Abstract
Background
There is no report on acute sensorineural hearing loss with congenital cytomegalovirus (cCMV) infection in basic experiments.
Aims/Objectives
The aim of this study was to evaluate the effect of dexamethasone, an anti-inflammatory steroid, on acute sensorineural hearing loss in the mouse cytomegalovirus (MCMV) infection model mice.
Material and Methods
Sensorineural hearing loss model mice were divided into two groups, one with and one without intratympanic dexamethasone. Dexamethasone was injected into the tympanic cavity of only the right ear, and hearing ability was assessed at the ages of three, six, and eight weeks by auditory brainstem response measurement.
Results
Among the 23 mice intratympanically injected with dexamethasone (15 μg/mouse) at the age of three weeks, five (21.7%) had a hearing improvement of at least 10 dB and 18 (78.3%) had no improvement at the age of six weeks. Among the 19 mice that did not receive a dexamethasone injection, one (5.3%) showed improvement and 18 (94.7%) showed no improvement (p = 0.129).
Conclusions and Significance
In this study, transtympanic infusion of dexamethasone into the tympanic cavity was effective in some mice with sensorineural hearing loss, suggesting that, in addition to angiogenesis, anti-inflammatory activity might be a mechanism of treatment for hearing loss.
Chinese Abstract
背景:没有关于在基础实验中先天性巨细胞病毒(cCMV)感染引起的急性感觉神经性听力损失的报道。
目的:本研究的目的是评估地塞米松(一种抗炎类固醇)对小鼠巨细胞病毒 (MCMV) 感染范例小鼠的急性感觉神经性听力损失的影响。
材料与方法:感音神经性听力损失范例小鼠分为两组, 一组鼓室内接受地塞米松, 另一组鼓室内没有接受地塞米松。地塞米松只注入右耳鼓膜腔, 在 3、6 和 8 周时通过听觉脑干反应测量对听力能力进行评估。
结果:在 23 只鼓室内注射地塞米松(15 lg/小鼠)的三周龄的小鼠中, 六周龄时五只 (21.7%) 听力改善了至少 10 分贝, 而 18 只 (78.3%) 没有改善。在未接受地塞米松注射的 19 只小鼠中, 1 只(5.3%)听力有改善, 18 只(94.7%)听力没有改善(p = 0.129)。
结论和意义:在本研究中, 经鼓室向鼓室腔注入地塞米松对某些感音神经性耳聋小鼠有效。这表明, 除了血管再生术, 抗炎活性可能是治疗听力损失的一种机制。
Introduction
Congenital cytomegalovirus (cCMV) infection is known to cause various diseases or signs/symptoms following mother-to-child transmission, including low birth weight, microcephaly, chorioretinitis, and sensorineural hearing loss (SNHL). cCMV is estimated to account for approximately 20% of cases of congenital hearing loss [Citation1].
There has been the previous report that intravenous administration of the antiviral agent ganciclovir and oral administration of the antiviral agent valganciclovir are effective in improving SNHL or preventing its progression in children who have developed cCMV infection and been diagnosed with SNHL [Citation2]. Ineffectiveness in some cases or occurrence of adverse drug reactions such as bone marrow suppression has also been reported [Citation2]. Thus, treatments for SNHL caused by cCMV have not been established, and considering new treatments is an issue.
Steroids are used for the treatment of sudden deafness or progressive hearing loss in daily clinical practice of otolaryngology, with their routes of administration including oral administration, intravenous infusion, and intratympanic injection (i.e. local administration in the inner ear). The synthetic glucocorticoid dexamethasone is reported to achieve much higher local concentrations in the inner ear with intratympanic injection than with intravenous infusion [Citation3] and is also well reported to be associated with a low incidence of systemic adverse drug reactions and its effectiveness against sudden deafness [Citation4]. Transtympanic infusion of steroid into the tympanic cavity seems to have anti-inflammatory and angiogenic effects, but previously reported studies in basic experiments are limited to the efficacy of dexamethasone on noise-induced hearing loss [Citation5] and the efficacy of prednisolone on cisplatin-induced hearing loss [Citation6], with no report on acute sensorineural hearing loss being yet available.
It has been reported that SNHL occurred in approximately 80% of model mice that were injected in the brain with CMV immediately after birth and was mediated by degeneration of myosin in outer hair cells [Citation7]. Myosin VI is regarded as an essential protein for auditory function, and its loss may lead to hearing loss [Citation8]. In addition, studies have also suggested that myosin degeneration is not attributable to direct cytotoxicity but caused by inflammatory reactions around infected cells around the spiral ganglion, meninges, and scala tympani, including macrophage activation [Citation8]. In addition, it has been previously reported that inflammasome is activated in response to MCMV infection in the inner ear of the mice to induce SNHL [Citation9], suggesting that administration of anti-inflammatory agents may inhibit the progression of SNHL.
