Abstract
Background
The mechanisms of association between diabetes and inner ear dysfunction are unknown, although endolymphatic hydrops may be involved. We have previously shown that insulin signaling components are expressed in human saccule and that insulin signaling takes place in HEI-OC1 auditory cells.
Aim
To explore Nedd4-2 as a target for insulin signaling.
Materials and methods
Effects of insulin were analyzed using western blot and confocal microscopy in HEI-OC1 auditory cells.
Results
Insulin induced phosphorylation of Nedd4-2 and increased the amount of ENaC at the plasma membrane. Also, protein kinase B (PKB) and NDRG1, a substrate for SGK1 (serum and glucocorticoid stimulated kinase), were phosphorylated in response to insulin. The SGK1 inhibitor GSK650394 prevented insulin-induced phosphorylation of NRDG1, but not of PKB and Nedd4-2, whereas the phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin and the PKB inhibitor MK2206 inhibited phosphorylation of all components. Ceramides prevented insulin-induced phosphorylation of PKB and NDRG1, but not of Nedd4-2. The ceramide metabolite sphingosine 1-phosphate induced phosphorylation of Nedd4-2.
Conclusions
Insulin induces phosphorylation of Nedd4-2, most likely involving PI3K/PKB signaling. Sphingosine 1-phosphate might protect Nedd4-2 against ceramide-induced insulin resistance.
Significance
Insulin-mediated regulation of Nedd4-2 might impact on inner ear sodium homeostasis with implications for diabetes-induced inner ear damage.
Chinese Abstract
背景:糖尿病与内耳功能障碍之间的关联机制是个未知数, 但可能涉及内淋巴积水。我们之前已经证明胰岛素信号成分在人球囊中有所表达, 胰岛素信号发生于 HEIOC1 听觉细胞之内。
目的:探索作为胰岛素信号传导的靶点的Nedd4-2。
材料和方法:使用蛋白质印迹和共聚焦显微镜分析胰岛素在 HEI-OC1 听觉细胞中的作用。
结果:胰岛素诱导了 Nedd4-2 的磷酸化并增加了血浆膜中 ENaC 的量。此外, 蛋白激酶 B (PKB) 和 NDRG1, 后者是SGK1(血清和糖皮质激素)的基底物, 因响应胰岛素而被磷酸化。 SGK1 抑制剂 GSK650394 阻止了胰岛素诱导的 NRDG1 磷酸化, 但不能阻止 PKB 和 Nedd4-2 的磷酸化, 而磷脂酰肌醇 3-激酶 (PI3K) 抑制剂渥曼青霉素和 PKB 抑制剂 MK2206 抑制了所有成分的磷酸化。神经酰胺阻止胰岛素诱导的 PKB 和NDRG1的磷酸化, 但不能阻止 Nedd4-2的磷酸化。神经酰胺代谢物 1-磷酸鞘氨醇诱导了 Nedd4-2 的磷酸化。
结论:胰岛素诱导 Nedd4-2 的磷酸化, 很可能涉及 PI3K/PKB 信号传导。1-磷酸鞘氨醇可能保护 Nedd4-2 免受神经酰胺诱导的胰岛素抗阻。
意义:胰岛素介导的 Nedd4-2 调节可能影响内耳钠稳态, 这对糖尿病引起的内耳损伤有意义。
Introduction
Ample evidence shows that type 1 and type 2 diabetes negatively impact on the function of the vestibular and auditory systems [Citation1–3]. Although several theories have been proposed [Citation4], the exact underlying mechanisms responsible for diabetes-induced damage remain uncertain.
In humans, insulin resistance, impaired fasting glucose and beta-cell dysfunction have been reported as independent risk factors for hearing impairment even before the onset of type 2 diabetes [Citation5]. Also, hyperinsulinemia has been suggested to explain differences in dysfunction of specific auditory pathways in type 1 and type 2 diabetes in mice [Citation6]. We have previously shown that mice fed a high fat diet, a model for insulin resistance and diabetes, develop inner ear endolymphatic hydrops [Citation7], a hallmark of inner ear dysfunction. Whether inner ear insulin resistance provides a mechanistic link between diabetes and inner ear dysfunction remains to be determined.
