Abstract
Background
High-resolution MR imaging enables the visualization of individual nerves in the internal auditory canal (IAC). Cochlear nerve deficiency (CND) is recognized as one of the major causes of sensory neural hearing loss (SNHL), especially in cases of unilateral hearing loss in childhood. Some patients with CND are thought to have accompanying vestibular nerve deficiency (VND). However, there have been few reports focusing on VND and vestibular function in these children.
Aims
The aim of this study was to evaluate the frequency of VND and vestibular dysfunction in children with unilateral SNHL caused by CND.
Material and Methods
Thirty-eight children with unilateral SNHL, who were diagnosed with CND by 3 T-MRI, were evaluated for VND and underwent caloric testing and cervical vestibular evoked potential (cVEMP).
Results
Fourteen of 38 patients (37%) had VND, and eleven (29%) of the patients [ten of the patients (71%) with VND] had at least one vestibular dysfunction. The patients with VND had significantly worse hearing and an IAC of smaller diameter than did patients without VND.
Conclusions and significance
We should pay attention to VND as well as vestibular dysfunction in hearing loss patients with CND.
Chinese abstract
背景:高分辨率 MR 成像可实现内耳道(IAC)中单个神经的可视化。耳蜗神经缺陷(CND)被认为是感觉神经性听力损失(SNHL)的主要原因之一, 特别是在儿童单侧听力损失的病例。一些CND 患者被认为伴有前庭神经缺陷 (VND)。然而, 很少有关于这些儿童的 VND 和前庭功能的报道。
目的:本研究的目的是评估 CND 引起的单侧 SNHL 儿童的VND 和前庭功能障碍的发生频率。
材料和方法:通过 3 次 TMRI 诊断为 CND 的38 名单侧 SNHL 儿童, 接受了VND评估 , 并接受了热量测试和颈前庭诱发电位 (cVEMP)测试。
结果:38 名患者中有 14 名 (37%) 患有 VND, 11 名 (29%) 患者 [其中10名(71%) 患有 VND] 至少有一个前庭功能障碍。与非VND 患者相比, VND 患者的听力明显更差, IAC 直径更小。
结论与意义:对于患有 CND 的听力损失患者, 我们应注意他们的VND及前庭功能障碍。
Introduction
The high-resolution MR imaging enables the visualization of individual nerves in the internal auditory canal (IAC), and cochlear nerve deficiency (CND) is recognized as one of the major causes of sensory neural hearing loss in childhood [Citation1,Citation2]. About 20% of hearing loss in childhood is thought to be caused by CND, and the frequency of CND is higher in children with unilateral hearing loss than in those with bilateral hearing loss [Citation3–5]. In our previous report of 210 children with congenital or early-onset unilateral hearing loss, hearing loss in 43.7% of the children was caused by CND, which was considered to be the most prevalent cause of unilateral hearing loss in children [Citation6]. As the IAC is composed of not only the cochlear nerve but also the vestibular nerves, some patients with CND are thought to have accompanying VND. However, there have been few reports focusing attention on the deficiency of the vestibular nerve and vestibular function in these children [Citation3,Citation7,Citation8]. In this study, we evaluated the frequency of VND and vestibular function in children with unilateral hearing loss caused by CND.
Material and methods
Subjects
Thirty-eight children (twenty males and eighteen females) with unilateral sensorineural hearing loss caused by CND were included in this study after obtaining informed written consent. The age at vestibular testing ranged from 7 to 16 years, with the mean age being 9.7 years. The study was carried out with the approval of the Shinshu University Ethical Committee.
Diagnose of CND by MRI
MRI findings were obtained by 3.0-Tesla MRI (Trio; Siemens, Munich, Germany) with a 3-dimensional fast spin-echo T2-weighted sequence. Images were acquired in the direct axial plane at a slice thickness of 0.5 mm. Reconstructed sagittal oblique images of the component through the IAC (cochlear, vestibular, and facial nerves) were obtained perpendicular to the long axis of the IAC (). Normal cochlear and vestibular nerves were considered equal to or greater in diameter than the facial nerve as visualized by reconstructed sagittal oblique images of the IAC. A deficient cochlear or vestibular nerve was diagnosed when smaller than the ipsilateral facial nerve or contralateral cochlear or vestibular nerve, or when the cochlear or vestibular nerve was not visualized. Patients with obvious inner ear malformation, such as Mondini dysplasia other than hypoplasia of modiolus, were excluded from this study. The diameter of the IAC was measured on the axial images at its midpoint.
