Since the first descriptions of superior canal dehiscence (SCD) syndrome, it has been widely demonstrated how a bony defect overlying the superior semicircular canal (SSC) can result in a low-impedance pathway for sound and pressure stimuli, resulting in a variety of audio-vestibular symptoms and signs consistent with a third window mechanism [Citation1–3]. Although physiologists and clinicians have clearly explained the mechanisms accounting for both low-frequencies conductive hearing loss on audiometry and enhanced amplitudes of vestibular-evoked myogenic potentials (VEMPs), mismatching results have been achieved in the interpretation of the vestibulo-ocular reflex (VOR) data for the affected SSC. In fact, whilst it has been reported how sound and/or pressure stimuli can result in eye movements aligning with the plane of the dehiscent SSC [Citation1,Citation2,Citation4], studies performed either with magnetic-scleral search coils [Citation2,Citation5] or with the video-head impulse test (vHIT) [Citation6,Citation7] observed that VOR-gain values for the affected SSC could be impaired. These questions have strongly arisen thanks to the growing accessibility of canal VOR-gain measurements in the high-frequency domain promoted by the recent introduction of the vHIT in clinical practice. Our aim is to point out some aspects resulting from our clinical experience in this field, and to offer further possible interpretations for some apparently mismatching data with a common theory accounting for overall measurements of SSC activity in case of SCD.
Correlations between SCD size and vHIT measurements
As already conducted for other cochlear and vestibular data in several investigations [Citation6,Citation8–10], VOR-gain values of the dehiscent SSC have also been related to SCD size. Even though dehiscence size did not correlate with vHIT measurements from the data analysis of a recently published investigation on 8 patients (11 ears) [Citation7], this finding is not in line with most data available in literature. In fact, besides previous studies on small-sized cohorts reporting how SSC activity for high-frequency inputs impairs in case of large dehiscences [Citation2,Citation5], a recent correlation analysis on a large series including 95 SCD (73 patients) clearly confirmed an inverse relationship between SCD size and SSC VOR-gain on vHIT [Citation6]. The reason for the discrepancy among results yielded in different studies could be attributed both to the wide difference in the size of cohorts analysed and to the possible different methods used for measuring the length of the bony defects. On the other hand, it could be considered that, during high-accelerations head impulses, an altered balance of fluid dynamics at the defect could occur in some patients, resulting in membranous canal indentations, generating a transient mechanical occlusion of the canal [Citation4]. Therefore, this condition might partly account for different vHIT measurements among subjects, irrespective to the size of the defect.
Possible explanations accounting for reduced SCC VOR-gain on vHIT in SCD
It has mainly been speculated that the functional impairment of SSC could be the result of a progressive herniation of middle fossa dura through the dehiscence, which might plug the membranous SSC, preventing dynamic responses for the affected canal, likewise a surgical plugging [Citation2,Citation5]. Nevertheless, despite it might be highly plausible [Citation11], it has been highlighted how these patients rarely exhibit VEMPs with normal/reduced amplitudes and/or normal/increased thresholds, near-normal air-conducted threshold for low frequencies on audiometry and lack of sound/pressure-induced nystagmus [Citation6,Citation7] as one would expect from a surgical canal repair [Citation5,Citation8,Citation12]. Conversely, most of them develop clinical and instrumental findings consistent with reduced inner ear impedance proper to a third window lesion, as if dehiscence was particularly “active” within a patent SSC (). In our opinion, these apparently mismatching data could be explained by two additional theories: incomplete canal plugging and dissipation of mechanical energy.
Table 1. Possible clinical/instrumental scenarios in case of intact SSC, small/wide SCD and surgically plugged SCD.