The insulin receptor, insulin receptor substrate 1(IRS-1), protein kinase B (PKB/Akt), the insulin-sensitive phosphodiesterase (PDE)3B, as well as targets such as the insulin-regulated glucose transporter (GLUT4), were recently shown to be expressed in the sensory epithelium of the human saccule [Citation8,Citation9] and insulin signaling was shown to take place in HEI-OC1 auditory cells [Citation7]. Still, the molecular targets for insulin signaling in inner ear cells remain to be identified.
The endolymphatic space of the inner ear contains luminal fluid with high potassium concentration and low sodium concentration which provides the ionic milieu needed for normal hearing and balance functions [Citation10]. By absorbing sodium from the endolymphatic fluid compartment of the inner ear, the epithelial sodium channel ENaC plays a critical role in inner ear sodium homeostasis [Citation10,Citation11]. It has been shown that insulin activates sodium transport most likely mediated via PI3K induced activation of SGK1 and/or PKB/Akt leading to phosphorylation and inhibition of the ubiquitin ligase Nedd4-2, a key negative regulator of ENaC [Citation11–15]. Whether insulin regulates Nedd4-2/ENaC, and thereby sodium homeostasis in the inner ear is not known.
The complex interactions between insulin signaling, sphingolipid signaling and diabetic pathologies have been extensively investigated. Particular attention has been paid to ceramides, a class of sphingolipids suggested to contribute to the development of systemic insulin resistance in obesity and type 2 diabetes [Citation16]. Also, there are several reports that highlight a role of different sphingolipids in the biology of the inner ear [Citation17]. For example, ceramides accelerates cochlear hair cell death induced by ototoxic agents, while sphingosine 1-phosphate (S1P) protects cochlear hair cells which appears to be mediated by S1P receptor subtype 2 (S1P2) [Citation17].
In the present study, we focus on Nedd4-2, an important regulator of ENaC and hence endolymph homeostasis. The impact of insulin signaling on Nedd4-2, and cross-talk between insulin and sphingolipid signaling is studied using House Ear Institute-Organ of Corti 1 (HEI-OC1) auditory cells, one of the few auditory cell lines available for research purposes [Citation18].
Materials and methods
HEI-OC1 auditory cells and culture
HEI-OC1 cells were cultured in 6-well or 12-well plates (TC plate, Standard, Sarstedt) under permissive conditions (33 °C, 10% CO2) in high-glucose Dulbecco’s Eagle’s medium (DMEM, Sigma Aldrich, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS, Sigma Aldrich) without antibiotics as previously described [Citation7,Citation18]. After reaching 70–80% confluence, cells were washed twice with phosphate-buffered saline (PBS) and then cultured in KRBB containing 2 mM glucose for 2 h. Thereafter fresh Krebs-Ringer biocarbonate buffer (KRBB) containing 2 mM glucose was added and cells were treated without or with insulin (Actrapid, Novo Nordisk, Bagsvaerd, Denmark), isoprenaline hydrochloride (Sigma Aldrich), sphingosine 1-phosphate (Cayman Chemicals, Ann Arbor, MI, USA), CYM 5478, a SIP2 agonist (Cayman Chemicals) and C2-ceramide (Avanti Polar Lipids) in the presence or absence of H89 (Enzo Life Science), a PKA inhibitor, MK2206 (MedChem Express), a PKB inhibitor, wortmannin (Sigma Aldrich), a PI3K inhibitor, GSK 650394 (Sigma Aldrich), a SGK1 inhibitor as described in the Results. After treatments, the cells were harvested in a buffer containing 50 mM TES, pH 7.4, 250 mM sucrose, 1 mM EDTA, 2 mM EGTA, 40 mM phenyl-phosphate, 5 mM sodium fluoride, 1 mM dithiothreitol (DTT), 50 µM sodium vanadate, Pefabloc (Sigma), Complete (Roche, Basel, Switzerland, protease inhibitors) and 1% NP40 (100 µl/well using 12 well plates or 200 µl per well using 6 well plates) and subjected to sonication (10 short pulses). The lysates were centrifuged for 5 min at 5000 × g, 4 °C, and the supernatants were used for further analysis.