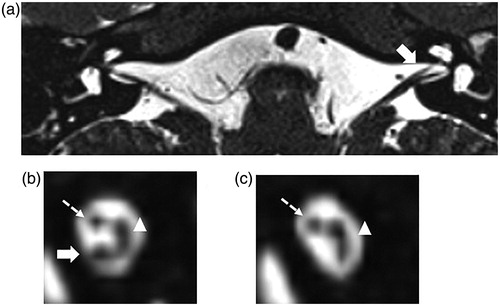
Figure 1. MRI images of the IAC in a CND case (Case No. 32). This case (Case No. 32) had right ear deafness and was diagnosed with CND. (a) shows an axial image of the IAC. The cochlear nerve in the IAC in the affected ear (left ear) could not be detected, while it can be detected in the IAC of the unaffected ear (right ear, solid arrow). (b) shows a sagittal section of the unaffected IAC in which the cochlear nerve (solid arrow), vestibular nerve (arrowed head), and facial nerve (dotted arrow) can all be detected. (c) shows the IAC on the affected side. The vestibular nerve (arrowed head) and facial nerve (solid arrow) can be detected and a defect in the cochlear nerve is shown.
Audiological evaluation
Hearing levels were determined by pure-tone audiometry (PTA). The average threshold in the conversation frequencies (0.5 kHz, 1.0 kHz, 2.0 kHz, 4.0 kHz) was calculated. When a patient did not respond to the maximum hearing level (105 dB) at a given frequency, 5 dB was added to the maximum hearing level.
Vestibular testing
The patients were examined by caloric testing and cVEMP to obtain data on the superior and inferior vestibular nerve functions, respectively.
In cVEMP testing, electromyography (EMG) was performed using a pair of surface electrodes mounted on the upper half and the sterna head of the sternocleidomastoid (SCM) muscle. The electrographic signal was recorded using a Neuropack evoked potential recorder (Nihon Kohden Co. Ltd, Tokyo, Japan). Clicks lasting for 0.1 ms at 105dBnHL were presented through a headphone. The stimulation rate was 5 Hz, the bandpass filter intensity was 20-2000 Hz, and analysis time was 50 ms. The responses to 200 stimuli were averaged twice. As the amplitude of the VEMP based on the unrectified EMG is correlated with the activity of the SCM muscle during the test [Citation9], we measured the activity of the SCM muscle using the background integrated EMG response, the area under the averaged rectified EMG curve, from −20 ms to 0 ms before the sound stimulation. For cVEMP, the amplitude was defined as the difference between the peaks p13 and n23. The correction of the p13-n23 amplitude was calculated as follows [Citation10]: corrected amplitude = amplitude of the averaged unrectified EMG (micro V)/background integrated EMG (micro V)
The cVEMP asymmetry was calculated as follows: asymmetry ratio (AR) = (larger amplitude - smaller amplitude) * 100/(larger amplitude + smaller amplitude). In this study, an asymmetry ratio of >30% was defined as decreased reaction, and no reaction in amplitude cVEMP as absent [Citation11].
In caloric testing, the maximum slow phase velocity (mSPV) was measured by cold water irrigation (20 °C, 5 ml, 20 s). We defined a value of mSPV below 10 deg/s of as areflexia and that between 10 to 20 deg/s as hyporeflexia [Citation12].
Statistical analysis
For all analyses, SPSS for Windows software (IBM Co., Chicago, IL, USA) was used and Man-Whitney U tests were applied when comparing differences in patients with and without VND.
Results
Vestibular testing, hearing threshold and width of IAC in all the patients are shown . In the present study, as shown in , fourteen of 38 CND patients (36.8%) showed VND (hypoplasia or an absence of vestibular nerve). Eleven of the CND patients (28.9%) had vestibular dysfunction based on the caloric testing and/or cVEMP. Ten (71%) of the 14 patients with VND based on the MR images showed vestibular dysfunction, and ten of 11 (90%) patients with vestibular dysfunction had VND. Eight of the 11 patients (72.7%) who had vestibular dysfunction showed pathological results on both the caloric testing and cVEMP, with two patients showing pathological results only on the caloric testing and one only on the cVEMP (). Among the 10 patients who had a pathological caloric response, 5 had areflexia and the other 5 had hypoflexia in the affected ears, while among the nine patients who had a pathological cVEMP response, 3 had no response and 6 had decreased response in the affected ears.