Incomplete canal plugging
As already hypothesized [Citation13], it might be assumed a condition of incomplete canal plug, in which a partial herniation of middle fossa contents could prevent high-frequency inputs within the SSC (as objectified by vHIT). On the contrary, the same condition could allow low-acceleration endolymphatic flows, such as sound pressure waves dissipating in the vestibular partition (accounting for low-frequency conductive hearing loss) and sound-induced endolymphatic movements (resulting in Tullio phenomenon). A similar functional dissociation pattern between high (impaired) and low-frequencies (spared) canal VOR has been implied in other conditions accounting for a third window mechanism, such as labyrinthine fistula [Citation14]. In fact, it has been observed how pressure-induced nystagmus (comparable to sound-evoked eye movements) aligning with the plane of the eroded canals could be evoked despite a global vestibular impairment on vHIT, suggesting a possible residual activity for canal afferents encoding low-velocity inputs [Citation14]. The hypothesis that a loss of sensitivity for high-acceleration head movements does not prevent the occurrence of vestibular signs driven by low-frequency canal afferents is also supported by experimental studies on animal models of SCD. In fact, it has been reported how Tullio phenomenon does not only arise from a sustained triggering of phase-locking irregularly-discharging sensors, but also to a slowly developing but sustained activation of regularly discharging afferents [Citation4,Citation15]. Similarly, cases with persistent positional downbeat nystagmus, where an incomplete canal plug (also called “incomplete canalith jam”) exerted by an otolith clot has been assumed as the underlying mechanism, have also been related to the same functional dissociation paradigm impairing high-velocity responses while sparing low-velocity VOR for the canal involved [Citation16,Citation17].
Dissipation of mechanical energy
Alternatively, we suggest that such vestibular signs could be more easily explained hypothesizing that SCD might act as a low-impedance pathway for overall acceleration inputs encoded by vestibular sensors, similar to how a bony dehiscence results in dissipation of acoustic energy accounting for conductive hearing loss [Citation3]. In fact, it may be assumed that mechanical energy conveyed to the endolymphatic fluids in both low and high-frequency domains (during sound/pressure stimuli and head impulses, respectively) could be shunted through the SSC opening where it could be wasted. This assumption may easily explain both excitation/inhibition of type-II hair cells of SSC ampulla in response to loud sounds or pressure changes and the reduced activation of type-I sensors of the affected SSC by residual energy during head impulses. In other words, while in normal conditions the vestibular compartment offers high impedance to loud sounds and pressure changes, it has been widely demonstrated how it becomes sensitive to this type of stimuli in SCD, as part of the fluid-mechanical waves is conveyed through the vestibule activating SSC afferents, thus generating eye movements aligning with the dehiscent canal plane [Citation2,Citation4,Citation15]. On the other hand, it could be supposed that while bony canal integrity is needed to allow high-acceleration energy to be correctly driven in the plane of the tested canal by head impulses, a canal dehiscence could likely dissipate part of this energy. This condition could result in a reduced amount of fluid-mechanical waves efficiently pulling the SSC cupula away from the utricle to excite canal afferents, thus accounting for impaired VOR-gain on vHIT (). The same low-impedance pathway allowing endolymphatic flows to be driven through the vestibule has also been implied in the genesis of other vestibular signs evoked by mechanical stimuli in the high-frequency domain, such as vertical/torsional nystagmus (aligning with the dehiscent canal) evoked by skull vibrations [Citation18,Citation19] and enhanced VEMPs amplitudes to air/bone-conducted sounds [Citation1,Citation13]. This latter hypothesis might also explain why large-sized SCD (where a wide third window lesion is expected to allow a greater waste of pressure energy than a small opening) are more prone to result simultaneously in sound/pressure nystagmus [Citation9,Citation10], abnormal VEMPs [Citation6,Citation8,Citation10] and reduced SSC VOR-gain values on vHIT [Citation2,Citation5,Citation6] compared to small-sized defects (). In short, the larger is the dehiscence, the greater should be the dissipation of mechanical energy within the vestibule. These data are also in line with measurements on humans and animal models confirming that sound pressure waves shunted from the oval window to an additional large opening increase the probability of a conductive hearing loss [Citation3,Citation6]. Obviously, different conditions of the dura mater - membranous canal interface at the dehiscence, likewise various SCD locations including possible contacts with the superior petrosal sinus, or even additional transient/persistent factors such as idiopathic intracranial hypertension might account for different and incomplete clinical/instrumental patterns and for mismatching correlation results among different investigations [Citation3,Citation5,Citation6,Citation8–10]. Mukherjee et al. [Citation7] hypothesized that a different behaviour of vestibular sensors (ampullary crests versus otolith organs) might account for mismatching results between vHIT measurements (impaired) and VEMPs responses (enhanced) in SCD, along with considering possible different activities of central compensation mechanisms. Although they might be somehow involved, we suggest to consider also the easier abovementioned pathomechanisms to explain overall vestibular signs in SCD. Nevertheless, these are only some suggestions to stimulate targeted clinical trials, as to date no clinical or experimental studies have been performed in this field. Therefore, further research is needed to confirm these hypotheses arising from our clinical practice.