This study evaluated the efficacy of transtympanic infusion of dexamethasone into the tympanic cavity in model mice that developed SNHL after injection of CMV in the brain to determine the effects of the anti-inflammatory dexamethasone on the progression of SNHL due to cMCV infection.
Materials and methods
Ethical review
This study was conducted with the approval of the ethical committee of the Animal Care and Use Committee in Fukushima Medical University.
Generation of SNHL model mice
SNHL model mice were generated by injection of 140 pfu of the virus in 3 µL viral suspension obtained by diluting the mouse cytomegalovirus (MCMV) Smith strain with Dulbecco’s Modified Eagle Medium (DMEM) in the right lateral ventricle of the cerebrum of neonatal BALB/c mice within 24 h of birth using a digital syringe (eVol XR, GL Sciences, Tokyo, Japan). This is the method already established by Ikuta et al. [Citation7].
Assessment of hearing loss
Hearing loss was assessed at three and six weeks after MCMV injection by auditory brainstem response (ABR) measurements. We evaluated it as hearing loss if the V wave (fifth) out of the five waveforms did not appear within the measurement time (up to about 8 to 10 msec on the horizontal axis). We also stepped down the sound pressure every 10 dB from 100 dB in a normal hearing mouse and set the "health threshold" 10 dB above the sound pressure at which the V wave disappeared. ABR thresholds were determined based on a series of responses obtained at intervals of 10 dB in the range from 60 dB sound pressure levels (SPLs) to 100 dB SPLs at 40 kHz, and mice were used as SNHL model mice when no response was obtained at 60 dB SPLs at the age of three weeks. A frequency of 12 kHz was used, and electrodes were grounded behind the right and left ears. When we evaluated the ABR, the mice were anesthetized with a mixture of three anesthetics of medetomidine, midazolam, and butorphanol. We used TDT system (Tucker-Davis Technologies, Alachua, FL, USA) for evaluation.
Therapeutic experiment in SNHL model mice
Mice were divided into two groups, one with and one without intratympanic dexamethasone, so that baseline hearing ability was well balanced between the groups at the age of three weeks. The mice in the intratympanic dexamethasone group were anesthetized with a mixture of three anesthetics of medetomidine, midazolam, and butorphanol and were transtympanically injected into the tympanic cavity with dexamethasone as steroid (DECADRON®, Aspen Japan K.K, Tokyo, Japan) at 15 µg/mouse using a 29 G microsyringe under a microscope. Dexamethasone was injected to the tympanic cavity of only the right ear at three times (at the ages of three, four, and five weeks), and hearing ability was assessed at the ages of three, six, and eight weeks by ABR measurement. After the study, all mice were anesthetized with isoflurane and euthanized by cervical dislocation.
Statistical analysis
Statistical analysis was performed using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria [https://www.R-project.org/]). The status of improvement in hearing ability at the age of six weeks was divided into an improvement of at least 10 dB and others and analyzed by chi-square testing. A two-sided significance level of 5% was applied to testing.
Results
Among the 71 mice injected with MCMV, 52 were divided into two groups of 26 mice and included in the experiment after excluding 19 mice that were dead or were not evaluable due to otitis media or other conditions at the age of three weeks. Of these mice, 23 and 19 in the groups with and without intratympanic dexamethasone, respectively, were measured for ABR up to the age of six weeks.
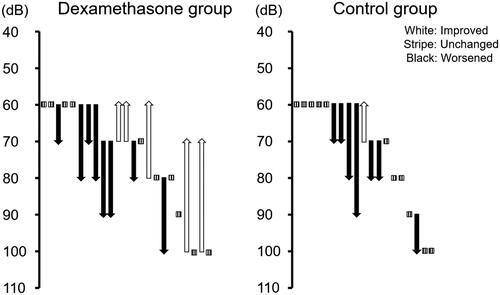
An assessment of changes in hearing ability from the age of three weeks (just before the start of treatment) to the age of six weeks found that, among the 23 mice in the group with intratympanic dexamethasone, five (21.7%) showed a hearing improvement of at least 10 dB (mean ± standard deviations are 20 ± 10) and 18 (78.3%) showed no improvement. In contrast, among the 19 mice in the group without intratympanic dexamethesone, one (5.3%) showed a hearing improvement of at least 10 dB and 18 (94.7%) remained unchanged, indicating a tendency toward hearing improvement in the group with intratympanic dexamethasone compared with the group without it (p = 0.129) (). Individual ABR changes, animals remaining unchanged, animals with improvement on the side of dexamethasone injection, animals with improvement on both sides, and animals with worsening hearing on both sides are shown in , respectively.
