Abstract
Background
Oropharyngeal squamous cell carcinoma (OPSCC) is dominated by tonsillar and tongue base carcinomas (TSCC/BOTSCC), but there are carcinomas at other sites, such as uvula/soft palate/pharyngeal wall here defined as other OPSCC. Human papillomavirus (HPV) positive TSCC/BOTSCC have favorable outcome, and the TNM-classification separates OPSCC into HPV mediated (p16INK4a overexpressing, p16+) and HPV unrelated OPSCC (p16INK4a non-overexpressing, p16-) cancer, but the prognostic role of p16+ in other OPSCC is unclear.
Aims/Objectives
This study therefore aimed to further investigate the prognostic role of p16+, presence of HPV DNA, or both combined in other OPSCC.
Material and methods
195 other OPSCC, from patients diagnosed 2000–2018 were tested for p16, and/or presence of HPV DNA and the data correlated to outcome.
Results
Neither overall survival, nor disease free survival correlated to presence of p16+ or HPV DNA in other OPSCC. p16+ and HPV DNA presence were correlated (p < .0001), but the sensitivity of p16 as a surrogate marker for presence of HPV DNA was low (49%).
Conclusions and significance
The data suggest that p16+ (and p16+/HPV DNA) positive other OPSCC should be analyzed cautiously and possibly separately from the HPV mediated OPSCC staging group.
Chinese abstract
背景:口咽鳞状细胞癌(OPSCC)主要为扁桃体和舌头基础癌(TSCC / BOTSCC), 但其它部位也会有癌, 例如悬雍垂/软腭/咽壁, 在此定义为其它OPSCC。人乳头瘤病毒(HPV)阳性TSCC / BOTSCC具有良好的结果, TNM分类将OPSCC分为HPV介导的(p16INK4a过表达, p16 þ)与HPV不相关的OPSCC(p16INK4a非过表达, p16-)癌症, 但是p16þ在其它OPSCC中的预后作用尚不清楚。
目的:本研究旨在进一步研究p16þ、HPV DNA的存在或两者在其他OPSCC中联合使用的预后作用。
材料和方法:对2000年至2018年确诊的195例其它OPSCC进行了p16、和/或HPV DNA存在以及与结果相关的数据的测试。
结果:在其它OPSCC中, 总体生存率和无病生存率均与p16þ或HPV DNA的存在无关。 p16þ和HPV DNA的存在是相关的(p <.0001), 但是作为HPV DNA存在的替代标志物, p16的敏感性很低(49%)。
结论和意义:数据表明, 其它OPSCC中的p16þ(以及p16þ/ HPV DNA)阳性应得以仔细分析, 并可能与HPV介导的OPSCC分期组分开进行分析。
Introduction
In many Western countries, a large proportion of oropharyngeal squamous cell carcinoma (OPSCC), which is dominated by tonsillar and base of tongue squamous cell carcinoma (TSCC/BOTSCC), is human papillomavirus positive (HPV+) [Citation1–5]. In addition, patients with HPV+ TSCC/BOTSCC have a more favorable clinical outcome than those with corresponding HPV negative (HPV-) cancer [Citation1,Citation6,Citation7]. This has also been proposed for all HPV+ OPSCC as compared to HPV- OPSCC [Citation8].
p16 INK4a overexpression (p16+) is used as a surrogate marker for presence of HPV in the latest American Joint Committee on Cancer/Union for International Cancer Control (AJCC-8/UICC-8) staging system for OPSCC, which separates the TNM classification of HPV mediated (p16+) and HPV unrelated (p16-) OPSCC [Citation9]. However, an estimated 10–20% of all OPSCCs are p16-positive, but HPV-, being most apparent in OPSCC arising outside the tonsils and base of tongue, such as e.g. other sites include the uvula/soft palate/pharyngeal wall, here defined as other OPSCC [Citation2,Citation10–13]. We and others have previously reported that presence of HPV DNA and p16+ was much less common in other OPSCC, and that presence of HPV DNA or p16+ in these tumors did not correlate well to each other, or to better clinical outcome [Citation2,Citation10–12].
