Abstract
Objectives: Existing fixation methods of automatic speaking valves (ASVs) suffer from shortcomings which partly are the result of insufficient conformity of the intratracheal fixation method’s shape to the tracheostoma anatomy. However, quantitative data are lacking and will be helpful to analyse solutions for airtight fixation. This article provides such data.
Patients and methods: The tracheostoma morphology was measured in computerized tomography scans of 20 laryngectomized patients. Measured were transverse and sagittal diameters, transition angle between skin level and tracheostoma lumen and between the tracheostoma lumen to the trachea, TE valve placement and stoma depth.
Results: The mean transverse and sagittal diameters of the stoma at the peristomal lip are 19.2 mm [standard deviation (SD 5.2 mm)] and 17.6 mm (SD 5.3 mm), respectively. The mean transition angles are 84.5° (SD 15.6°) at skin level and 153.6° (SD 11.7°) into the trachea. The mean distance between TE valve and peristomal lip is 13.5 mm (SD 7.0 mm). The mean stoma depth is 14.0 mm (SD 6.4 mm).
Conclusions: Due to the large variation, no ‘average tracheostoma morphology’, suitable for shaping a generic intratracheal fixation device, can be defined. Therefore, providing an airtight fixation in each patient would require a large range of different sizes, customization or a new approach.
Chinese abstract
目的:现有的自动口腔阀(ASV)的固定装置存在缺陷, 部分原因是气管内固定装置的形状与气管切开解剖特征不够吻合。然而, 定量数据缺乏, 这种数据将有助于分析气密固定的解决方案。本文提供了这样的数据。
患者和方法:在20例喉切除患者的计算机断层扫描中测量气管瘤形态。经测量的是横向和矢状直径、皮肤层面与气管腔内腔之间以及气管内腔与气管之间的转换角度、TE阀位置和造口深度。
结果:腹膜周围的气孔的平均横向和矢状径分别为19.2 mm(标准偏差(SD 5.2 mm))和17.6 mm(SD 5.3 mm)。平均过渡角在皮肤层面为84.5°(SD 15.6°), 在气管内为153.6°(SD 11.7°)。 TE阀和周围唇缘之间的平均距离为13.5 mm(SD 7.0 mm)。平均造口深度为14.0mm(SD 6.4mm)。
结论:由于变异度大, 无法确定”平均气管瘤形态”, 来制作一般性的气管内固定装置。因此, 为每个患者提供气密固定装置将需要大量的不同尺寸、量身定制或一种新方法。
Introduction
One-fourth of the total patient group diagnosed with a form of larynx cancer has to undergo a total laryngectomy (TL), when radiation, chemotherapy or minimally invasive surgery is not or insufficiently successful [Citation1]. This procedure has severe consequences for the patient, impacting the quality of life and perception of physical and psychological integrity. Most obvious to the patient’s environment is the loss of speech. However, because the patient now breathes through the tracheostoma instead of their nose or mouth, the functionality of the nose (pre-heating, filtration and moisturization of the air) is also lost, which can lead to pulmonary problems. Finally, there are social consequences to the procedure, such as a changed self-image, reduced sexuality, problems with social relationships and isolation. These severely affect the patient’s quality of life, more than the loss of speech [Citation2–4].
To help patients regain the lost functions and increase their quality of life, multiple rehabilitation devices have been developed, such as the heat-and-moisture exchange (HME) filter. This filter is placed in the tracheostoma opening and is kept in place by either peristomal (around the stoma) or intratracheal (inside the stoma) fixation methods. To regain the ability of speech, a voice prosthesis is placed between the trachea and esophagus and the container with the HME filter has to be occluded manually to enable tracheoesophageal (TE) speech. Currently, automatic speaking valves (ASVs) are being developed, which are containers which close automatically during speech. This improves hygiene and removes the emphasis on the patient’s disability. Permanent hands-free speech is considered to be the optimal end result for TL rehabilitation [Citation5,Citation6].