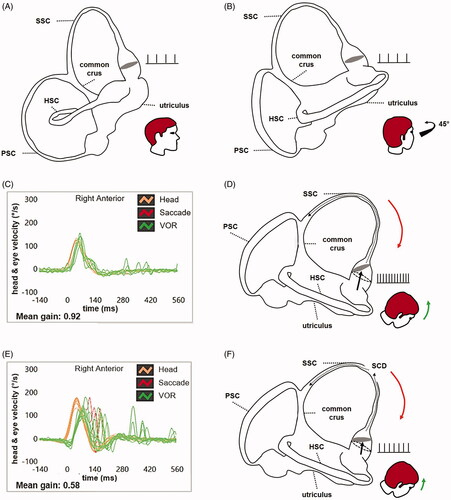
Figure 1. Schematic drawing of the right-sided labyrinth aligned with the sagittal (A) and the RALP plane (B) with corresponding representation of the resting firing rate of SSC afferents. Video-HIT measurements performed using the ICS Impulse device (Otometrics, Natus Medical Inc, Denmark) assessing the VOR-gain (eye velocity/head velocity) of the right SSC (C), with corresponding schematic drawing of the labyrinth during downward head impulses (D), in a case of intact SSC. Orange lines and arrows correspond to downward head impulses for the right SSC and green lines and arrows represent upward eye movements induced by the activation of the VOR following each impulse. A mean value of VOR-gain within normal limits (0.92) is shown, as during downward head impulses overall endolymphatic flows are driven upward along the SSC tested (black dotted arrow) maximally pulling utriculofugally the cupula from its original resting position (black dashed circle). In this physiological condition, ampullary afferents are maximally excited leading to a consistent increase in the resting firing rate. Video-HIT data (E) and corresponding schematic drawing of the labyrinth during downward head impulses (F) in a case of large-sized SCD. In this condition, during downward head impulses part of the fluid-mechanical wave is wasted at the dehiscence (black dotted arrows) reducing the amount of energy pulling the cupula utriculofugally. Therefore, the cupula is less displaced with respect to its original position (black dashed circle) increasing to a lesser extent the resting firing rate of the ampullary afferents. An impaired mean value of VOR-gain (0.58) is shown and red lines correspond to corrective saccades needed to compensate for the hypoactive SCC VOR. HSC: horizontal semicircular canal; PSC: posterior semicircular canal; RALP: right anterior-left posterior; SCD: superior canal dehiscence; SSC: superior semicircular canal; vHIT: video-head impulse test; VOR: vestibulo-ocular reflex.
Inner ear disorders accounting for isolated impairment of SCC on vHIT
Finally, we would also suggest to always warrant further investigations for patients presenting with isolated loss of SSC function on vHIT, as SCD might not be the sole underlying disorder as it has been found among 300 patients in a recently published study [Citation7]. In fact, along with anecdotal reports implicating Meniere’s disease in the acute stage [Citation20], Castellucci et al. have reported how benign positional paroxysmal vertigo (BPPV) presenting with positional downbeat nystagmus due to an involvement of a vertical canal might lead to a selective impairment of the affected canal VOR-gain, including the SSC [Citation16,Citation17]. Probably, the unlikelihood to examine patients either during the acute attack of Meniere’s disease or with SSC-BPPV (accounting for less than 10% of overall BPPV) could represent one of the reasons for this discrepancy. Moreover, it could also be assumed that patients with BPPV do not usually receive vHIT testing, even in those challenging cases with persistent positional downbeat nystagmus where instrumental tests could be of extreme help in the detection of the affected canal. Additionally, a possible asynchronous or incomplete recovery pattern of canal function from an acute vestibular loss due to a superior vestibular neuritis or labyrinthine ischemia might account for an early restoration of VOR-gain for lateral canal while the SSC remains hypoactive. Nevertheless, although we fully agree in considering SCD as the most likely disorder resulting in selective SSC loss on vHIT, we would stress the importance of recommending further investigation to confirm the suspicion, as SCD diagnosis could only be provided by a comprehensive vestibular assessment.
