Abstract
Background: Human papillomavirus-positive (HPV+) base of tongue squamous cell carcinoma (BOTSCC) has a better outcome than corresponding HPV− cancer. TLR5 and TLR7 expression was previously shown to differ depending on HPV − status and correlate with outcome in oropharyngeal squamous cell carcinoma.
Aims/objectives: For validation, TLR5 and TLR7 were analyzed in a BOTSCC-cohort for correlation with HPV, survival, CD4+ and CD8+ tumor-infiltrating lymphocyte (TIL) counts, the latter being a well-documented prognostic marker.
Materials and methods: BOTSCC biopsies, (49HPV+/28HPV−) were analyzed by immunohistochemistry for TLR5 and TLR7, and correlated with the above parameters.
Results: TLR5 expression was more frequently absent/weak than medium/strong in HPV+ compared to HPV− BOTSCC (p < .001). The opposite was observed for TLR7 (p < .007). TLR5 and TLR7 expression did not correlate to survival in either the HPV− or HPV+ cases, or to CD4+ TILs. TLR5, (but not TLR7) expression was correlated to CD8+ TIL counts (p = .023).
Conclusion and significance: Absent/weak TLR5 and medium/strong TLR7 expression was validated as more frequent in HPV+ compared with HPV− BOTSCC. A correlation between CD8+ TIL counts, and TLR5 expression was disclosed, but not with TLR7. Therefore, it could be useful to investigate TLR7 further as a potential independent prognostic marker.
Chinese abstract
背景:舌鳞状细胞癌(BOTSCC)的人乳头瘤病毒阳性(HPV+ )基础具有比相应的HPV−癌更好的结果。以前曾显示, TLR5和TLR7表达根据HPV状态的不同而不同, 并且与口咽鳞状细胞癌的结果相关。
目的:为了验证, 在BOTSCC组中对TLR5和TLR7进行分析, 研究他们与HPV、存活率、CD4+ 和CD8+ 肿瘤浸润淋巴细胞(TIL)计数的相关性, 后者是被充分证明的预后标志。
材料和方法:对BOTSCC活检(49HPV+ / 28HPV−)进行免疫组织化学分析, 来研究TLR5和TLR7, 并将其与上述参数相关联。
结果:与HPV−BOTSCC相比, HPV+中TLR5表达缺失/弱比中/强更频繁(p <.001)。对于TLR7, 观察到相反的情况(p <.007)。 TLR5和TLR7表达与HPV或HPV+ 病例或CD4 + TIL的存活率无关。 TLR5(但不是TLR7)表达与CD8+ TIL计数相关(p = .023)。
结论和意义:与HPV−BOTSCC相比, 缺乏/弱TLR5和中/强TLR7表达在HPV+中被证实更频繁。发现了CD8+ TIL计数与TLR5表达之间的相关性, 但与TLR7表达的相关性还未发现。因此, 进一步研究TLR7作为潜在的独立预后标志可能是有用的。
Introduction
Patients with human papillomavirus-positive (HPV+) oropharyngeal squamous cell carcinoma (OPSCC) have a much more favorable clinical outcome than those with HPV negative (HPV−) OPSCC [Citation1–3]. In the Western world the incidences of tonsillar and base of tongue squamous cell carcinoma (TSCC/BOTSCC), the OPSCC subtypes where HPV is most common, have increased [Citation4–7]. Notably, treatment for head and neck squamous cell carcinoma include chemo-radiotherapy, targeted therapy, and surgery either alone or in combination. These treatment modalities expose the patients to risks of therapy-related side effects and complications. Most HPV+ TSCC/BOTSCC patients do not need intensified treatment, and to de-escalate treatment and reduce side effects, would be of benefit for most patients [Citation3,Citation8,Citation9]. To identify patients who will respond readily to protocol treatment, attempts have been made to find additional predictive markers, e.g. age, stage, high CD8+ tumor-infiltrating lymphocyte (TIL) counts, HPV16 E2 mRNA expression, absent/weak CD44, or high LRIG1 or CD98 expression and use mathematical models [Citation3,Citation9–14]. In addition, we recently explored the impact of Toll-like receptors (TLR) in OPSCC and their influence on survival [Citation15]. In that study tumor biopsies of 202 OPSCC diagnosed in Helsinki, Finland, were analyzed for the presence of TLR5, 7 and 9, and the expression of some of these TLRs was correlated with clinical outcome. TLR5, 7 and 9 expression differed between HPV+ and HPV− OPSCC, and in general HPV+ OPSCC exhibited lower TLR5 and higher TLR7 expression levels than HPV− OPSCC [Citation15]. Furthermore, high expression of TLR5 and low expression of TLR7 correlated with worse disease-specific survival (DSS) in HPV+ OPSCC [Citation15]. To validate the data in a new cohort in another Nordic population and in a specific OPSCC subsite, biopsies from 77 BOTSCC patients from Stockholm, Sweden, were analyzed for TLR5 and TLR7 expression and the data were correlated with clinical outcome. Since TLRs initiate immune responses [Citation15–17], we also examined if TLR5 and/or TLR7 expression was correlated with CD4+ and/or CD8+ TIL counts in the tumors, because the presence of high CD8+ TIL counts is reported to be a good prognostic marker in HPV+ OPSCC [Citation9,Citation12,Citation13].
