/UC-F, C-F/UCHA-F and UC-F/UCHA-F.
Table 3. Demographic data from series
The Press-Fit Condylar (PFC) modular, posterior-cruciate-retaining prosthesis with a posterior lipped polyethylene insert (Johnson and Johnson Orthopaedics, New Milton, UK) was used in all cases. The PFC tibial prosthesis has a modular tray of titanium alloy (Ti6A14V) with no screw holes, a cylindrical stem with a polyethylene (UHMWPE) plug distally, and a three-flanged keel.
For the C-F group, the implants had a smooth undersurface and were cemented only under the tibial plateau. Vacuum-mixed, gentamicin-loaded cement (Palacos cum Gentamicin; Schering-Plough, Kenilworth, NJ) was used after routine lavage.
The UC-F components had a layer of sintered beads of Ti6A14V on the undersurface, but not on the stem or keel. The beads were spherical and measured 425–710 μm in diameter. The sintered coating had a porosity of about 40% with primary pores of 120–440 μm and interconnection pores measuring 200–240 μm on average.
The UCHA-F implants had the same beaded porous undersurface, with the addition of a 55-μm-thick plasma-sprayed hydroxyapatite layer (CAMCERAM: CAM Implants BV, Leiden, the Netherlands). The hydroxyapatite coating had a crystallinity of 62–72%, a calcium to phosphorus ratio of 1:67, contained no less than 95% (w/w) hydroxyapatite, and no more than 5% (w/w) calcium or phosphate salts, which was confirmed by the manufacturer by X-ray diffraction.
All the PFC femoral components had a beaded deep surface identical to that of the UC-F tibial components, and were implanted with or without cement. According to the preference of the surgeons, the femoral component was cemented in 36 of the 146 knees operated and left uncemented in 110. Although according to the protocol the patella should not be resurfaced, a surgeon chose to implant a patellar component in 1 patient randomized to UCHA-F and 2 patients randomized to C-F (both in series I).
The surgical procedure and the postoperative regimen have been described previously (Önsten et al. Citation1998).
The manufacturer supplied the tibial implants with 5 1.0-mm tantalum markers in the metal tray, 1 0.8-mm marker in the polyethylene plug at the end of the tibial stem, and 5 0.8-mm tantalum markers in the polyethylene inserts. About 10 0.8-mm tantalum markers were placed in trabecular bone in the proximal tibia.
Biplanar RSA with the patient supine was performed within 1 week of the operation, and thereafter at 3, 12, 24, 36 and 60 months. The films were digitized manually on a Hasselblad measurement table, and calculations were done using UMRSA software (RSA Biomedical, Umeå, Sweden). We computed RSA results both as the vector length of translation of the marker in the polyethylene insert that had moved the most (MTPM), and as absolute values of rotation around the 3 orthogonal axes. All markers in the tibial components were used for these latter computations.
The quality of the RSA was assessed at 60 months by the number of valid markers in the tibia and prosthesis, by the mean error of rigid body (Selvik Citation1989) and by a configuration number (). The mean error of rigid body fitting describes the pertubation (in mm) of the markers in the bone and implant, from the postoperative to the latest examination. The configuration number describes the spatial spread of the markers. A low number indicates a wide spatial orientation, and a high number a close-to-linear orientation. The precision of our RSA technique was ± 0.1° for rotations around the transverse and longitudinal axes and ± 0.09° around the sagittal axis (Önsten et al. 1988).
Table 4. RSA quality factors at 60 months in series I and II. Median (range)
Standard antero-posterior and lateral radiographs, with the beam tangential to the joint line, were obtained postoperatively and at 12, 24, 36 and 60 months. These radiographs were evaluated for any osteolytic lesions or any progressive implant-bone or cement-bone lucencies using the method of Ewald (Citation1989), in which the interface around the tibial component is analyzed in ten zones, on AP and lateral radiographs.
We used the Knee Society clinical rating system (Insall et al. Citation1989) to obtain knee and functional scores for clinical evaluation preoperatively, and at 60 months.