However, the current use of ASVs in TL patients is very low, with a compliance of only 25% to as low as 15% of the TL patients [Citation6–8], due to the fact that ASVs exert more stress on the fixation methods during speech than manually closed valves and due to the many disadvantages of the current fixation methods, such as dislodgement, traumatization of the peristomal or tracheal tissue and, in case of the Provox Larybutton (Atos Medical Inc., Hörby, Sweden), applicability only in the presence of a prominent peristomal lip [Citation4–9]. Therefore, to promote the use of ASVs and its ultimate hands-free speech, improvements are required in user comfort, strength of fixation and proper airtight sealing [Citation4,Citation6,Citation9]. For this purpose, intratracheal fixation appears to be the method of choice. A modification of the commercially available intratracheal buttons alone, as described by Lemon et al. [Citation10] or Lewin et al. [Citation11], is not sufficient for all patients to reach a perfect margin fit with an airtight seal. Therefore, the path to optimal airtight fixation is adjustment of the intratracheal button to the trachea, as many studies emphasize [Citation4,Citation9–12].
To be able to do this this, we first need to know what the required shape is. Two studies [Dirven et al. (2009) [Citation5], van der Houwen et al. (2012) [Citation4]] were found in literature that present data of the tracheostoma geometry. However, these only cover the peristomal geometry in laryngectomy patients. Parameters such as TE valve placement, prominence of the peristomal lip and the transition angle between tracheostoma lumen and the trachea’s natural course are also important structures to consider when designing or customizing an airtight intratracheal fixation technique. Some qualitative data has been provided by Müller et al. (2015) [Citation13]. However, to our knowledge, no quantitative data regarding the internal morphology are provided in literature. The aim of this study is to obtain quantitative data of and gain insight in the tracheostoma geometry by measuring the above-mentioned intratracheal stoma parameters as well as the peristomal parameters in the computerized tomography (CT) scans of laryngectomized patients.
Patients and methods
Patients
For the study, CT scans of 20 patients who underwent a TL were examined retrospectively. The patients were randomly selected out of the patient database of the Antoni van Leeuwenhoek Hospital (Amsterdam, The Netherlands). Inclusion criteria were time of surgery (between 2010 and 2016) and availability of a post-operative CT scan (made as part of the regular after-care protocol of laryngectomy patients).
Measurements
The CT scans were loaded and viewed in the hospital’s DICOM viewer software (Vue PACS, Carestream Health Inc., Rochester, NY). In the DICOM viewer software, the distance or angle between two user-specified point can be calculated automatically. The following stoma parameters were measured in both the sagittal and transversal plane, .
Figure 1. Schematic drawing of the measured values in CT scans of laryngectomized patients. (a) S1 till S4 measure the diameter of the stoma and trachea in the sagittal plane, T1 till T4 (not shown in the drawing) measure the diameter of the stoma and trachea in the transversal plane (perpendicular to S1 till S4). (b) The angles w1 and w2 in the sagittal plane measure the course/bend of the trachea. (c) The value ‘TE-1’ measures the distance between the skin level (S1) and the middle of the TE puncture, and the value ‘TE-2’ measures the distance between the peristomal lip (S2) and the middle of the TE puncture.
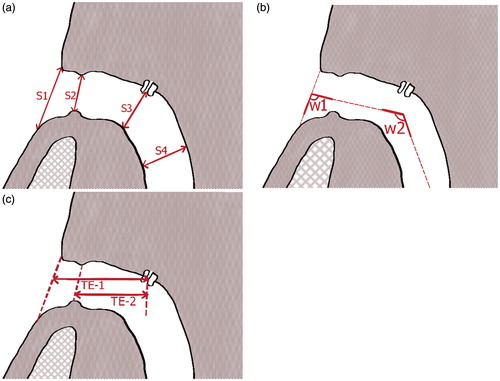
Transverse (i.e. in the transversal plane) and sagittal (i.e. in the sagittal plane) diameters
The sagittal diameters were vertically measured in the sagittal plane:
At the skin level in front of the stoma: along an extended line over the skin’s surface at the base of the patient’s neck (S1)
At the peristomal lip: the distance between the caudal and cranial wall of the tracheostoma directly at the peristomal lip (S2)
At the transition of the trachea at the TE puncture: cross sectional (S3)
At a level of one third of the sternum: cross sectional across the trachea (S4), .
The diameters T1 till T4 (not shown in ) were measured in the transversal plane perpendicular to the midpoints of S1 till S4.
Transition angles between tracheostoma lumen and trachea
The angles between the skin level and the tracheostoma lumen (w1) and the tracheostoma lumen and the trachea (w2) were quantified in the sagittal plane, in the slice at the middle of the TE valve ().