SDS-PAGE and Western blotting
HEI-OC1 cell lysates (20–30 µg protein as measured by Bradford) were mixed with LDS sample buffer 4× (Invitrogen, Carlsbad, MA) containing 300 mM DTT and subjected to electrophoresis on 4–12% bisacrylamide gels (Novex, Invitrogen). Proteins were transferred to Immobilon-P, PVDF membranes (Merck, Millipore, Billerica, MA), blocked with 10% milk in Tris-buffered saline tween-20 (TBST) containing 50 mM Tris, pH 7.6, 150 mM NaCl and 0.1% Tween-20 for 30 min and incubated at 4 °C overnight with primary antibodies as indicated. Membranes were incubated with secondary antibody conjugated with horseradish peroxidase (HRP) for 1 h at room temperature, incubated with SuperSignal West Pico ECL (enzymatic chemiluminescence) reagent (Thermo Scientific, Waltham, MA, USA) for 5 min followed by imaging (Molecular Imager ChemiDoc XRS+, Bio-Rad Laboratories, Hercules, CA, USA) and quantification (Image lab software, version 3.0, Bio-Rad Laboratories).
Antibodies
pNEDD4-2 serine 328 antibodies (ab95399) (raised against the synthetic peptide KPRSLS(P)SPTV), NEDD4-2 antibodies (ab 46521), and NDRG1 antibodies (ab196621) were from Abcam, pNDRG1 threonine 264 antibodies (#3217), pPKB serine 473 antibodies (#9271), PKB antibodies (#9272) and antibodies against PKA consensus phosphosequences (#96245) were from Cell Signaling. αENaC antibodies (PA-920A) were from Thermo Fisher (Waltham, MA USA). The antibodies were used for Western blotting and confocal microscopy as indicated.
Confocal microscopy
HEI-OC1 cells were culture as described, and incubated for 5 h with 2 µM dexamethasone (Sigma Aldrich), and 10 nM insulin. Cells were fixed using 4% paraformaldehyde, and incubated with primary antibody against ENaC, followed by fluorescence-conjugated (Alexa Fluor) secondary antibody in a buffer containing 1% BSA, 1% goat serum, and 0.05% saponin 1–2 h. Image acquisition was performed using a Nikon A1 plus confocal microscope with a 60× Apo DIC oil immersion objective with a NA of 1.40 (Nikon Instruments, Tokyo, Japan) and appropriate filter sets. Images were acquired with NIS-Elements, version: 4.50.02 (Laboratory Imaging). Images were taken slightly elevated from the glass bottom surface to get the membrane signal for the whole cell. For analysis FIJI was used. A line was drawn across the membrane, generating a signal profile. The average of the membrane adjacent signal was estimated based on 2–3 plots/cell.
Statistical analysis
Results are expressed as mean ± SEM. Differences between groups were tested for statistical significance using paired or non-paired Student’s t test. p values less than .05 were considered to denote statistical significance.
Results
Insulin induces phosphorylation of Nedd4-2 in HEI-OC1 auditory cells and increases the amount of ENaC at the plasma membrane
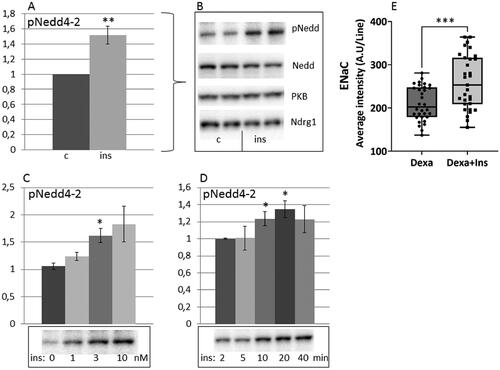
As shown in , stimulation of HEI-OC1 auditory cells with insulin induced serine 328 phosphorylation of the ubiquitine ligase Nedd4-2 in a dose and time dependent manner. Also, insulin increased the level of the sodium transporter ENaC, a Nedd4-2 substrate, at the plasma membrane ().
Figure 1. Insulin induces phosphorylation of Nedd4-2 and increases the amount of ENaC at the plasma membrane of HEI-OC1 auditory cells. HEI-OC1 cells were stimulated with 10 nM insulin for 10 min (n = 7) (A,B), with 0.1–10 nM insulin for 10 min (n = 3) (C) and with 10 nM insulin for 2–40 min (n = 4) (D). Nedd4-2 phosphorylation, total Nedd4-2, total PKB and total NDRG1 were analyzed by western blotting. Blots are representative of 3–7 independent experiments. Western blot signals were quantified and normalized to control samples not stimulated with insulin. Confocal imaging was performed on HEI-OC1 cells stimulated with 2 µM dexamethasone without or with 100 nM insulin for 5 h prior to fixation. Cells were stained with ENaC antibodies and the signal quantified as described in Material and methods, 30 cells/condition (E). Data are presented as means ± SEM. *p < .05, **p<.01, ***p < .001 versus control.
