Abstract
Background
In surgical resection of squamous cell carcinoma of the oral tongue (SCCOT), achieving clear margins is important for prognosis. Insufficient histopathological margins are common, particularly deep margins.
Aims/objectives
The aim of the present study was to determine whether ultrasound (US)-assisted resection could decrease the proportion of insufficient histopathological deep margins in SCCOT.
Material and methods
34 patients with SCCOT undergoing US-assisted resection (study group) were compared to 76 whose resections were performed without US (conventional group). Outcome measures were insufficient deep histopathological resection margins and mean difference in deep margins.
Results
Insufficient deep resection margins (<5.0 mm) were seen in 8 of 34 (23.5%) in the study group, compared to 31 of 76 (40.8%) in the conventional group, unadjusted RR 0.58 [95% CI 0.30–1.12; p = .11], adjusted RR 0.82 [95% CI 0.35–1.92; p = .64]. Unadjusted mean difference was 1.4 mm (95% CI 0.1–2.7, p = .04), adjusted mean difference 1.1 mm (95% CI −2.7 to 0.5, p = .19).
Conclusions
Intraoperative US can visualize the deep resection margins in T1/T2 SCCOT. US-assisted resection seems to decrease the number of insufficient histopathological deep margins, though the results are not statistically significant. Comparatively good results in the conventional group is one explanation for the lack of significance.
Clinicaltrials.gov ID
NCT04059861
Chinese Abstract
背景:过敏性鼻炎 (AR) 是西方化世界的一种常见病症, 被认为比典型的“Th2”炎症在免疫学上更为复杂, 需要新的方法来解释这种复杂性。
目的:在本项研究中, 我们探索了一种基于组织学的新型分析法, 用于分析 16 名在花粉季节之外和期间患有季节性 AR 的患者的循环血液白细胞特征。
材料和方法:用尽量少的离体人工制品纯化白细胞, 将其包埋于琼脂糖石蜡颗粒中, 用于基于免疫组织化学的免疫细胞分析, 并进行了血液白细胞制图。
结果:与淡季基线相比, 在花粉季节收集的样本具有统计学意义上的嗜酸性粒细胞、嗜中性粒细胞、单核细胞和 CD8+ 淋巴细胞的增加。 相反, CD20+ B 淋巴细胞和 CD3+ T 淋巴细胞没有观察到任何变化。 CD4+ T 辅助细胞的亚分类表明, 在花粉季节, Th2 和 Th17 细胞显示平行且显著的扩增, 而 Th1 细胞保持不变。 在花粉季节, 尽管嗜碱性粒细胞的绝对数量没有改变, 但嗜碱性粒细胞标记物 GATA2 和 CPA3有所增加。
结论和意义:本研究介绍了一种新的适用于系统免疫细胞筛选的方法, 并提供了季节性 AR 的复杂且平行的 Th2 和 Th17 免疫特征的进一步证据。 它还提出将 GATA2 和 CPA3 作为持续过敏性炎症的潜在生物标志物。
Introduction
The first line of treatment for squamous cell carcinoma of the oral tongue (SCCOT) is surgical resection with clear margins. What constitutes a sufficient margin is a matter of debate. Traditionally, a histopathological margin of 5 mm is considered a clear resection margin, whereas negative margins less than 5 mm are considered a close margin. Positive margins with tumours at the resection border are indisputably a negative prognostic factor, decreasing local recurrence-free survival (LRFS) [Citation1–4]. However, the definitions of positive margins differ. The College of American Pathologists consider tumours at the resection margin as a positive margin [Citation5], whereas The Royal College of Pathologists define a resection margin <1 mm as positive [Citation6]. Recent studies have questioned the definition of close margins, reporting that clear margins less than 5 mm had similar outcomes to those with margins more than 5 mm [Citation2,Citation3,Citation7,Citation8]. Zanoni et al. reported that the optimal cut-off between clear and close margins in SCCOT was 2.2 mm, since the group with resection margins 2.3–5 mm had a LRFS similar to the group with resection margins 5 mm or more [Citation3]. In addition, patients with a margin of 0.01–2.2 mm had a worse LRFS outcome, but significantly better than those with positive margins.