Figure 2. The frequencies of VND and vestibular dysfunction in CND cases. Fourteen of 38 CND patients (36.8%) had VND, and eleven of 38 cases (28.9%) had vestibular dysfunction on either caloric testing, cVEMP or both (a). The eight of 11 patients (73%) with vestibular dysfunction showed a pathological response on both caloric testing and cVEMP (b). Caloric test and cVEMP: vestibular dysfunction on both. Caloric test: Vestibular dysfunction on caloric testing only. cVEMP: Vestibular dysfunction on cVEMP only.
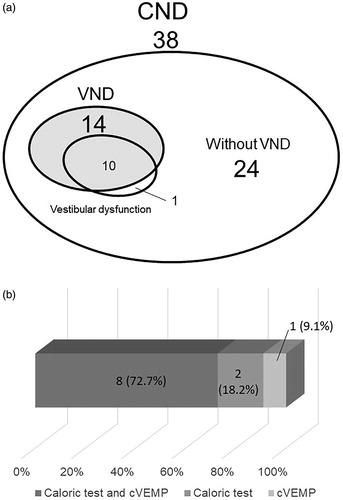
Table 1. Vestibular function, hearing levels and IAC diameters in CND cases.
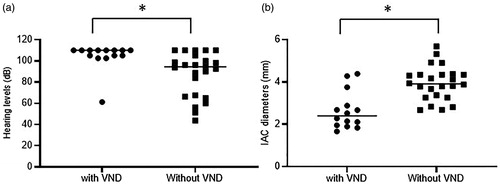
When comparing hearing levels between the patients with and without VND (), median hearing levels were 110 dB in the patients with VND (n = 14) and 94.4 dB in those without VND (n = 24). The hearing levels in the patients with VND were significantly worse than those in the patients without VND (p = 0.0016). We also compared the width of the IAC between the patients with (n = 14) and without VND (n = 24). The patients with VND showed a smaller diameter of IAC (median width: 2.40 mm) than did those without VND (median width: 3.95 mm), and this difference was statistically significant (p = 0.0001, ).
Figure 3. A comparison of hearing levels and IAC diameters between patients with and without VND. There was a statistically significant difference in hearing levels between the patients with (n = 14) and without VND (n = 24) (a). The IAC diameters were significantly smaller in patients with VND than in those without VND (b). *p < 0.05 in Mann-Whitney U test.
None of the 38 patients with unilateral SNHL caused by CND complained of vestibular symptoms such as vertigo or dizziness.
A typical example (Case 17) is shown below.
Case 17: a 16 y.o. female
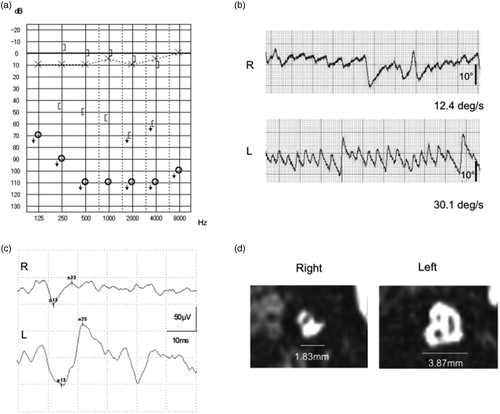
She was first diagnosed at 6 y.o. in her initial school check up, and her hearing threshold in pure-tone audiometry was off the scale at all frequencies in her right ear (). In the caloric testing (), the maximum slow phase velocity was 12.4 deg/s in the affected ear and 30.1 deg/s in the unaffected ear (CP% 41.6). The asymmetry ratio of the cVEMP was 34.8%, revealing a pathological cVEMP response in the affected ear (). In the MRI study, a narrow IAC (1.83 mm width) was observed in the affected ear (3.87 mm in the contralateral ear), and the cochlear nerve, vestibular nerve and facial nerve could not be distinguished ().
Figure 4. Case No. 17. Case No. 17 had profound hearing loss at all frequencies in the right ear. (a) Vestibular testing revealed hyporeflexia on caloric testing (b), and cVEMP results showed a decreased response (AR = 34.8%) (b). In the MR images of the sagittal IAC section, a narrow IAC (1.83mm in width) is seen in the affected ear and the cochlear nerve, vestibular nerve and facial nerve cannot be distinguish, while all nerves with a normal IAC width can be detected in the unaffected ear (b).