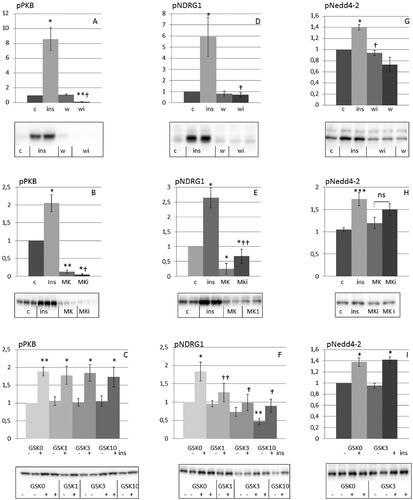
As a next step, we evaluated the role of PI3K, PKB and SGK1 in mediating the insulin-dependent phosphorylation of Nedd4-2. SGK1 activity was monitored by assaying the phosphorylation status of residue threonine 346 within NDRG1, a physiological substrate for SGK1 and not for other related kinases including PKB [Citation13]. As insulin had no effect upon total protein expression of Nedd4-2, PKB and NDRG1under the conditions used (), in the following, data on changes in phosphorylation of these proteins were normalized to the phosphorylation state measured in hormone deprived cells (control).
Initially we characterized the inhibitory effects of wortmannin, the PKB inhibitor MK2206 and the SGK1 inhibitor GSK650394 on insulin induced phosphorylation of PKB and NDRG1. As shown in , wortmannin as well as MK2206 inhibited insulin-induced phosphorylation of PKB and NDRG1. Thus, MK2206 cannot be used to distinguish between PKB and SGK1 signaling. On the other hand, GSK650394 inhibited insulin-induced phosphorylation of NDRG1, whereas phosphorylation of PKB was not affected (). As shown in ), wortmannin and MK2206 inhibited insulin-induced phosphorylation of Nedd4-2, which was not the case for GSK650394, implicating a role for PKB rather than SGK1 in mediating the effect of insulin on Nedd4-2.
Figure 2. Insulin induces phosphorylation of Nedd4-2 via phosphorylation of PKB rather than via SGK1 in HEI-OC1 auditory cells. HEI-OC1 cells were preincubated with or without 100 nM wortmannin (A,D,G) for 30 min (n = 3–5), 10 µM MK2206 (B,E,H) for 1 h (n = 3–12) and 1–10 µM GSK650394 (C,F,I) for 1 h (n = 3–5) and then stimulated without or with 10 nM insulin for 10 min. Phosphorylation of PKB, NDRG1 and Nedd4-2 were analyzed by Western blotting. Blots are representative of 3–12 independent experiments. Western blot signals were quantified and normalized to untreated control samples. Data are presented as means ± SEM. *p < .05, **p<.01, ***p < .001 versus control, †p < .05, ††p < .01 versus insulin only. wi: wortmannin plus insulin; MKi: MK2206 plus insulin.
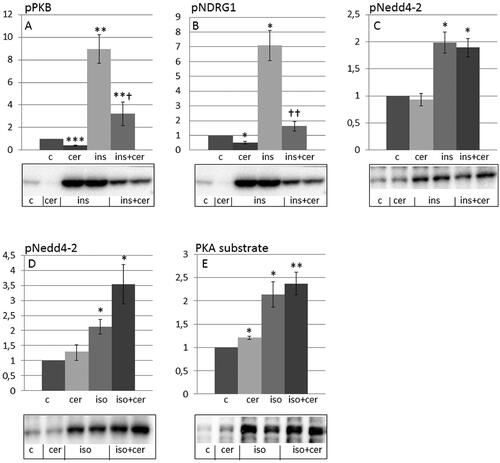
Ceramide inhibits insulin-induced phosphorylation of PKB and NDRG1 but not phosphorylation of Nedd4-2 in HEI-OC1 auditory cells
Ceramides inhibited insulin-induce phosphorylation of PKB and the SGK1 substrate NDRG1 (). However, ceramides did not prevent insulin induced phosphorylation of Nedd4-2 (), indicating the involvement of another Nedd4-2 kinase. Also, ceramides did not prevent isoprenaline-induced phosphorylation of Nedd4-2 and PKA substrates ().