In surgical resection of SCCOT, the deep resection margin is where it is most difficult to achieve clear margins [Citation4,Citation9]. During surgery, the mucosal margin can be inspected and marked under direct vision 10–15 mm from the macroscopic tumour border. In contrast, the deep tumour border is assessed by palpation and preoperative radiologic imaging but is not directly visible.
Intraoral ultrasound (US) is able to determine the tumour thickness in SCCOT [Citation10,Citation11]. This means that the technique can visualize the deep border of the tumour and potentially also be used to assist in surgical resection [Citation12]. In a pilot study with 12 patients, Tarabichi et al. suggested that the use of intraoperative US as a non-invasive method improves the deep resection margins of SCCOT [Citation13].
The aim of the present study was to determine whether US-assisted resection could decrease the proportion of insufficient margins in oral tongue cancer. Outcome measure was insufficient deep histopathological resection margins in the US-assisted study group compared to the conventional group where surgical resection was performed without the use of US.
Materials and methods
The study was approved by the Swedish Medical Ethics Committee, 2019-00246.
Population
The study population consisted of patients with biopsy-proven primary SCCOT stage T1–T3 according to TNM8 [Citation14] who presented to Örebro University Hospital between May 2019 and May 2021. Patients consecutively provided written consent after receiving oral and written information about the study. The exclusion criteria were earlier surgery, radiotherapy in the oral cavity, stage T4 tumours or patients unsuitable for surgery.
The conventional group consisted of all patients with corresponding inclusion and exclusion criteria treated at the Centre for Head and Neck Oncology in Örebro between May 2015 and May 2019. These patients were identified in the Örebro Head and Neck Cancer Register. The T stage for patients who were classified according to TNM7 was converted to TNM8.
Method
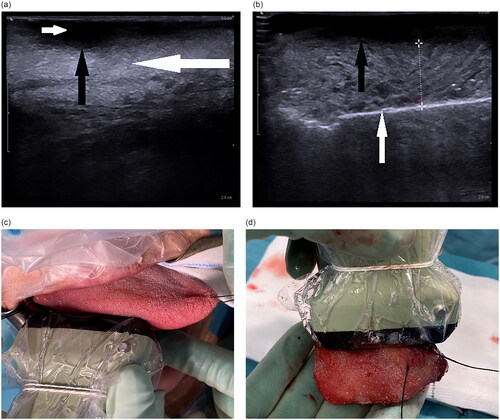
In the study group, The BK Medical Flex Focus 500 US system with the high frequency linear 8870 probe (Peabody, MA) was used in all patients. An US frequency of 18 MHz and a gain between 50% and 65% were used along with water-based Eco gel and a transducer cover. The deep tumour margin was evaluated by US (). A mucosal resection margin of at least 10 mm was marked with monopolar diathermy. Deep resection was performed with either monopolar diathermy or harmonic scalpel (Ethicon Harmonic Focus + Shears, Bridgewater, NJ), again aiming for at least a 10 mm macroscopic margin. Approximately one-quarter into the resection, US examination was performed (US in-vivo), and the deep resection margin was measured in millimetres. A metal spatula was placed at the resection border for increased contrast of the US image (can be excluded since air at the resection border produces a contrast as well). The smallest pressure with the probe necessary to achieve an adequate image was applied, since different pressures could alter the assessed margin by several millimetres. This assessment was repeated approximately two- and three-quarters into the resection and finally on the whole surgical specimen after resection (US ex-vivo); the latter was used for analysis. If the US margin was under 5 mm, a wider resection was considered after inspection and palpation of the specimen. Frozen-section analysis was only used in a few resections of stage T3 tumours. The conventional group were operated on by more senior surgeons compared to the study group.