Discussion
Recently, advances in diagnostic imaging technology have made it possible to diagnose CND, which has been recognized as a cause of SNHL[Citation1,Citation2]. It is proposed that CND results from developmental disorders in the embryonic stage and/or inner ear neuropathy in the early post-natal period [Citation2]. The inner ear nerves consist of the cochlear nerve, and the upper and lower vestibular nerves. Based on the possibility of a developmental disorder, not only CND but also VND is expected. However, there have been few reports to date focusing on VND. McClay reported that hypoplasia or an absence of the vestibular nerve was observed in 12 of 49 ears (24%) with CND [Citation3], and Adunka also reported that a loss of vestibular nerve was observed in 11 of 20 (55%) ears in CND cases [Citation7]. In the present study, 36.8% of patients showed a vestibular nerve that was absent or smaller than that on the contralateral side (), suggesting that majority of the cases had only cochlear nerve deficiencies without VND.
Embryologically, the inner ear nerve is divided into the upper and lower branches from 4 to 5 weeks; the upper branch begins to differentiate into the utricle, and the superior and lateral semicircular canals and, then at 5-6 weeks, the lower branch is further subdivided with the upper half forming the saccule and posterior semicircular canal, and the lower half forming the cochlea [Citation13]. As the vestibular nerve is thought to develop at an earlier stage than the cochlear nerve, a deficiency in the vestibular nerve may always be accompanied by a deficiency in the cochlear nerve. The higher frequency of CND without VND is presumed to be due to the higher frequency of damage to the auditory nerve after vestibular nerve differentiation than before vestibular nerve differentiation, and it is difficult to believe that vestibular nerve deficiency alone might occur. The present results are in-line with this developmental theory.
With regard to the frequencies of vestibular dysfunction, 11 (28.9%) of the total number of cases had vestibular dysfunction (). In addition, 10 (71%) of the 14 cases with VND based on the MR images showed vestibular dysfunction, and all but one patient with vestibular dysfunction had VND, suggesting that vestibular dysfunction is likely to accompany VND.
In the present study, 8 (72.7%) of 11 patients showed vestibular dysfunction on both caloric testing and cVEMP, which may reflect superior and inferior vestibular nerve function, respectively (). The remaining three patients had pathological results on either cVEMP or the caloric test. It is suggested that vestibular dysfunction involves both the superior and inferior vestibular nerve in the majority of CND cases with vestibular dysfunction. Ito et al. reported pathological caloric responses in 5 of 10 patients with unilateral CND, although cVEMP showed normal responses in all 9 patients, suggesting that the superior vestibular nerve is more frequently affected than the inferior vestibular nerve [Citation8]. Unlike previous reports, the present study clearly showed that both the superior and inferior vestibular nerves may frequently be impaired.
Regarding the severity of the vestibular dysfunction in CND patients, only two (Case 16 and Case 18) of the eleven patients with vestibular dysfunction had a completely absent reaction on both the caloric testing and cVEMP, with the other 9 cases showing deterioration of vestibular function, which implies that vestibular function remained in most cases. Similar morphological and functional discrepancies were reported for the relationship between hearing and CND [Citation14,Citation15]. This suggested that even if the vestibular nerve could not be detected or was extremely small on MR images, the vestibular nerve exists and functions to some extent in most cases with VND.
In the present study, comparisons were made regarding hearing levels between the patients with and without VND (). As a result, the patients with VND showed worse hearing levels than did those without VND. Clemmen et al. reported that a smaller cochlear nerve induced a worse hearing level [Citation16]. As the vestibular nerve differentiates earlier than the cochlear nerve in embryos, if not absent or if hypoplasia of the vestibular nerve exists, the cochlear nerve is presumed not to be formed or smaller in diameter, resulting in a worse hearing level in patients with VND.
Moreover, we also focused on the IAC diameter (). In the cases with VND, the IAC diameter was significantly smaller compared with the cases without VND. A previous report has shown that when the diameter of the IAC is smaller, deterioration of vestibular function tends to occur [Citation8]. When the IAC is smaller, it is often difficult to distinguish and ascertain the presence of the nerve in the IAC even with high-resolution MRI, as in Case No. 17 (), and in these cases, it is quite possible for both the cochlear nerve and vestibular nerve to be absent or hypoplastic. It has been reported that the vestibular nerve could not be identified because of an inability to distinguish the inner ear nerves in approximately 90% of patients with IAC stenosis [Citation9]. It is known that mesodermal tissue accumulates around auditory nerve fibres from 6 weeks of embryonic development, and cartilage is then formed at around 9 weeks. As a result, the IAC is formed along the nerve fibres [Citation17]. However, if the auditory nerve fibres are absent or hypoplastic, it is thought that the mesodermal tissue forms a narrow inner ear canal in accordance with the diameter of the auditory nerve fibres, resulting in IAC stenosis [Citation2,Citation17]. Therefore, when the development of the vestibular nerve is impaired during the embryonic period, it may be more likely to be associated with IAC stenosis.