Materials and methods
Patients and tumor characteristics
Patients diagnosed from 2000 to 2011 at the Karolinska University Hospital with BOTSCC (ICD-10 code C01.9), were analyzed for TLR5 and TLR7 expression by immunohistochemistry (IHC) in order to retrospectively compare TLR5 and TLR7 expression in patients with HPV+ and HPV− tumors. Details of the patients and their tumors are presented in together with p values. HPV+ BOTSCC was defined as having an HPV DNA positive tumor with p16INK4A overexpression (p16+) [Citation18]. In total, 49 HPV+ BOTSCCs and 28 HPV− BOTSCCs fulfilling these criteria according to our previous studies [Citation5,Citation7,Citation14] were analyzed for TLR5 and TLR7 expression [Citation15]. The study was performed in compliance with permission (2009/1278-31/4) granted by the Regional Ethical Review Board in Stockholm.
Table 1. Patient and tumor characteristics.
Analysis of HPV DNA, p16 overexpression, and CD4+ and CD8+ TILs
All samples had been tested for HPV DNA by a PCR-based bead-based multiplex-assay on a MagPix instrument (Luminex Inc.) as described before [Citation19]. p16 had been assayed using the monoclonal antibody (mAb) clone JC8 (Santa Cruz Biotech, Santa Cruz, CA), or the E6H4™ mouse mAb clone (CINtec®, Ventana, Tucson, AZ) and CD4+ and CD8+ with the mAb clone 1FC and 4B11 respectively, (Leica Biosystems, Wetzlar, Germany) [Citation19].
Immunohistochemistry for TLR 5 and 7
TLR5 and 7 expression by IHC on 4 μm FFPE sections were investigated and has been reported before [Citation15]. Monoclonal mouse anti-human TLR5 (1:200, IMG-664A Imgenex) and monoclonal rabbit anti-human TLR7 (1:300, IMG-581A Imgenex) were used. All tissue samples were evaluated separately for TLR5 and 7 expression by light microscopy, by two researchers (LH and AN), of which the latter is a resident in surgical pathology. The samples were evaluated independently, without the knowledge of clinical parameters. The scoring of the two researchers was similar in the majority of the cases, and when not, a consensus was reached. TLR5 intensity, mainly localized to the cell membranes, was scored as: absent (0), weak (1), medium (2), and strong (3). TLR7 intensity, mainly localized to the nuclear membrane and nucleus, was scored as absent (0) if no TLR7 was observed, weak (1) if some nuclear membranes were positive, medium (2) if all nuclear membranes and some nuclei were stained, and strong (3) if nuclear membranes and nuclei were stained substantially. Cases, where the staining was not possible to be evaluated, were excluded.
Statistical analysis
Categorical variables (e.g. TLR5 and TLR7 expression by IHC) were compared with the Chi2-test or Fisher's exact test, and continuous variables (CD8+ TIL counts) with Student’s t-test. Clinical outcome was measured as 3-year disease-free survival (DFS) or 3-year disease-specific survival (DSS). Only patients treated with intent to cure were included in the analysis. All patients were followed up for 3-years and then censored. DFS was defined as the time from diagnosis until date of relapse in disease. Patients being never tumor-free were censored on day 0, while patients dying without prior recurrence were censored at the time-point, when assessing DFS. DSS was defined as the time from diagnosis until date of relapse or death with the disease. Survival curves with 3-year DFS and DSS were calculated using the Kaplan–Meier method and differences in survival were tested using the log-rank test. All statistical tests were performed using SPSS (SPSS Statistics for Mac, Version 25. Armonk, NY: IBM Corp.).
Results
Analysis of TLR5 and TLR7 expression by IHC in HPV+ and HPV− BOTSCC
Totally, 49 HPV+ and 28 HPV− BOTSCC samples were examined for the expression of TLR5 and TLR7 by IHC (). Absent/weak TLR5 staining was observed in 41/49 (84%) HPV+ BOTSCC and 12/28 (43%) of HPV− BOTSCC (p < .0001). In contrast, TLR7 was more often absent/weak in HPV− BOTSCC (26/28, 93%) as compared with HPV+ BOTSCC (32/49, 65%) (p = .007).