Figure 3. Ceramide inhibits insulin-induced phosphorylation of PKB and NDRG1 but not phosphorylation of Nedd4-2 in HEI-OC1 auditory cells. HEI-OC1 cells were preincubated with C2-ceramide (100 µM, 30 min) and thereafter stimulated without or with 10 nM insulin for 10 min (A-C) and 30 nM isoprenaline for 10 min (D,E). Phosphorylation of PKB (A) (n = 3), NDRG1 (B) (n = 4), Nedd4-2 (C,D) (n = 4) and PKA substrates (E) (n = 5) were analyzed by western blotting. Blots are representative of 3–5 independent experiments. Western blot signals were quantified and normalized to untreated control samples. Data are presented as means ± SEM. *p < .05, **p<.01, ***p < .001 versus control, †p < .05, ††p < .01 versus insulin only.
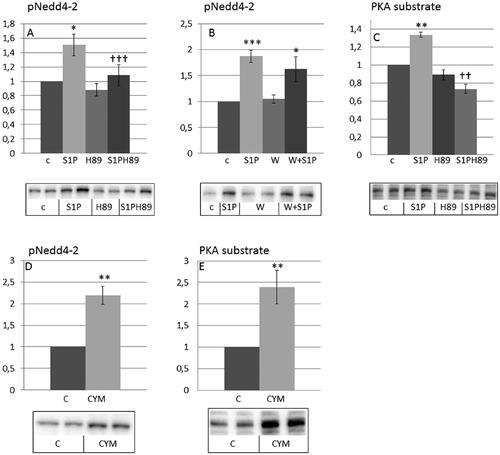
Sphingosine 1-phosphate rescues Nedd4-2 from ceramide induced insulin resistance in HEI-OC1 auditory cells
Ceramides are known to generate a number of sphingolipids among them sphingosine 1-phosphate (S1P). As shown in , SIP induced phosphorylation of Nedd4-2, which was prevented by H89, a PKA inhibitor, but not by wortmannin. CYM5478, a SIP2 agonist, induced phosphorylation of Nedd4-2 (). Both S1P () and CYM5478 () induced phosphorylation of PKA substrates. These results are in agreement with Nedd4-2 being phosphorylated in response to S1P in a SIP2 and PKA-dependent manner.
Figure 4. Sphingosine 1-phosphate and the S1P2 agonist CYM5478 induce phosphorylation of Nedd4-2 and PKA substrates in HEI-OC1 auditory cells. HEI-OC1 cells were incubated without or with 20 µM H89 for 30 min (A,C) (n = 4) and 100 nM wortmannin for 30 min (B) (n = 6) and then stimulated without or with 1 µM sphingosine 1-phosphate (SIP) for 10 min or HEI-OC1 cells were incubated with 100 µM CYM5478 for 20 min (D,E) (n = 3). Phosphorylation of Nedd4-2 (A,B,D) and PKA substrates (C,E) were analyzed by western blotting. Western blot signals were quantified and normalized to untreated control samples. Blots are representative of 3–6 independent experiments. Data are presented as means ± SEM. *p < .05, **p<.01, ***p < .001 versus control, ††p < .01, †††p < .001 versus S1P only.
Discussion
In previous studies, we have shown that the insulin receptor and insulin signaling components are expressed in the human saccule sensory epithelium [Citation8,Citation9], and that early events of insulin signaling takes place in auditory HEI-OC1auditory cell [Citation7]. In the present study, we show that (a) Nedd4-2 is a target for insulin signaling in HEI-OC1 cells, (b) PI3K/PKB rather than PI3K/SGK1 mediates the effect of insulin on Nedd4-2 phosphorylation, and (c) sphingolipids interfere with insulin signaling in both beneficial and unfavorable ways.