To avoid spurious results from repetitive statistical testing between the three groups of implants, we made an adjustment according to Bonferoni-Holm (Holm Citation1979) in all comparisons. Thus, for the first sequence of comparison the given p-value was used as the adjusted p-value, for the second sequence twice p was used, and for the third sequence 3 times p was used as the adjusted p-value. An adjusted p-value of < 0.5 was considered significant. The order of analysis between implants was set by the order of differences between groups, starting with the largest difference.
To achieve data reduction of the measured rotations at up to 5 time points, we performed a regression over time for each variable in the individual case, forcing the regression slope origo, i.e. without an intercept. The logarithms of the absolute values of the rotations were used, and the regression coefficients were found to have a close to normal distribution. The regression coefficients express the per cent difference in rotation per month between groups. For cases with incomplete follow-up, the regression coefficients were based on data up to the latest examination. We used unpaired and paired t-tests to compare rotations between groups in series I and II, respectively.
The MTPM was used to compare groups at the latest examination (60 months), using the post-operative examination as a reference, and from 12 to 24 months and 24 to 60 months, using the first time point as a reference. We used this procedure because the MTPM represents the length of a vector, which cannot be subjected to regular addition, subtraction or regression over time unless a possible change in direction of the vector is being considered. Mann-Whitney and Wilcoxon tests were used to compare MTPM between groups in series I and II, respectively.
Clinical scores were compared between groups by Mann-Whitney and Wilcoxon tests in series I and II, respectively.
Results
Series I
1 knee in the UC-F group was excluded because of revision due to deep infection. At 60 months, 6 patients had died and another 6 refused examination (). There were no significant differences between groups regarding rotations around the transverse axis. The UC-F rotated 2% (95% CI 1–3) more per month around the longitudinal axis than the C-F, with no further differences between groups regarding this variable. There were no differences between groups regarding rotations around the sagittal axis ().
Figures 1–3. Absolute rotations (means) around the three orthogonal axes in degrees and over time for the different implant groups. Series I and II merged.Error bars indicate standard error
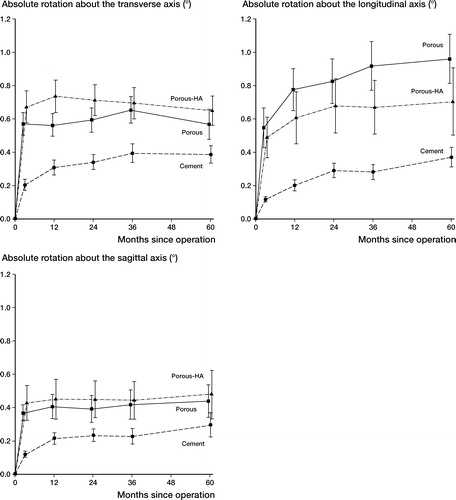
Table 5. Series I. Number of patients examined by RSA at the different time points, and reasons for exclusion stratified to the three fixation groups
The MTPM at 60 months showed the C-F to have migrated median 0.3 mm less than the UC-F (adjusted p = 0.004), with no further significant differences between groups ().
Figure 4. Maximum total point motion (MTPM) in mm against time for the different implant groups. Series I and II merged. Error bars indicate standard error.
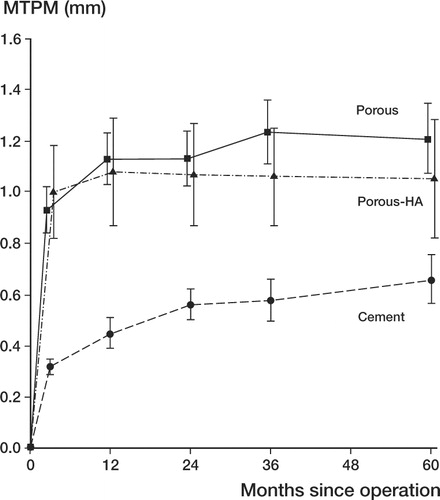
The MTPM from 12 to 24 months showed both the UCHA-F and the C-F to have migrated less than the UC-F (median differences: 0.08 and 0.06 mm; adjusted p = 0.02 and 0.04, respectively). There was no difference between the C-F and the UCHA-F (). The MTPM from 24 to 60 months showed the UCHA-F to have migrated median 0.04 mm less than the UC-F (adjusted p = 0.02), with no further differences between groups (). No difference was found between groups for the RSA quality factors ().