It has been suggested that it should be possible to de-escalate today’s more intensified treatment, i.e. chemo-radiotherapy, targeted therapy, and surgery either alone or in combination for patients with HPV+ OPSCC, in order to reduce therapy-related side effects and complications [Citation1]. Patients with other OPSCC are often included into the same studies and treatment protocols as patients with TSCC/BOTSCC, even though earlier studies by us and others have indicated that prevalence, clinical significance and the correlation between HPV and p16+ is markedly lower in other OPSCC [Citation2,Citation11,Citation13]. Since TSCC/BOTSCC dominates OPSCC with roughly 90% of all cases, there is an obvious risk that the results from patients with other OPSCC are concealed and misinterpreted [Citation2]. Thus, if the basis of selection i.e. p16+ as a surrogate marker for HPV, does not apply to better clinical outcome for patients with other OPSCC as indicated before by us and others, this definitely presents a problem [Citation1,Citation11,Citation12,Citation14].
Based on the above, we wanted to examine if p16 overexpression, indeed was an adequate surrogate marker for HPV, as well as prognostic favorable factor in OPSCC other than TSCC and BOTSCC. For this purpose, we extended our previous study of patients diagnosed 2000-2008 with OPSCC at sites outside the tonsil and base of tongue (other OPSCC) [Citation11], and included patients diagnosed from 2000 until 2018 to examine if p16 overexpression is an accurate marker for HPV as well as a prognostic favorable factor in OSCC other than TSCC and BOTSCC.
Material and methods
Patients and tumor samples
195 patients diagnosed 2000–2018 with other OPSCC, i.e. including cancer of the uvula, the soft palate and the pharyngeal walls (ICD-10: C10.0–C10.9 and C50.1–C50.8) at Karolinska University Hospital were included in the analysis. The diagnosis and TNM stage was established at a multidisciplinary conference. The diagnosis was based on physical examination, ultrasound, with fine needle aspiration in case of N+, computer tomography, and/or magnetic resonance imaging scans followed by physical examinations (panscopy) in anesthesia and biopsy material and exscised tumor material were subjected to pathological diagnostics. Patient case reports were analyzed, and age, gender, TNM-stage, treatment and survival were recorded (LM, LH). Treatment was categorized as surgery, radiotherapy or chemoradiotherapy, with the vast majority of patients receiving radiotherapy, or chemoradiotherapy. Patients were evaluated for tumor progression every 3 months the first 2 years, and then every 6 months for a total of 5 years. From these, 75 patients, diagnosed 2000–2008, had 69 tumor biopsies available, previously analyzed for p16 expression and presence of HPV DNA, and 61 of these, treated with curative intent, had earlier been included in a survival analysis [Citation11]. Here, for an additional 120 tumor patients, tumor biopsies were analyzed as depicted below, for p16 overexpression and/or presence of HPV DNA. This, allowed for in total 124 patients (with tumors with data on p16 overexpression) treated curatively to be included in the survival analysis (). The study was performed according to permissions (2009/1278-31/4 and 2018/870-32) from the Stockholm Regional Ethical Review Board.
Table 1. Patient and tumor characteristics separated by tumor p16 expression.
Analysis of HPV DNA, p16 overexpression, other OPSCC samples
The previously non-analyzed samples were tested for DNA of 27 HPV types, including all high-risk types, by a PCR-based bead-based multiplex-assay on a MagPix instrument (Luminex Corp., Austin, TX, USA) as described before [Citation15]. p16 overexpression (p16+), i.e. >70% of the tumor cells being strong cytoplasmic and nuclear p16 positive, was tested by immunohistochemistry using the monoclonal antibody (mAb) clone JC8 (Santa Cruz Biotech, Santa Cruz, California, USA), or the E6H4TM mouse mAb clone (CINtec®, Ventana, Tucson, Arizona, USA) [Citation15].
Statistical analysis
Differences in categorical data were examined by Chi2 test, and continuous data were assessed by two-sided student t-test. Outcome was analyzed as disease free survival (DFS) or overall survival (OS). DFS was defined as day of diagnosis until day of any relapse. Patients never tumor-free were censored day 0, and patients dying without recurrence were censored at the time-point, when assessing DFS. OS was defined as day of diagnosis until day of death irrespective of cause of death. Survival curves with DFS, and OS were calculated using the Kaplan–Meier method. Differences in survival were calculated using the log-rank test. In addition, because of treatment differences in these patient groups, we performed a subgroup analysis in patients with WHO performance status 0–1. Only patients treated curative intent, and that completed their treatment, were included in the survival analysis. All statistical tests were performed using SPSS (SPSS Statistics for Mac, Version 25. Armonk, NY: IBM Corp. USA).