Figure 1. (a) US of tongue cancer before resection. The tumour is seen hypodense (small white arrow), while normal tongue muscle deep to the tumour is isodense (large white arrow). The deep border of the tumour is clearly seen (black arrow). (b) US-assisted surgery measuring the deep resection margin. Deep tumour border (black arrow). Metal spatula marking the deep resection border (white arrow). (c) In-vivo intraoperative US examination of SCCOT. (d) Measurement ex-vivo by US of the deep resection margin in the resected specimen.
A clear margin was defined as 5.0 mm or more, whereas a clear margin of 0.01–4.9 mm was classified as close. We also performed an analysis using an alternative clear margin definition of 2.3 mm or more, and a clear margin of 0.01–2.2 mm classified as close.
Invasive tumours at the resection border were classified as positive (involved) margins. Dysplasia and carcinoma in situ were not classified as tumour and were not included in these definitions.
Statistical analysis
Differences in age and mean deep histopathological resection margin between the US-assisted surgery and control cohorts were analysed by Student’s t test and linear regression, and differences in categorical variables were analysed by the Chi-square test or Fisher’s exact test when appropriate.
Unadjusted and adjusted Poisson regression with robust standard errors were performed for the two binary definitions of close margins (<5.0 mm and ≤2.2 mm) to compare the two cohorts. Adjustment was made for potential confounders: T stage, free flap, Brandwein (BW) classification and harmonic scalpel. As nine patients had missing BW classifications, the adjusted models were constructed with (n = 101) and without (n = 110) BW classifications. This modified Poisson regression gives relative risks (RR) with 95% confidence intervals (CIs) as association measures. A p value lower than 0.05 was regarded as statistically significant, and analyses were performed in IBM SPSS (Armonk, NY) version 25 and Poisson regression in STATA release 17 (StataCorp, TX).
Results
A total of 34 patients were included in the study group and 76 patients in the conventional group. Details of these cohorts are presented in .
Table 1. Patient characteristics.
Insufficient deep resection margins defined as <5.0 mm, were seen in 8 of 34 (23.5%) US-assisted resections compared to 31 of 76 (40.8%) resections without US, unadjusted RR 0.58 [95% CI 0.30–1.12; p = .11], adjusted RR 0.82 [95% CI 0.35–1.92; p = .64] (). Higher T-stage was significantly associated with insufficient margins.
Table 2. Unadjusted and adjusted Poisson regression for insufficient deep margin <5.0 mm.
Insufficient deep resection margins defined as ≤2.2 mm, were seen in 2 of 34 (5.9%) US-assisted resections compared to 13 of 76 (17.1%) resections in the controls, unadjusted RR 0.34 [95% CI 0.08–1.45; p = .15], adjusted RR 0.28 [95% CI 0.09–0.87; p = .027].
The deep resection margins in the study group was 6.5 ± 3.2 mm (mean ± SD) (n = 34), and in the conventional group it was 5.1 ± 3.1 mm (n = 76). The unadjusted mean difference was 1.4 mm (95% CI 0.1–2.7; p = .04), while the adjusted mean difference was not significantly different between the groups (). Mean difference between deep margin by US ex-vivo and histopathological deep margin was 0.8 mm (95% CI −0.3 to 1.9; p = .1).
Table 3. Histopathological resection margins in mm for the study group and the conventional group. Ultrasound deep margins for study group.
Deep margins >10 mm were seen in two (5.9%) and three (3.9%) patients respectively in the study group and the conventional group, not significantly different.
The use of US to assist resection in this non-invasive method increased the time of the procedure by approximately 5–10 min.
Discussion
In the present study, US-assisted resection in patients with SCCOT resulted in proportionally fewer patients with insufficient histopathological deep margins compared to the control group, 23.5% and 40.8% respectively, but not significantly different. In the literature, data on deep resection margins are scarce; overall margins are more often reported. Several studies have reported 73–85% overall margins <5.0 mm in resections of SCCOT [Citation3,Citation7,Citation8,Citation15,Citation16]. In comparison, overall margins <5.0 mm in our study group was 41.2% and 59.2% in the conventional group, see . The fact that our conventional group had a fairly low percentage of insufficient margins, could be one explanation for the lack of significant difference when comparing with the US-assisted group.