Figures 5–8 The marker within the box indicates median, and the height of the box indicates the distance between 25 and 75 percentiles (the intequartile range). Whisker indicates non-outlier range. Dots and stars mean outliers defined as above the 75 percentile plus 1.5 and 3 times the interquartile range, respectively
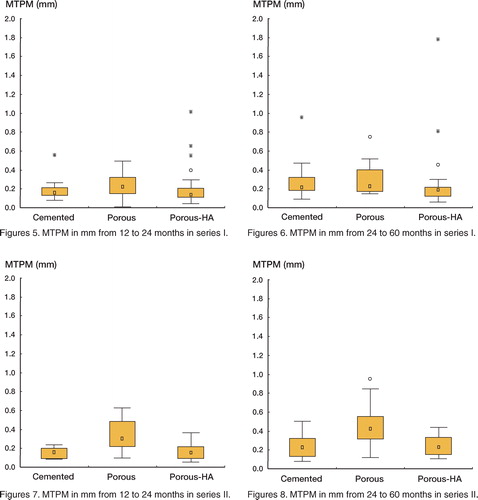
The median knee and functional scores at 60 months were 86 (33–100) and 65 (0–100), respectively, with no significant differences between groups. No progressive radiolucencies over 1 mm or any osteolytic lesions were seen in any subject at 60 months. 1 case in the UCHA-F group showed a continuous migration from the postoperative examination onwards. At 60 months, it had migrated with a MTPM of 9.8 mm (representing the most distant outlier in and ), and rotated 7.8° around the longitudinal axis and 6° around the transverse axis. The patient had a knee score of 33 and a functional score of 0. This case is a pending failure.
Series II
In series II, 1 C-F knee was reoperated due to instability and was excluded. 4 patients refused examination at 60 months and in 1 patient RSA did not function at 60 months (). The C-F rotated 3% less (95% CI, 1.5–4.5; adjusted p = 0.02) per month around the transverse axis compared to the UCHA-F, and 2% less (95% CI, 0.5–3.5; adjusted p = 0.03) per month compared to the UC-F. There was no difference between the UC-F and UCHA F. The C-F rotated 3% less (95% CI, 2–4; adjusted p = 0.0005) per month around the longitudinal axis compared to the UC-F, with no further differences between groups regarding this variable. There were no differences between groups regarding rotations around the sagittal axis ().
Table 6. Series II. Number of patients examined by RSA at the different time points, and reasons for exclusion stratified to the three pairwise fixation groups
The MTPM at 60 months showed the C-F to have migrated median 0.6 mm less than the UC-F (adjusted p = 0.01), with no further differences between groups (). The MTPM from 12 to 24 months showed that both the UCHA-F and the C-F had migrated less than the UC-F (median differences 0.14 mm for both; adjusted p = 0.03 and 0.03, respectively). There was no difference between C-F and UCHA-F (). The MTPM from 24 to 60 months showed that C-F had migrated (median) 0.18 mm less than the UC-F (adjusted p = 0.05), with no differences between the UCHA-F and UC-F, or between C-F and UCHA-F (). No difference was found between groups for the RSA quality factors (). The median knee and functional scores at 60 months were 91 (60–100) and 80 (10–100), respectively, with no significant differences between groups. No progressive radiolucencies over 1 mm or any osteolytic lesions were seen in any subject at 60 months.
Discussion
At the 2-year follow-up, we concluded that hydroxyapatite-coated porous (UCHA-F) implants were more stable than porous implants without an hydroxyapatite coating between 12 and 24 months postoperatively. After 5 years, there was a small but statistically non-significant difference in migration between hydroxyapatite-coated and porous implants. Also Regnér et al. (Citation2000) observed that porous-coated knee prostheses with an hydroxyapatite coating had migrated less than those without an hydroxyapatite coating after 5 years. Both hydroxyapatite-coated and porous implants show an initial rapid migration, from 0 to 3–6 months. After that, they stabilized and showed very little migration over time, whereas cemented implants showed a continuous migration. This migration behaviour has been described previously (Önsten et al. Citation1998, Nilsson et al. Citation1999) and appears to be a typical and crucial point in the use of cementless fixation in the knee. Although the cemented implants showed continuous migration, they showed less migration at 60 months compared to hydroxyapatite-coated and porous implants—both of which showed about the same tendency to migrate (measured as MTPM) ().