Regarding the insulin sensitive kinase mediating the effect on Nedd4-2, our data support a main role for PKB in HEI-OC1 cells which is in agreement with some previous studies in other cell types, for examples in alveolar cells [Citation15], however, most studies give support for SGK1 as a major Nedd4-2 upstream kinase, for example [Citation11–14]. Thus, in HEI-OC1 cells, GSK650394 prevented insulin-induced phosphorylation of the SGK1 substrate NDRG1, but not of PKB and Nedd4-2. One should stress that PKB and SGK1 appear to display a high level of overlapping substrate specificity, which complicates the attribution of specific biological functions to either of them. For example, PKB and SGK1 share the same optimal target motif, Arg-X-Arg-X-X-Ser/Thr, for example see [Citation13] and references therein, and also share the essential features of their activation mechanism involving mTOR Complex 2, PDK1 and PI3K [Citation11,Citation14] which is in agreement with our result that the PKB inhibitor MK2206 also prevented the phosphorylation of the SGK1 substrate NDRG1. However, it has been demonstrated that activation of SGK1 and PKB occurs at distinct subcellular compartments which provides a mechanism for the selective activation of these functionally distinct mTORC2 targets [Citation19].
Nevertheless, Nedd4-2 appears to be a target for insulin signaling in HEI-OC1 cells, which has implications for insulin regulation of sodium transport and, hence endolymphatic homeostasis. Hypoabsorption of sodium from the endolymph has been suggested to be associated with endolymphatic hydrops, the pathological base of Meniere’s disease [Citation10]. Defects in inner ear insulin signaling leading to reduced Nedd4-2 phosphorylation could thus lead to lower amounts of ENaC at the plasma membrane which could contribute to hypoabsorption of sodium and possibly contribute mechanistically to the link between diabetes and inner ear dysfunction.
Increased levels of tissue ceramides are believed to contribute to insulin resistance in obesity and type 2 diabetes [Citation16]. Also ceramides have been shown to have negative effects on inner ear function [Citation17]. The finding that ceramides inhibit insulin-induced phosphorylation of PKB in HEI-OC1 cells is in agreement with previous studies in classical target tissues for insulin action [Citation16]. Nevertheless, insulin-induced phosphorylation of Nedd4-2 was not reduced by ceramide treatment, however completely blocked by wortmannin. These results indicate that ceramides per se induce signaling events, resulting in the phosphorylation of Nedd4-2. Thus, we tested the ability of S1P, a molecule generated from ceramides, to phosphorylate Nedd4-2. We found that SIP induced phosphorylation of Nedd4-2, an effect which was mimicked by CYM5478, a S1P2 agonist and antagonized by the PKA inhibitor H89. In agreement with PKA-dependent phosphorylation of Nedd4-2 by S1P, also isoprenaline induced phosphorylation of Nedd4-2. Thus, insulin, cAMP-increasing hormones as well as SIP phosphorylate Nedd4-2 at serine 328. Indeed Nedd4-2 has been suggested to be an important point of convergence between receiving input from various pathways [Citation11,Citation20]. In addition, the different pathways may intersect at multiple levels in addition to Nedd4-2 phosphorylation resulting in differences in final physiological responses by the different combinations of stimuli [Citation11,Citation19].
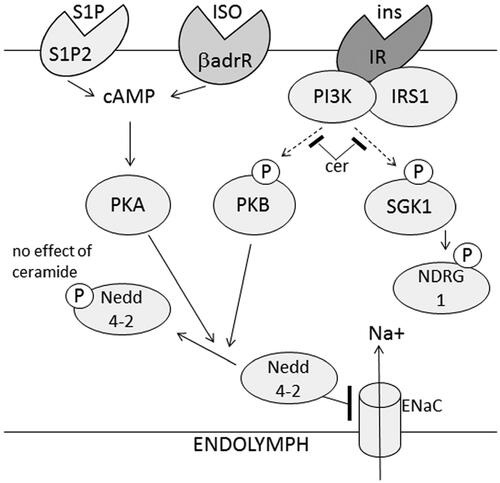
In conclusion, insulin induces phosphorylation of Nedd4-2 and increased expression of ENaC at the plasma membrane in a PI3K and PKB-dependent manner with implications for sodium and fluid homeostasis (). Using ceramides to mimic an insulin resistant state, up-stream insulin signaling was blocked in HEI-OC1 cells, however, ceramides did not block insulin-induced phosphorylation of the down-stream target Nedd4-2. Thus, in the insulin-resistant situation created by ceramides, it appears as if S1P can rescue phosphorylation of Nedd4-2 in a PKA-dependent manner. More studies are needed to further elucidate the role of inner ear insulin signaling in the regulation of inner ear ion and fluid homeostasis in normal physiology and in diabetes.