TE valve placement and stoma depth
The distances between the middle of the TE puncture and the skin level (TE-1) and the tracheostoma opening at the peristomal lip (TE-2) were measured. The difference between TE-1 and TE-2 can be regarded as the stoma depth ().
Of the 20 CT scans, one CT scan was excluded because of the absence of a TE valve. The means and standard deviations of the remaining 19 patients were calculated and compared to the values found in literature.
Results
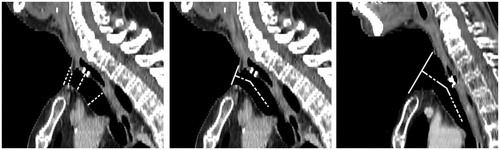
The measurements in the CT scan of two patients are used as an example, : the measurements of the sagittal tracheostoma diameters in the sagittal plane of one single patient (, left image), and the measurement of the angles of transition between the tracheostoma lumen and trachea in the sagittal plane in two patients (, middle and right image).
Figure 2. (Left) Measurement of the (vertical) sagittal diameters in the sagittal plane in Patient 8. (Middle) Measurement of the transition angles between tracheostoma lumen and trachea in the sagittal plane in Patient 8. (Right) Measurement of the transition angles between tracheostoma lumen and trachea in the sagittal plane in Patient 9.
The measurements of the different parameters is shown in . The mean tracheostoma shape is described as an elliptical shape with the longest axis in the sagittal plane at the tracheostoma opening at skin level, and as an elliptical shape with the longest axis in the transversal plane inside the tracheostoma lumen; at the peristomal lip and towards the trachea, . The mean angle w1 is 84.5° and has a large variation, thus the tracheostoma opening at skin level and the course of the tracheostoma lumen are not perpendicular to each other. Because of the significant differences in TE valve placement no cross-correlation or volumetric differences in tracheostoma morphology could be calculated between different patients.
Figure 3. The mean tracheostoma shape at the different measurement sites. The mean shape at the tracheostoma opening is described as an elliptical shape with the longest axis in the sagittal plane, and described as an elliptical shape with the longest axis in the transversal plane inside the tracheostoma lumen; at the peristomal lip and towards the trachea.
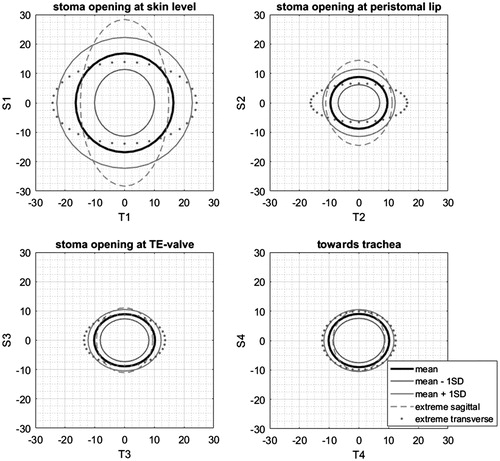
Table 1. Intratracheal morphology of the tracheostoma.
Discussion
The data outcomes show significant variation between patients. In particular, the neck opening at skin level and the stoma depth show large variation, which could most likely have occurred due to anatomically differences between patient’s neck geometry or differences in surgical procedures [Citation4,Citation5].
The mean transverse and sagittal diameters at the peristomal lip are 19.17 mm [standard deviation (SD) 5.2 mm] and 17.59 mm (SD 5.3 mm), respectively. These values are in the same order of magnitude as the horizontal and vertical tracheostoma diameters as found in literature, even though the measuring protocol differs between studies [Citation4,Citation5]. However, in this study the mean tracheostoma shape inside the tracheostoma lumen (at the peristomal lip and towards the trachea) is described as an elliptical shape with the longest axis in the transverse plane, instead of the described elliptical shape from literature with the longest axis in the sagittal plane [Citation4]. Still, for half of the measured patients, the individual transverse and sagittal diameters at the peristomal lip do correspond to the general elliptical shape as described in literature: an elliptical shape with the longest axis in the sagittal plane.
Commercially available buttons have a cylindrical shape, where the end surfaces are perpendicular to the longitudinal axis. The data outcomes show that the overall morphology of the tracheostoma has an elliptical shape, with a vertical ellipse at the tracheostoma opening at skin level and a horizontal ellipse inside the tracheostoma lumen (at the peristomal lip and towards the trachea). Also, the tracheostoma opening and the course of the tracheostoma lumen are not perpendicular to each other.