Historically, cementless tibial fixation—both porous and hydroxyapatite-coated—has yielded a higher 24-month MTPM compared to cemented fixation. This difference may be due in part to the fact that after the initial period when the cementless tibial component moves, it stabilizes and remains stable whereas the cemented tibia continues to move. In this way, at least theoretically, early failures with cementless tibial components will be noticed, as will later failures with the cemented tibial components. One key issue is to obtain initial fixation with the cementless tibial components in order to minimize the early migration, and in doing so, to minimize early failures due to loosening. Recent studies have shown no difference between cemented and hydroxyapatite-coated tibial components regarding fixation at 5 years postoperatively (Nilsson et al. Citation1999).
In our material, we had a low overall revision rate up to 5 years, but we still had a significant difference in migration between cemented implants on the one hand, and porous and hydroxyapatite-coated fixated implants on the other. This could be the result of many different factors, such as tibial component design and the varied experience of different surgeons in using cementless designs.
Hydroxyapatite crystals have been shown to cause third body wear in hip implants (Bloebaum et al. Citation1994, Morscher et al. Citation1998) and it has been speculated that this could happen also in knee replacements using hydroxyapatite. In our study, we could not detect any signs of impaired longterm durability due to hydroxyapatite-related third body wear. As previously shown by our group, hydroxyapatite-coated PFC tibial components performed better than porous-coated components at 5 years also but the difference was not significant. Cemented tibial components display less migration (measured as MTPM) than hydroxyapatite-coated and porous-coated implants, even at 5 years. Recent studies with other tibial designs have shown similar results between hydroxyapatite-coated and cemented implants, and it is possible that the migration curves may meet if the PFC prosthesis is given more time.
One important question is how to obtain initial fixation with the cementless prostheses, in order to avoid the initial migration shown by all cementless tibial designs. If this can be achieved, they may perform similarly to cemented implants, and over time even better than the cemented implants. The reason is that the hydroxyapatite-coated implants seem to settle firmly over time, whereas the cemented implants continue to migrate.
- Bloebaum R D, Beeks D, Dorr L D, Savory C G, Du Pont J A, Hofmann A A. Complications with hydroxyapatite particulate separation in total hip arthroplasty. Clin Orthop 1994; 298: 19–26
- Ewald F C. The knee society total knee arthroplasty roent genographic evaluation scoring system. Clin Orthop 1989; 248: 9–12
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70
- Insall J N, Dorr L D, Scott R D, Scott W N. Rationale of the Knee Society clinical rating system. Clin Orthop 1989; 248: 13–4
- Morscher E W, Hefti A, Aebi U. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular coating. J Bone Joint Surg (Br) 1998; 80: 267–72
- Nilsson K G, Kärrholm J, Carlsson L, Dalén T. Hydroxyapatite coating versus cemented fixation of the tibial component in total knee arthroplasty: Prospective randomized comparison of hydroxyapatite-coated and cemented tibial components with 5-year follow-up using radiostereometry. J Arhroplasty 1999; 1: 9–20
- Önsten I, Nordqvist A, Catlsson Å S, Besjakov J, Shott S. Hydroxyapatite augmentation of the porous coating improves fixation of tibial components: A randomised RSA study in 116 patients. J Bone Joint Surg (Br) 1998; 80: 417–25
- Regnér L, Carlsson L, Kärrholm J, Herberts P. Tibial component fixation in porous and hydroxyapatite-coated total knee arthroplasty: A radiostereometric evaluation of migration and inducible displacement after 5 years. J Arthroplasty 2000; 6: 681–9
- Selvik G. Roentgen stereophotogrammetry: a method for the study of the kinematics of the skeletal system. Acta Orthop Scand 1989; 1–51, (Suppl 232)