TLR5 and TLR7 expression, and CD8+ TILs, in relation to outcome in HPV+ and HPV− BOTSCC
The presence of absent/weak TLR5 and TLR7 expression was then correlated with prognosis among the 68 patients in the whole cohort, and separately among the 47 patients with HPV+ BOTSCC, and the 21 patients with HPV− BOTSCC, who had been treated with curative intent, although in the latter group the patient number was limited.
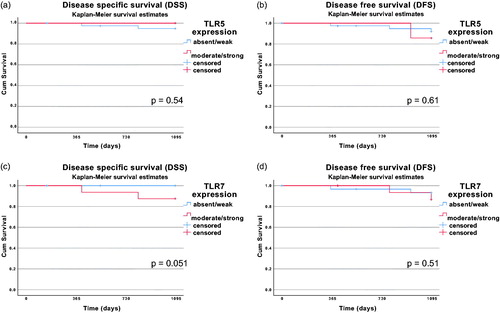
In all the examined cases, absent/weak TLR5 or TLR7 did not have a statistically significant correlation with clinical outcome defined as OS, DFS or DSS. For HPV+ BOTSCC, Kaplan–Meier graphs for DFS and DSS are depicted for absent/weak and moderate/high TLR5 expression, as well as for absent/weak and moderate/high TLR7 expression in .
Figure 1. Kaplan–Meier curves with 3-year disease-specific survival (DSS) and 3-year disease-free survival (DFS) for patients with HPV+ BOTSCC stratified for absent/weak vs. medium/strong TLR5 expression (A and B) and absent/weak vs. medium/strong TLR7 expression (C and D). Differences in survival was analyzed with the log-rank test. No differences in survival were observed (TLR5 DSS: p = .54; TLR5 DFS: p = .61; TLR7 DSS: p = .051; TLR7 DFS: p = .51).
TLR5 and TLR7 expression in correlation to CD8+ and CD4+ TIL counts
The presence of TLR5 and TLR7 expression in correlation with CD4+ and CD8+ TIL counts was examined in all patients. Patients with absent/weak TLR5 expression had significantly higher CD8+ TIL counts (p = .023), but no correlation was found for CD4+ TIL counts (p = .17). In addition, for patients with HPV+ BOTSCC the correlation between absent/weak TLR5 expression and high CD8+ TIL counts remained (p = .038), while this was not the case for corresponding HPV− BOTSCC cases p = .92. No correlation could be observed either between TLR7 expression and CD4+ TIL or CD8+ TIL counts (p = .43 and p = .58, respectively), or when dichotomizing the patients into those with HPV+ or HPV− BOTSCC (p = .17 and p = .94, respectively).
Discussion
This study aimed to validate whether TLR5 and TLR7 expression differed between HPV+ and HPV− BOTSCC (i.e. in a specific subtype of OPSCC), as has been previously shown in HPV+ and HPV− OPSCC and whether TLR5 and TLR7 expression would correlate with survival in HPV+ BOTSCC [Citation15]. Our results confirmed that absent/weak TLR5 and medium/strong TLR7 expression was more frequently found in HPV+ compared with HPV− BOTSCC [Citation15,Citation20].
However, it was not possible to confirm with statistical significance that moderate/strong TLR5 or absent/weak TLR7 expression would correlate with worse outcome irrespective of tumor HPV status. TLR5 and TLR7 expression was also analyzed for correlation with CD4+ and CD8+ TIL counts and a statistically significant correlation between absent/weak TLR5 expression and the presence of high CD8+ TIL counts in all patients, as well as in patients with HPV+ BOTSCC, was shown. There was, however, no correlation either between TLR7 expression and CD8+ TIL counts or between CD4+ TIL counts, and TLR5 and TLR7 expression.
Consequently, in this Scandinavian BOTSCC cohort, we confirmed similar results compared with the previous study in an OPSCC cohort [Citation15] stating that the expression of TLR5 and TLR7 varied according to HPV status, but not that moderate/strong TLR5 and absent/weak TLR7 expression correlated with survival. In fact, the trend was the opposite for DSS and TLR7 expression. The reasons for the latter discrepancies in clinical outcome and TLR5 and TLR7 expression between this study and the aforementioned study on OPSCC are unknown. The most plausible explanation could be that in this study the number of events was limited due to the favorable outcome for patients with HPV+ BOTSCC. Furthermore, the limited size of the cohort had an obvious effect on possibilities to encounter such events [Citation15]. Nevertheless, as discussed further below, the potential role of TLR7 expression on survival should be studied further in a larger HPV+ BOTSCC cohort, and if possible with proteomic and transcriptomic analysis.
For HPV− BOTSCC no correlation between TLR5 and TLR7 expression was found, similarly to previously reported results [Citation15]. This could, however, be due to that in our study the number of HPV− cases were limited.