Table 1. Changes in hearing ability from the ages of three weeks to six weeks.
Discussion
This study indicated that transtympanic infusion of dexamethasone into the tympanic cavity might be effective in model mice with SNHL induced by MCMV. Although no significant difference was found between the groups (p = 0.129), evidence of an adequate tendency was demonstrated.
Oral steroid therapy or intravenous steroid infusion is generally used for the treatment of sudden deafness or progressive hearing loss. Because the glurocorticoid receptor is present in the inner ear [Citation10], and dexamethasone, which has high affinity for glurocorticoid, has been found to reduce intracochlear inflammation [Citation11], steroids have been used for the treatment of sudden deafness attributable to various causes. Intratympanic steroids have received attention because transport of high concentrations of steroids to the inner ear is expected to bring about higher efficacy, but drug transport is inhibited by the blood-labyrinth barrier with oral therapy or intravenous infusion [Citation12]. Intratympanic steroids have recently demonstrated evidence of efficacy and are currently studied in clinical settings [Citation4,Citation13].
It has been reported that acute inner ear disorder is mediated by the inflammatory cytokines IL-6, IL-1β, and TNFα [Citation14], and that the expression of these cytokines is mediated by an increase in the nuclear factor kappa B (NFκB) [Citation15]. In addition, Kanzaki et al. reported that a high level of fibrinogen was a poor prognostic factor of sudden deafness [Citation16]. In this study, intratympanic dexamethasone likely inhibited these factors to improve hearing ability.
The observed improvement in hearing ability is likely to be due to not only the anti-inflammatory effect of the steroid but also the improvement of blood flow disturbance by its angiogenic effect. Steroids have been reported to increase microvascular blood flow in the cochlea [Citation17], and this increase in blood flow is thought to be due to activated potassium ions associated with angiogenesis in the stria vascularis [Citation18].
Possible mechanisms of impaired hearing due to CMV include viral meningitis, virus proliferation and the accumulation of activated macrophages in the spiral ganglion, around the meninges, and around the scala tympania at the age of one week, and the loss of myosin expression in outer hair cells at the age of 3 weeks, leading to auditory impairment. However, the study suggested that transtympanic infusion of dexamethasone into the tympanic cavity was effective against SNHL induced by MCMV infection and that therapy with dexamethasone might therefore have the potential to inhibit the progression of SNHL induced by inflammation of the inner ear due to cCMV. The study also indicated that improvement of impaired hearing by dexamethasone might be based on both the angiogenic and anti-inflammatory effects of the drug.
In this study, improvement in hearing ability in SNHL was observed only in some mice, but not in all mice, likely due to individual differences. Impaired hearing is caused by a blood flow disturbance resulting from inflammation. Mice that showed improvement of impaired hearing are likely to be those in which the blood supply from the stria vascularis was not completely interrupted. In mice with more rapid progression, it is likely that myosin degeneration was marked, and it was too late. Improvement was also observed in the ear on the other side in some mice (data not shown), likely due to the rudimentary status of the blood-labyrinth barrier. Suzuki et al. suggested that the blood-labyrinth barrier matured in rats up to the age of 14 days [Citation19]. This study included mice that likely had a poorly developed blood-labyrinth barrier because they were used as SNHL model mice soon after birth.
Many uncertainties still remain about impaired hearing and inflammation due to CMV infection. Loss of myosin expression, generation of active oxygen due to accumulation of activated macrophages, and disruption of hearing-related genes due to chromosomal damage have been thought to be caused by CMV infection and remain to be elucidated.
In mice with SNHL induced by intraventricular inoculation of MSMV, Ikuta et al. reported that the viral antigen and DNA were detected in the spiral ganglion, around the meninges, and in the perilymphatic region at one week after infection, but were lost at two weeks or later, and that, at three weeks or later, when impaired hearing started to progress, cells infected with the virus and inflammatory evidence disappeared, indicating a short duration of inflammation [Citation20]. This study suggested that intratympanic dexamethasone administered at three, four, and five weeks to SNHL model mice generated by injection of MCMV in the right lateral ventricle of the cerebrum showed improved hearing ability at the age of six weeks. This study suggested that residual inflammation observed in the tympanic cavity was improved by dexamethasone. It should be noted that the condition of mice may vary with the site of MCMV injection. In the future, more accurate studies will be possible with markers of inflammation.