Acknowledgements
In memory of Prof. Giovanni Carlo Modugno and Dr. Erik Ulmer.
Disclosure statement
The authors report no conflict of interest.
References
- Brantberg K, Bergenius J, Mendel L, et al. Symptoms, findings and treatment in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol. 2001;121(1):68–75.
- Cremer PD, Minor LB, Carey JP, et al. Eye movements in patients with superior canal dehiscence syndrome align with the abnormal canal. Neurology. 2000;55(12):1833–1841.
- Rosowski JJ, Songer JE, Nakajima HH, et al. Clinical, experimental, and theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004;25(3):323–332.
- Iversen MM, Zhu H, Zhou W, et al. Sound abnormally stimulates the vestibular system in canal dehiscence syndrome by generating pathological fluid-mechanical waves. Sci Rep. 2018;8(1):10257.
- Carey JP, Migliaccio AA, Minor LB. Semicircular canal function before and after surgery for superior canal dehiscence. Otol Neurotol. 2007;28(3):356–364.
- Castellucci A, Piras G, Del Vecchio V, et al. The effect of superior canal dehiscence size and location on audiometric measurements, vestibular-evoked myogenic potentials and video-head impulse testing. Eur Arch Otorhinolaryngol. 2021;278(4):997–1015.
- Mukherjee P, Chiarovano E, Cheng K, et al. Video-head impulse test in superior canal dehiscence. Acta Otolaryngol. 2021;141(5):471–475.
- Welgampola MS, Myrie OA, Minor LB, et al. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008;70(6):464–472.
- Rajan GP, Leaper MR, Goggin L, et al. The effects of superior semicircular canal dehiscence on the labyrinth: does size matter? Otol Neurotol. 2008;29(7):972–975.
- Pfammatter A, Darrouzet V, Gärtner M, et al. A superior semicircular canal dehiscence syndrome multicenter study: is there an association between size and symptoms? Otol Neurotol. 2010;31(3):447–454.
- Brandolini C, Modugno GC. Do signs of natural plugging of superior semicircular canal dehiscence exist? Am J Otolaryngol. 2012;33(2):268–271.
- Ward BK, Agrawal Y, Nguyen E, et al. Hearing outcomes after surgical plugging of the superior semicircular canal by a middle cranial fossa approach. Otol Neurotol. 2012;33(8):1386–1391.
- Manzari L, Burgess AM, MacDougall HG, et al. Enhanced otolithic function in semicircular canal dehiscence. Acta Otolaryngol. 2011;131(1):107–112.
- Castellucci A, Botti C, Bettini M, et al. Case report: could Hennebert's sign be evoked despite global vestibular impairment on video head impulse test? Considerations upon pathomechanisms underlying pressure-induced nystagmus due to labyrinthine fistula. Front Neurol. 2021;12:634782.
- Carey JP, Hirvonen TP, Hullar TE, et al. Acoustic responses of vestibular afferents in a model of superior canal dehiscence. Otol Neurotol. 2004;25(3):345–352.
- Castellucci A, Malara P, Delmonte S, et al. A possible role of video-head impulse test in detecting canal involvement in benign paroxysmal positional vertigo presenting with positional downbeat nystagmus. Otol Neurotol. 2020;41(3):386–391.
- Castellucci A, Malara P, Martellucci S, et al. Feasibility of using the video-head impulse test to detect the involved canal in benign paroxysmal positional vertigo presenting with positional downbeat nystagmus. Front Neurol. 2020;11:578588.
- Aw ST, Aw GE, Todd MJ, et al. Three-dimensional vibration-induced vestibulo-ocular reflex identifies vertical semicircular canal dehiscence. J Assoc Res Otolaryngol. 2011;12(5):549–558.
- Dumas G, Tan H, Dumas L, et al. Skull vibration induced nystagmus in patients with superior semicircular canal dehiscence. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136(4):263–272.
- Martinez-Lopez M, Manrique-Huarte R, Perez-Fernandez N. A puzzle of vestibular physiology in a Meniere's disease acute attack. Case Rep Otolaryngol. 2015;2015:1–5.