Results
Analysis of p16 and HPV in other OPSCC
Data on either p16 expression, and/or presence of HPV were obtained for totally 195 patients with other OPSCC, when summing up data obtained earlier for 69 patients [Citation11]. The data and patient characteristics are shown in according to the p16 status of the tumors. p16 expression was obtained for 147 cases; data on presence/absence of HPV was available for 153 tumors; and data for both was obtained for 128 tumors. p16+ was found in 35/148 (24%) of the cases; HPV DNA (all from high-risk HPV types) was present in 31/154 (20%) cases; and both p16+ and HPV DNA were present in in 17/128 (13%) of the cases, while 82/128 (64%) were both p16- and HPV DNA negative (). HPV DNA positive tumors were p16+ significantly more often than HPV DNA negative tumors (p < .0001) (). However, the sensitivity for p16+ as a surrogate marker for presence of HPV DNA was low 17/35 (49%), while specificity was 88%.
Table 2. Correlation between p16 expression and HPV DNA status.
p16, HPV DNA and combined p16 and HPV DNA status in relation to outcome in other OPSCC
Of all the 195 patients, 148/195 (76%) were treated with curative intent and of those 75 (51%) had a 3-year OS. Treatment and TNM-7 stage were both in uni- and multivariable analysis correlated with 3-year OS, but not to 3-year DFS (). Age (below/above mean age) was not correlated to neither 3-year OS nor 3-year DFS (data not shown).
Table 3. Uni- and multivariable analysis with overall and disease-free survival in patients with other OPSCC.
Among patients with other OPSCC and data on p16, comparing those with p16+ to those with p16-, 18/31 (58%) vs. 40/93 (43%) had a 3-year OS (log-rank test: p = .2) (). Similarly, no difference in 3-year DFS was observed between patients with p16+ as compared to those with p16- tumors, 77% vs. 78%; log rank test: p = .8 ().
Figure 1. Overall survival (OS) and disease free survival (DFS) in correlation to presence or absence of p16 overexpression and HPV DNA in non-tonsillar and non-base of tongue oropharyngeal squamous cell carcinoma. (A) OS in correlation to p16 overexpression or not; (B) DFS in correlation to p16 overexpression or not; (C) OS in correlation to presence or absence of HPV DNA; (D) DFS in correlation to presence or absence of HPV DNA; (E) OS in correlation to both presence of p16 overexpression and presence of HPV DNA or remaining cases; and (F) DFS in correlation to both presence of p16 and presence of HPV DNA or remaining cases.
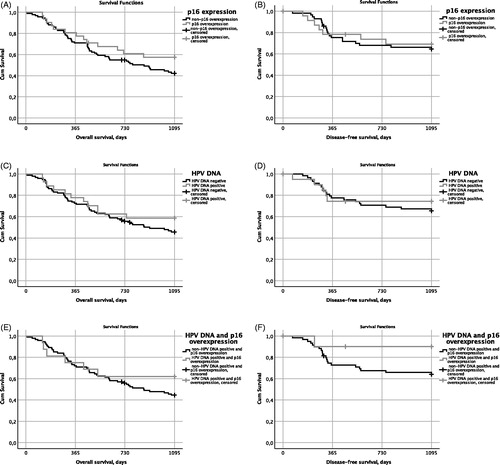
Analyzing patients with other OPSCC with data on HPV DNA, and comparing the ones with HPV DNA positive tumors to those with HPV DNA negative tumors, 16/27 (59%) vs. 44/95 (46%) had a 3-year OS (p = .3) (). No differences were observed in 3-year DFS between patients with HPV DNA positive and negative tumors (82% vs. 79%, log rank test: p = .6) ().
For patients with data on both HPV DNA and p16 expression, comparing those with p16+ and HPV DNA positive tumors to the remaining patients, 10/16 (63%) vs. 42/93 (45%) had a 3-year OS (log rank test: p = .3) (). Also, for this category, no differences were observed in 3-year DFS between patients with HPV DNA positive and p16+ tumors and the remaining patients (94% vs. 77%, log rank test: p = .1) ().
Furthermore, no differences were observed in 3-year OS or 3-year DFS between patients younger or older than the mean age of the study population, or with regard to gender (data not shown). Moreover, p16 expression did not discriminate OS or DFS even if patients with smaller tumors and larger tumors were analyzed separately (T1-T2: p16+ vs. p16-: log rank test: p = .3 and p = .1 respectively, T3-T4: p16+ vs. p16-: log rank test, p = .3 and p = .3 respectively) (data not shown). Finally, when adjusting for (WHO) performing status according to p16 status, no changes in statistical significance were observed within any of the groups (data not shown).