In this report, TLR5 and TLR7 expression was also investigated for possible correlation with the presence of CD4+ and CD8+ TIL counts since TLRs recognize molecular patterns, which can initiate immunological defense mechanisms against bacteria and viruses [Citation17]. High CD8+ TIL counts correlated with absent/weak TLR5 expression. This finding was not entirely unexpected since both absent/weak TLR5 expression, as well as high CD8+ TIL counts, are correlated with the presence of HPV [Citation11,Citation15].
There are some limitations in this study. Here, only BOTSCC samples were examined and no TSCC cases were included. More importantly, this gave us an opportunity to really focus on one tumor subtype and clearly, the association between absent/weak TLR5 and medium/strong TLR7 expression with HPV− positivity remained. This confirms that the HPV+ and HPV− BOTSCC behave differently regarding their immunological response, and thus are justly separated to different entities according to the latest WHO Head and Neck Pathology book (WHO Classification of Head and Neck tumors, Lyon 2017). Furthermore, due to the few events in this cohort among the HPV+ BOTSCC cases, we could not evaluate the correlation of the expression results with survival.
To conclude, in accordance with previous studies we could validate and confirm the presence of absent/weak TLR5 and medium/strong TLR7 expression in the HPV+ BOTSCC subtype, but not their correlation with clinical outcome. However, since TLR7 expression contrary to TLR5, was not correlated to CD8+ TIL counts, which itself being a very strong predictive marker, more studies on TLR7 as a prognostic marker are warranted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mellin H, Friesland S, Lewensohn R. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89:300–304.
- Attner P, Du J, Nasman A, et al. Human papillomavirus and survival in patients with base of tongue cancer. Int J Cancer. 2011;128:2892–2897.
- Dalianis T. Human papillomavirus and oropharyngeal cancer, the epidemics, and significance of additional clinical biomarkers for prediction of response to therapy. Int J Oncol. 2014;44:1799–1805.
- Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers?. Cancer. 2007;110:1429–1435.
- Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma?. Int J Cancer. 2009;125:362–366.
- Attner P, Du J, Nasman A, et al. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int J Cancer. 2010;126:2879–2884.
- Nasman A, Nordfors C, Holzhauser S, et al. Incidence of human papillomavirus positive tonsillar and base of tongue carcinoma: a stabilisation of an epidemic of viral induced carcinoma?. Eur J Cancer. 2015;51:55–61.
- Mirghani H, Amen F, Blanchard P, et al. Treatment de-escalation in HPV − positive oropharyngeal carcinoma: ongoing trials, critical issues and perspectives. Int J Cancer. 2015;136:1494–1503.
- Nasman A, Bersani C, Lindquist D, et al. Human papillomavirus and potentially relevant biomarkers in tonsillar and base of tongue squamous cell carcinoma. Anticancer Res. 2017;37:5319–5328.
- Lindquist D, Ährlund-Richter A, Tarjan M, et al. Intense CD44 expression is a negative prognostic factor in tonsillar and base of tongue cancer. Anticancer Res. 2012;32:153–161.
- Nordfors C, Grun N, Tertipis N, et al. CD8+ and cD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013;49:2522–2530.
- Tertipis N, Hammar U, Nasman A, et al. A model for predicting clinical outcome in patients with human papillomavirus-positive tonsillar and base of tongue cancer. Eur J Cancer. 2015;51:1580–1587.
- Bersani C, Mints M, Tertipis N, et al. A model using concomitant markers for predicting outcome in human papillomavirus positive oropharyngeal cancer. Oral Oncol. 2017;68:53–59.
- Bersani C, Sivars L, Haeggblom L, et al. Targeted sequencing of tonsillar and base of tongue cancer and human papillomavirus positive unknown primary of the head and neck reveals prognostic effects of mutated fgfr3. Oncotarget. 2017;8:35339–35350.
- Jouhi L, Mohamed H, Mäkitie A, et al. Toll-like receptor 5 and 7 expression may impact prognosis of HPV − positive oropharygeal squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1619–1629.
- Janeaway CAJR. Approaching the asymptote. Evolution and revolution in immunology. Cold Spring Harb Syp Quant Biol. 1989;54:1–13.
- Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–344.
- Smeets SJ, Hesselink AT, Speel E-JM, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121:2465–2472.
- Dalianis T, Grun N, Koch J, et al. Human papillomavirus DNA and p16(ink4a) expression in hypopharyngeal cancer and in relation to clinical outcome, in stockholm, sweden. Oral Oncol. 2015;51:857–861.
- Jouhi L, Datta N, Renkonen S, et al. Expression of toll-like receptors in HPV − positive and HPV − negative oropharyngeal squamous cell carcinoma–an in vivo and in vitro study. Tumor Biol. 2015;36:7755–7764.