Based on the measured morphology, the angle between the ASV fixation and the intratracheal part of the commercially available button, as well as the overall button shape should be adjusted. However, due to the significant variation between patients, there is no ‘average tracheostoma morphology’ which can be used as a golden standard for new intraluminal fixation methods. Therefore, providing an airtight fixation and promoting the use of ASVs in all patients would either require a patient-specific customization as proposed in literature [Citation4,Citation9–12], a large range of different anatomically redesigned intratracheal buttons, or even a completely new fixation approach, which can deal with the variety in tracheostoma morphology.
There might be a discrepancy between the actual tracheostoma morphology and the one measured, because CT scans are acquired in a lying position. Also, the transition angles between the tracheostoma lumen itself and the natural course of the trachea was one of the hardest parameters to determine, as well as the exact location of the peristomal lip. For patients without a peristomal lip, the parameters S2 and T2 were difficult to determine and in that case the two diameters were measured at the site of the smallest stoma opening between the opening at skin level and the TE-valve. The large deviations in the parameters could also have occurred due to the bias of the study, because only a small number of CT scans were measured, only one observer measured the parameters and the used software does not select the ROI automatically, which could lead to intra-observer variability. In future studies this could be improved by performing the measurements multiple times on the same set of CT scans or by performing the measurements by multiple observers, as done in the study of Dirven et al. [Citation5]. For this study we think the parameters are clearly visible and no large differences are expected which could lead to different conclusions.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Chen AY, Fedewa S, Zhu J. Temporal trends in the treatment of early- and advanced-stage laryngeal cancer in the united states, 1985-2007. Arch Otolaryngol Head Neck Surg. 2011;137:1017–1024.
- Debry C, Dupret-Bories A, Vrana NE, et al. Laryngeal replacement with an artificial larynx after total laryngectomy: the possibility of restoring larynx functionality in the future. Head Neck. 2014;36:1669–1673.
- Boscolo–Rizzo P, Maronato F, Marchiori C, et al. Long-term quality of life after total laryngectomy and postoperative radiotherapy versus concurrent chemoradiotherapy for laryngeal preservation. Laryngoscope. 2008;118:300–306.
- Van der Houwen EB. Development of a handsfree speech valve for laryngectomy patients. [Dissertation]. The Netherlands: University of Groningen; 2012.
- Dirven R, Hilgers FJ, Plooij JM, et al. 3D stereophotogrammetry for the assessment of tracheostoma anatomy. Acta Otolaryngol. 2008;128:1248–1254.
- Hilgers FJM, Ackerstaff AH. Development and evaluation of a novel tracheostoma button and fixation system (Provox LaryButton and LaryClip adhesive) to facilitate hands-free tracheoesophageal speech. Acta Otolaryngol. 2009;126:1218–1224.
- Lorenz KJ, Groll K, Ackerstaff AH, et al. Hands-free speech after surgical voice rehabilitation with a Provox((R)) voice prosthesis: experience with the Provox FreeHands HME tracheostoma valve((R)) system. Eur Arch Otorhinolaryngol. 2007;264:151–157.
- Op de Coul BM, Ackerstaff AH, van As-Brooks CJ, et al. Compliance, quality of life and quantitative voice quality aspects of hands-free speech. Acta Otolaryngol. 2005;125:629–637.
- Ten Hallers EJ, Marres HA, Rakhorst G, et al. Difficulties in the fixation of prostheses for voice rehabilitation after laryngectomy. Acta Otolaryngol. 2005;125:804–813.
- Lemon JC, Lewin JS, Chambers MS, et al. Modification of the Barton button for tracheoesophageal speech: an innovative maxillofacial prosthetic technique. J Prosthet Dent. 2002;87:236–239.
- Lewin JS, Montgomery PC, Hutcheson KA, et al. Further experience with modification of an intraluminal button for hands-free tracheoesophageal speech after laryngectomy. J Prosthet Dent. 2009;102:328–331.
- Meyer JB Jr, Knudson RC. Fabrication of a custom recessed tracheostoma valve retainer for the total laryngectomy patient. J Prosthet Dent. 1990;63:182–186.
- Müller R, Meißner H, Böttcher G, et al. Development and first data of a customized short tracheal cannula based on digital data. Support Care Cancer. 2015;23:3089–3093.