Discussion
In this study, we show that most other OPSCC, was p16-, lacked presence of HPV DNA and was not p16+ and HPV DNA positive, and despite there was a correlation between p16+ and presence of HPV DNA, the sensitivity of p16+ as a predictor for presence of HPV DNA was poor. More importantly, there were no statistically significant differences in survival between patients with HPV DNA positive/negative, p16+/p16-, or p16 and HPV DNA positive/remaining other OPSCC treated with curative intent.
Our results thereby confirm previous data by others and us, where we showed that p16 overexpression and presence of HPV DNA was markedly less frequent in other OPSCC compared to TSCC/BOTSCC [Citation2,Citation11,Citation13]. Moreover, although p16 and HPV status were significantly correlated to each other, the sensitivity of p16 as a surrogate marker of HPV infection was lower here in other OPSCC, as compared to the sensitivity reported in TSCC/BOTSCC [Citation16]. More specifically, only 61% (17/28) of the HPV DNA positive tumors also overexpressed p16, and only 49% (17/35) of p16+ tumors were also HPV DNA positive. This observation may be of great importance since p16 is regarded as a surrogate marker for active HPV infection in OPSCC, and that the AJCC (8th Ed) TNM-staging system now separates HPV mediated (p16+) OPSCC from HPV unrelated (p16-) OPSCC. While the latter may hold true for most, but not all cases of TSCC and BOTSCC, this is likely not the case for other OPSCC [Citation11,Citation12].
The low prevalence of p16 overexpression and presence of HPV DNA in other OPSCC, in this study (24% and 20% respectively) were very similar to those obtained in our previous study, i.e. 25% and 17% respectively, as well as to the data in the systematic analysis study [Citation2,Citation11]. Furthermore, combining HPV DNA and p16 overexpression in other OPSCC, similar to the suggested golden standard (HPV E6 and E7 mRNA positivity) regarded as indicating active HPV positive status[Citation12], the proportion was even lower (13%). Also, here the trend was similar to the data obtained in our previous study (12%) as well as to the data in the metanalysis study [Citation2,Citation11]. Nevertheless, the low p16 overexpression and HPV prevalence in other OPSCC, irrespective of how it was assayed for, was not unexpected, since the epithelial tissue of these tumors differs from that of TSCC and BOTSCC, that are mainly lymphoepithelial origin [Citation2].
Similar to our previous study, neither p16 overexpression, nor presence of HPV DNA alone, in other OPSCC, were correlated to better clinical outcome, since no significant differences were disclosed for either OS or DFS [Citation11]. Moreover, when smaller and larger other OPSCC were analyzed separately, still no difference in survival between p16+ other OPSCC and p16- other OPSCC was observed. Combined presence of p16 overexpression and HPV DNA present in 13% of the samples, was not correlated to better OS or DFS either, whether this was due to the very low numbers of patients or not, needs likely to be confirmed in an even larger cohort. Nonetheless, the data suggest that this patient group, should be regarded with some caution and not be the first group, to introduce treatment de-escalation.
It has been suggested that it should be possible to de-escalate today’s more intensified treatment, i.e. chemo-radiotherapy, targeted therapy, and surgery either alone or in combination for patients with HPV+ OPSCC, in order to reduce therapy-related side effects and complications [Citation1]. Attempts have also been made to find more predictive markers to use in combination with HPV-status, e.g. age, stage, high CD8+ tumor infiltrating lymphocyte (TIL) counts, HPV16 E2 mRNA expression, absent/weak CD44, or high LRIG1 or CD98 expression to better select patients for de-escalated therapy [Citation1]. Today there are several ongoing clinical trials including patients with OPSCC based on p16-status [Citation17,Citation18]. If the basis of selection, i.e. HPV status, or p16 status as a surrogate marker for HPV, does not apply to better clinical outcome for other OPSCC, this definitely presents a problem [Citation1,Citation11]. Hence, it may be suboptimal to stratify patients with other OPSCC to de-escalation studies based on evaluation of a single biomarker (i.e. p16 alone) due to the risk of misclassification of tumors and thereby misallocation of patients with an undesired prognosis [Citation19].