There are the limitations of the study. This study is at the stage of animal experiments in mice, and the mechanism of SNHL generally varies even if the virus is the same between animals and humans. Therefore, basic reports of cCMV infection in humans will be important in the future. In addition, dexamethasone therapy regimens in SNHL due to cCMV infection in humans, including the method or frequency of injection, are not standardized, and remain to be determined in the future.
This study is only an experiment in animals, and the effect of intratympanic administration of steroids on deafness caused by CMV and the proof of its mechanism are limited. It is necessary to prove the hypothesis by showing human data in the future.
Conclusion
This study indicated that transtympanic infusion of dexamethasone into the tympanic cavity might be effective in mice with SNHL induced by MCMV, suggesting that this therapy might improve SNHL associated with cCMV infection.
Disclosure statement
The authors declare that there is no conflict of interest.
Data availability statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Morton CC, Nance WE. Newborn hearing screening-a silent revolution. N Engl J Med. 2006;354(20):2151–2164.
- Kimberlin DW, Lin CY, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the Central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25.
- Chandrasekhar SS, Rubinstein RY, Kwartler JA, et al. Dexamethasone pharmacokinetics in the inner ear: Comparison of route of administration and use of facilitating agents. Otolaryngol Head Neck Surg. 2000;122:521–528.
- Zhao D, Tong B, Wang Q, et al. A comparison of effects of systemic and intratympanic steroid therapies for sudden sensorineural hearing loss: a Meta-analysis. J Otol. 2016;11(1):18–23.
- Han MA, Back SA, Kim HL, et al. Therapeutic effect of dexamethasone for noise-induced hearing loss: Systemic versus intratympanic injection in mice. Otol Neurotol. 2015;36(5):755–762.
- Ramaswamy B, Roy S, Apolo AB, et al. Magnetic nanoparticle mediated steroid delivery mitigates cisplatin induced hearing loss. Front Cell Neurosci. 2017;11:268.
- Ikuta K, Ogawa H, Hashimoto H, et al. Restricted infection of murine cytomegalovirus (MCMV) in neonatal mice with MCMV-induced sensorineural hearing loss. J Clin Virol. 2015;69:138–145.
- Friedman TB, Sellers JR, Avraham KB. Unconventional myosins and the genetics of hearing loss. Am J Med Genet. 1999;89(3):147–157.
- Zhuang W, Wang C, Shi X, et al. MCMV triggers ROS/NLRP3-associated inflammasome activation in the inner ear of mice and cultured spiral ganglion neurons, contributing to sensorineural hearing loss. Int J Mol Med. 2018;41(6):3448–3456.
- Rarey KE, Luttge WG. Presence of type I and type II/IB receptors for adrenocorticosteroid hormones in the inner ear. Hear Res. 1989;41(2-3):217–222.
- Lyu AR, Kim DH, Seung LH, et al. Effects of dexamethasone on intracochlear inflammation and residual hearing after cochleostomy: a comparison of administration routes. PLoS One. 2018;13(3):e0195230.
- Nyberg S, Abbott NJ, Shi X, et al. Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med. 2019;11:eaao0935.
- Kordiš Š, Battelino S. The role of high dose intratympanic dexamethasone as salvage therapy for idiopathic sudden sensorineural hearing loss. J Int Adv Otol. 2017;13(3):318–321.
- Fujioka M, Kanzaki S, Okano HJ, et al. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res. 2006;83(4):575–583.
- Masuda M, Nagashima R, Kanzaki S, et al. Nuclear factor-kappa B nuclear translocation in the cochlea of mice following acoustic overstimulation. Brain Res. 2006;1068(1):237–247.
- Kanzaki S, Sakagami M, Hosoi H, et al. High fibrinogen in peripheral blood correlates with poorer hearing recovery in idiopathic sudden sensorineural hearing loss. PLoS One. 2014;9(8):e104680.
- Shirwany NA, Seidman MD, Tang W. Effect of transtympanic injection of steroids on cochlear blood flow, auditory sensitivity, and histology in the guinea pig. Am J Otol. 1998;19:230–235.
- Umaru B, Pyriochou A, Kotsikoris V, et al. ATP-sensitive potassium channel activation induces angiogenesis in vitro and in vivo. J Pharmacol Exp Ther. 2015;354(1):79–87.
- Suzuki M, Yamasoba T, Kaga K. Development of the blood-labyrinth barrier in the rat. Hear Res. 1998;116(1-2):107–112.
- Ikuta K, Suzutani T. Congenital cytomegarovirus infection and hearing loss. Fukushima Med J. 2017;67:7–15. (In Japanese)