There were no cases showing vestibular symptoms such as obvious vertigo or dizziness among the CND cases with vestibular dysfunction. Vestibular dysfunction, whether congenital or from the juvenile stage, is considered to be the result of insufficient vestibular compensation. However, if there is any inner ear impairment to the healthy side in the future, the loss of QOL associated with bilateral vestibular dysfunction may be high. It is, therefore, necessary to pay attention to vestibular function in cases with CND.
Conclusion
We found that 36.8% of patients with CND had accompanying VND, and the majority of the patients with VND had vestibular dysfunction (both superior and inferior vestibular nerve dysfunction). Thus, in patients with CND, we should pay attention to associated VND as well as vestibular dysfunction.
Disclosure statement
The authors have no conflicts of interest to disclose in relation to this article.
Additional information
Funding
References
- Casselman JW, Offeciers FE, Govaerts PJ, et al. Aplasia and hypoplasia of the vestibulocochlear nerve: diagnosis with MR imaging. Radiology. 1997;202(3):773–781.
- Glastonbury CM, Davidson HC, Harnsberger HR, et al. Imaging findings of cochlear nerve deficiency. AJNR Am J Neuroradiol. 2002;23(4):635–643.
- McClay JE, Booth TN, Parry DA, et al. Evaluation of pediatric sensorineural hearing loss with magnetic resonance imaging. Arch Otolaryngol Head Neck Surg. 2008;134(9):945–952.
- Miyasaka M, Nosaka S, Morimoto N, et al. CT and MR imaging for pediatric cochlear implantation: emphasis on the relationship between the cochlear nerve canal and the cochlear nerve. Pediatr Radiol. 2010;40(9):1509–1516.
- Nakano A, Arimoto Y, Matsunaga T. Cochlear nerve deficiency and associated clinical features in patients with bilateral and unilateral hearing loss. Otol Neurotol. 2013;34(3):554–558.
- Usami SI, Kitoh R, Moteki H, et al. Etiology of single-sided deafness and asymmetrical hearing loss. Acta Otolaryngol. 2017;137(sup565):S2–S7.
- Adunka OF, Roush PA, Teagle HF, et al. Internal auditory canal morphology in children with cochlear nerve deficiency. Otol Neurotol. 2006;27(6):793–801.
- Ito K, Ishimoto SI, Karino S. Isolated cochlear nerve hypoplasia with various internal auditory meatus deformities in children. Ann Otol Rhinol Laryngol. 2007;116(7):520–524.
- Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57(2):190–197.
- Shojaku H, Takemori S, Kobayashi K, et al. Clinical usefulness of glycerol vestibular-evoked myogenic potentials: preliminary report. Acta Otolaryngol Suppl. 2001;545:65–68.
- Murofushi T, Matsuzaki M, Mizuno M. Vestibular evoked myogenic potentials in patients with acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1998; May124(5):509–512.
- Midorikawa C, Takahashi M, Tsujita N, et al. A simple cold caloric test. Nihon Jibiinkoka Gakkai Kaiho. 1984;87(9):1111–1119.
- Gulya AJ, et al. Developmental anatomy of the temporal bone and skull base. In: Gulya AJ, Minor LB, Poe D, editors. Surgery of the ear: Glasscock-Shambaugh. 6th ed. Shelton (CT): People's Medical Publishing House-USA; 2010. p. 3–27.
- Taiji H, Morimoto N, Matsunaga T. Unilateral cochlear nerve hypoplasia in children with mild to moderate hearing loss. Acta Otolaryngol. 2012;132(11):1160–1167.
- Miyanohara I, Miyashita K, Takumi K, et al. A case of cochlear nerve deficiency without profound sensorineural hearing loss. Otol Neurotol. 2011;32(4):529–532.
- Clemmens CS, Guidi J, Caroff A, et al. Unilateral cochlear nerve deficiency in children. Otolaryngol Head Neck Surg. 2013;149(2):318–325.
- Ferreira T, Shayestehfar B, Lufkin R. Narrow, duplicated internal auditory canal. Neuroradiology. 2003; May45(5):308–310.