This study has limitations. First of all, it is a retrospective clinical analysis study with prospectively collected patient data. However, given the small number of cases, it would require a large multicenter approach to run this study prospectively. Secondly, the different treatment approaches are not adjusted for. Finally, we could not report smoking data, since these are not given in detail in our patients case reports. Given the fact that there is a great variation and fairly few cases with few ‘events’, we decided to use low performance status (WHO performance score 0-1) as a proxy for curative treatment in its true sense. This did not however, change the deduction.
To summarize, in this expanded study of other OPSCC, the sensitivity for p16+ as a surrogate marker for presence of HPV DNA was low, and although presence of p16 overexpression and HPV DNA correlated, neither p16+ status nor HPV DNA positive status correlated to OS or DFS.
The obtained data, i.e. that the sensitivity of p16+ as a surrogate marker for presence of HPV DNA was low, and that neither p16+ nor HPV DNA positive status correlated to OS or DFS in other OPSCC suggest that other OPSCC differs from TSCC/BOTSCC and should be analyzed separately and cautiously. However, it is important that additional studies with larger cohorts of other OPSCC are conducted. Should additional studies also confirm our data, then it would be safer to exclude other OPSCC from the p16 overexpressing OPSCC group in the recent AJCC 8th Edition. In the meanwhile, we suggest that greater caution for this group of patients is warranted.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nasman A, Bersani C, Lindquist D, et al. Human papillomavirus and potentially relevant biomarkers in tonsillar and base of tongue squamous cell carcinoma. Anticancer Res. 2017;37(10):5319–5328.
- Haeggblom L, Ramqvist T, Tommasino M, et al. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res. 2017;4:1–11.
- Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–1435.
- Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125(2):362–366.
- Attner P, Du J, Nã¤Sman A, et al. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int J Cancer. 2010;126(12):NA–84.
- Attner P, Du J, Nasman A, et al. Human papillomavirus and survival in patients with base of tongue cancer. Int J Cancer. 2011;128(12):2892–2897.
- Mellin H, Friesland S, Lewensohn R, et al. Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer. 2000;89(3):300–304.
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720.
- Amin MB, Edge SB, Gress DM, et al., editors. American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging manual. 8th ed. Chicago, IL: American Joint Committee on Cancer, Springer; 2017.
- Ljokjel B, Lybak S, Haave H, et al. The impact of HPV infection on survival in a geographically defined cohort of oropharynx squamous cell carcinoma (OPSCC) patients in whom surgical treatment has been one main treatment. Acta Otolaryngol. 2014;134(6):636–645.
- Marklund L, Nasman A, Ramqvist T, et al. Prevalence of human papillomavirus and survival in oropharyngeal cancer other than tonsil or base of tongue cancer. Cancer Med. 2012;1(1):82–88.
- Smeets SJ, Hesselink AT, Speel EJ, et al. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer. 2007;121(11):2465–2472.
- Gelwan E, Malm IJ, Khararjian A, et al. Nonuniform distribution of high-risk human papillomavirus in squamous cell carcinomas of the oropharynx: rethinking the anatomic boundaries of oral and oropharyngeal carcinoma from an oncologic HPV perspective. Am J Surg Pathol. 2017;41(12):1722–1728.
- Tham T, Ahn S, Frank D, et al. Anatomical subsite modifies survival in oropharyngeal squamous cell carcinoma: National Cancer Database study. Head Neck. 2020;42(3):434–445.
- Dalianis T, Grun N, Koch J, et al. Human papillomavirus DNA and p16(INK4a) expression in hypopharyngeal cancer and in relation to clinical outcome, in Stockholm, Sweden. Oral Oncol. 2015;51(9):857–861.
- Nasman A, Andersson E, Marklund L, et al. HLA class I and II expression in oropharyngeal squamous cell carcinoma in relation to tumor HPV status and clinical outcome. PLoS One. 2013;8(10):e77025.
- Fakhry C, Zhang Q, Gillison ML, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: Implications for risk-based therapeutic intensity trials. Cancer. 2019;125(12):2027–2038.
- Jones DA, Mistry P, Dalby M, et al. Concurrent cisplatin or cetuximab with radiotherapy for HPV-positive oropharyngeal cancer: Medical resource use, costs, and quality-adjusted survival from the De-ESCALaTE HPV trial. Eur J Cancer. 2020;124:178–185.
- Craig SG, Anderson LA, Schache AG, et al. Recommendations for determining HPV status in patients with oropharyngeal cancers under TNM8 guidelines: a two-tier approach. Br J Cancer. 2019;120(8):827–833.