Previous studies have indicated that US is promising in assisting the resection, both with and without invasive techniques to mark the margin [Citation12,Citation13]. Baek et al. included 20 patients and compared them to 20 historical controls [Citation12]. Their outcome measure was the mean deep margin (9.8 ± 5.2 mm SD in the US group, 4.0 ± 2.0 mm SD in the controls, p < .01). In comparison, the present study group had a mean deep margin of 6.5 ± 3.2 mm SD, and the controls 5.1 ± 3.1 mm SD (mean difference 1.4 mm [95% CI 0.1–2.7], p = .04, adjusted analyses not significant). Tarabichi used a non-invasive approach where US visualized the deep border of the tumour and the deep resection margin [Citation13]. In line with Baek et al. they used the mean deep margin as an outcome measure (9.7 mm ± 1.2 SD, no control group). De Koning compared 40 US-assisted resections to 96 conventional resections and found significant improvement in the US-assisted resections, 55% clear deep margins vs 15% [Citation16]. In the present study 76.5% of the US-assisted resections had clear deep margins. The mean margin in their study was 4.9 ± 2.6 mm in the US-assisted resections and 3.4 ± 2.0 mm in the conventional group, p = .002. Moreover, they found 8 mm to be a better cut-off than 5 mm on US ex-vivo for assessment of adequate resection margins. In agreement with our study, overtreatment (>10 mm margin) did not seem to be more common in the study group, supporting that US can adequately tailor the resections.
The comparison of the mean deep margin can be questioned. The purpose of oncological resection is not as wide a margin as possible, since it would have detrimental effects on the postoperative function of patients. Rather, the aim is clear margins but saving as much healthy tissue as possible. Therefore, we find it relevant to classify the margins as involved, close or clear, since excessive margins in some resections could mislead the results. Recent studies have questioned the necessity of 5 mm resection margins and suggest margins of 2–3 mm to be a better cut-off between clear and close margins in SCCOT [Citation2,Citation3,Citation7,Citation8,Citation17]. These data also imply that definitions should include involved margins (defined as invasive tumour at the resection border) and specify close margins in millimetres. The possibility of comparing studies suffers from a lack of uniform definition of involved, close and clear margins. The definition of what constitutes a close margin should be based on risk for LRFS and the need for adjuvant treatment [Citation18,Citation19]. We included an analysis with the definition of close margins as ≤ 2.2 mm, showing statistically significantly less inadequate resection margins in the US-assisted group (5.9%) compared to the control group (17.1%), see .
Of potential confounders in the present study, stage T3 was a strong predictor for inadequate margins, adjusted RR 3.63 [95% CI 1.41–9.37], p = .0076). In 56.0% of the stage T3 tumours, clear deep margins were not achieved. US could not overview these larger tumours and underestimated the deep borders. The present data support the potential of US-assisted surgery in stage T1 and T2 tumours, which is in line with the results reported by Noorlag et al. [Citation20]. In stage T3 tumours, US can still be useful, but awareness of the shortcomings in larger stage T3 tumours is important. Since stage T3 per se is a known risk factor for inadequate resection margins in comparison to stage T1/T2 [Citation4], this might explain the worse outcome, regardless of the use of US.
Harmonic scalpels were much more frequently used in the study group. This did not seem to affect the proportion of insufficient margins, but the harmonic scalpel was predominantly used in stage T3 tumours (90.0% usage in stage T3 tumours vs. 37.5% usage in stage T1/T2 tumours in the study group), which complicates the conclusions.
In summary, the results of the present and the abovementioned studies indicate that US-assisted surgery for T1/T2 SCCOT can improve the deep resection margins. Being non-invasive and fast, the method seems promising, but larger studies are needed to possibly confirm a statistically significant difference in adequate resection margins.