Figure 5. Schematic drawing of the effects of insulin on sodium transport in HEI-OC1 auditory cells. Insulin induces phosphorylation of Nedd4-2 via PKB signaling and increases the amount of ENaC at the plasma membrane. Ceramides prevent insulin-induced phosphorylation of PKB and NDRG1, but not phosphorylation of Nedd4-2. The ceramide metabolite sphingosine 1-phosphate induces phosphorylation of Nedd4-2 in a PKA-dependent manner which is also the case for isoprenaline and could explain the maintained phosphorylation of Nedd4-2 in the presence of ceramides. βadrR: βadrenergic receptor; iso: isoprenaline.
Acknowledgments
HEI-OC1 auditory cells were a kind gift from Dr Federico Kalinec. E. D., A. P., K. G. S. and B. M. are affiliated with Lund University Diabetes Center (LUDC).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Teng ZP, Tian R, Xing FL, et al. An association of type 1 diabetes mellitus with auditory dysfunction: a systematic review and meta-analysis. Laryngoscope. 2017;127(7):1689–1697.
- Akinpelu OV, Mujica-Mota M, Daniel SJ. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope. 2014;124(3):767–776.
- D'Silva LJ, Lin J, Staecker H, et al. Impact of diabetic complications on balance and falls: contribution of the vestibular system. Phys Ther. 2016;96(3):400–409.
- Elangovan S, Spankovich C. Diabetes and auditory-vestibular pathology. Semin Hear. 2019;40(4):292–299.
- Seo M, Lee YS, Moon SS. Association of hearing impairment with insulin resistance, β-cell dysfunction and impaired fasting glucose before onset of diabetes. Diabet Med. 2016;33(9):1275–1282.
- Hong BN, Kang TH. Distinction between auditory electrophysiological responses in type 1 and type 2 diabetic animal models. Neurosci Lett. 2014;566:309–314.
- Pålbrink AK, Kopietz F, Morén B, et al. Inner ear is a target for insulin signaling and insulin resistance: evidence from mice and auditory HEI-OC1 cells. BMJ Open Diab Res Care. 2020;8(1):e000820.
- Degerman E, Rauch U, Lindberg S, et al. Expression of insulin signalling components in the sensory epithelium of the human saccule. Cell Tissue Res. 2013;352(3):469–478.
- Degerman E, In 't Zandt R, Pålbrink A, et al. Inhibition of phosphodiesterase 3, 4, and 5 induces endolymphatic hydrops in mouse inner ear, as evaluated with repeated 9.4T MRI. Acta Otolaryngol. 2017;137(1):8–15.
- Kim SH, Marcus DC. Regulation of sodium transport in the inner ear. Hear Res. 2011;280(1–2):21–29.
- Rotin D, Staub O. Function and regulation of the epithelial Na+ channel ENaC. Compr Physiol. 2021;11(3):2017–2045.
- Mansley MK, Wilson SM. Effects of nominally selective inhibitors of the kinases PI3K, SGK1 and PKB on the insulin-dependent control of epithelial Na + absorption. Br J Pharmacol. 2010;161(3):571–588.
- Inglis SK, Gallacher M, Brown SG, et al. SGK1 activity in Na + absorbing airway epithelial cells monitored by assaying NDRG1-Thr346/356/366 phosphorylation. Pflugers Arch. 2009;457(6):1287–1301.
- Gleason CE, Frindt G, Cheng CJ, et al. mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity. J Clin Invest. 2015;125(1):117–128.
- Mattes C, Laube M, Thome UH. Rapid elevation of sodium transport through insulin is mediated by AKT in alveolar cells. Physiol Rep. 2014;2(3):e00269.
- Sokolowska E, Blachnio-Zabielska A. The role of ceramides in insulin resistance. Front Endocrinol . 2019;10:577.
- Romero-Guevara R, Cencetti F, Donati C, et al. Sphingosine 1-phosphate signaling pathway in inner ear biology. Front Aging Neurosci. 2015;7:60.
- Kalinec GM, Webster P, Lim DJ, et al. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol. 2003;8(4):177–189.
- Snyder PM, Olson DR, Kabra R, et al. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem. 2004;279(44):45753–45758.
- Gleason CE, Oses-Prieto JA, Li KH, et al. Phosphorylation at distinct subcellular locations underlies specificity in mTORC2-mediated activation of SGK1 and akt. J Cell Sci. 2019;132(7):jcs224931.