Methodological considerations/limitations
Limitations in this study include small numbers in the study and conventional group, making it difficult to reach significance if differences do exist. Wide confidence intervals for the association measures (RR) decreases the strength of the conclusions. The nonrandomized study design and comparison to a retrospective conventional group have inbuilt weaknesses, even though we tried to adjust for different confounders by regression analyses. The present study benefits from the fact that the US examinations were performed exclusively by head and neck surgeons, adding support to the feasibility of US-assisted resection of SCCOT.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue–clinicopathologic features affecting outcome. Cancer. 2012;118(1):101–111.
- Nason RW, Binahmed A, Pathak KA, et al. What is the adequate margin of surgical resection in oral cancer? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(5):625–629.
- Zanoni DK, Migliacci JC, Xu B, et al. A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol Head Neck Surg. 2017;143(6):555–560.
- Sutton DN, Brown JS, Rogers SN, et al. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2003;32(1):30–34.
- Seethala R, Weinreb I, Bullock M, et al. Protocol for the examination of specimens from patients with Carcinomas of the Lip and Oral Cavity version LipOralCavity 4.0.0.1. College of American Pathologists (CAP). 2017. Homepage | College of American Pathologists (https://www.cap.org)
- Helliwell T, Woolgar J. Dataset for histopathology reporting of mucosal malignancies of the oral cavity. London: The Royal College of Pathologists; 2013.
- Tasche KK, Buchakjian MR, Pagedar NA, et al. Definition of “close margin” in oral cancer surgery and association of margin distance with local recurrence rate. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1166–1172.
- Dik EA, Willems SM, Ipenburg NA, et al. Resection of early oral squamous cell carcinoma with positive or close margins: relevance of adjuvant treatment in relation to local recurrence: margins of 3 mm as safe as 5 mm. Oral Oncol. 2014;50(6):611–615.
- Klein Nulent TJW, Noorlag R, Van Cann EM, et al. Intraoral ultrasonography to measure tumor thickness of oral cancer: a systematic review and meta-analysis. Oral Oncol. 2018;77:29–36.
- Tarabichi O, Bulbul MG, Kanumuri VV, et al. Utility of intraoral ultrasound in managing oral tongue squamous cell carcinoma: systematic review. Laryngoscope. 2019;129:662–670.
- Yesuratnam A, Wiesenfeld D, Tsui A, et al. Preoperative evaluation of oral tongue squamous cell carcinoma with intraoral ultrasound and magnetic resonance imaging-comparison with histopathological tumour thickness and accuracy in guiding patient management. Int J Oral Maxillofac Surg. 2014;43(7):787–794.
- Baek CH, Son YI, Jeong HS, et al. Intraoral sonography-assisted resection of T1-2 tongue cancer for adequate deep resection. Otolaryngol Head Neck Surg. 2008;139(6):805–810.
- Tarabichi O, Kanumuri V, Juliano AF, et al. Intraoperative ultrasound in oral tongue cancer resection: feasibility study and early outcomes. Otolaryngol Head Neck Surg. 2018;158(4):645–648.
- Brierley J, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. Chichester (UK): John Wiley & Sons, Inc.; 2017.
- Smits RW, Koljenovic S, Hardillo JA, et al. Resection margins in oral cancer surgery: room for improvement. Head Neck. 2016;38(Suppl 1): e2197–203.
- de Koning KJ, van Es RJJ, Klijn RJ, et al. Application and accuracy of ultrasound-guided resections of tongue cancer. Oral Oncol. 2022;133:106023.
- Brinkman D, Callanan D, O’Shea R, et al. Impact of 3 mm margin on risk of recurrence and survival in oral cancer. Oral Oncol. 2020;110:104883.
- Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–178.
- Ch’ng S, Corbett-Burns S, Stanton N, et al. Close margin alone does not warrant postoperative adjuvant radiotherapy in oral squamous cell carcinoma. Cancer. 2013;119(13):2427–2437.
- Noorlag R, Klein Nulent TJW, Delwel VEJ, et al. Assessment of tumour depth in early tongue cancer: accuracy of MRI and intraoral ultrasound. Oral Oncol. 2020;110:104895.
