Abstract
The World Health Organization (WHO) believes that the advent of highly pathogenic avian influenza A (H5N1) has moved the world closer to a further global pandemic of human influenza than at any time since the Hong Kong (H3N2) pandemic of 1968–1969. The immediacy of the perceived threat is underscored by the current classification of the world at Phase 3 of WHO's operative six-phase system of pandemic alert, with H5N1 having met all the prerequisites for the onset of a human pandemic but one: the efficient and sustained person-to-person transmission of the virus. With preparations for an anticipated pandemic now recognized as a global health priority, the purpose of this article is to provide a foundation for geographical research on avian influenza A (H5N1). The article introduces geographers to the complex nature and ecology of H5N1, the principal data sources available to analyze the global occurrence of the virus in birds and humans, and evidence regarding its geographical origins and international dispersal during the first thirty months of the ongoing panzootic in wild birds and poultry, from December 2003 through May 2006. Key epidemiological facets of the disease in humans are examined. We conclude with a review of the incurred and projected economic costs of H5N1, global plans for pandemic aversion and mitigation, and prospects for the future geographical expansion of the virus. Areas in which geographers can make an effective contribution to knowledge about the virus and the disease are considered.

La Organización Mundial de la Salud (World Health Organization, WHO) cree que el advenimiento de la altamente patogénica gripe aviar A (H5N1) ha acercado al mundo hacia una pandemia de gripe humana más que en ninguna otra época desde la pandemia de Hong Kong (H3N2) de 1968 a 1969. La inmediatez de la amenaza percibida ha sido recalcada por la clasificación actual del mundo en la Fase 3 del sistema operativo de seis fases de alerta pandémica de la WHO, habiendo H5N1 cumplido todos los requisitos para el inicio de una pandemia humana, excepto uno: la eficiente y sostenida transmisión del virus de persona a persona. Con las preparaciones para una pandemia anticipada ahora reconocida como una prioridad global de salud, el propósito de este artículo es proporcionar una base para la investigación geográfica de la gripe aviar A (H5N1). El artículo presenta a los geógrafos la naturaleza y ecología complejas de H5N1, las principales fuentes de datos disponibles para analizar la ocurrencia global del virus en las aves y en los seres humanos, y la evidencia referente a sus orígenes geográficos y su dispersión internacional durante los primeros treinta meses transcurridos desde la panzootia en curso entre aves silvestres y de corral, desde diciembre de 2003 a mayo de 2006. Se examinan los factores epidemiológicos claves de la enfermedad en los seres humanos. Concluimos con una revisión de los costos económicos proyectados e incurridos por la gripe H5N1, los planes globales de la aversión y mitigación pandémica, y las perspectivas de la futura expansión geográfica del virus. Se consideran las áreas en las que los geógrafos pueden hacer una contribución efectiva al conocimiento sobre el virus y la enfermedad.
关键词:
Palabras claves:
The emergence of novel subtypes of influenza A virus, to which the human population has little or no existing immunity, underpins the great influenza pandemics that have periodically rolled around the world (). Historically, these events have been associated with large—sometimes massive—population losses. At an extreme, the Spanish influenza pandemic of 1918–1919 is estimated to have killed between 20 million and 50 million or more worldwide (CitationJordan 1927; CitationOxford 2000; CitationJohnson and Mueller 2002).Footnote 1 Other influenza pandemics have resulted in more modest death tolls, with the combined global excess mortality due to the Asian (1957–1958) and Hong Kong (1968–1969) pandemics estimated at 3 million to 4 million (CitationWorld Health Organization 2005a). Whatever the associated mortality, however, the broader social and economic impacts of pandemic influenza are always substantial. The overloading of health services, high levels of worker absenteeism, the disruption of essential services, and the interruption of trade and commerce underline the status of influenza pandemics as global public health emergencies (CitationGust, Hampson, and Lavanchy 2001).
Table 1 Pandemics and probable pandemics of influenza, 1500–2000
Pandemics and probable pandemics of influenza have occurred at irregular intervals over the last several centuries, although the evidence in yields an average interval of approximately twenty-five years (range = 2 to 139 years) between major events. Although Asia in general—and southern China in particular—has been implicated as the likely source of many of these (CitationShortridge and Stuart-Harris 1982; CitationK. D. Patterson 1986), considerable uncertainty surrounds the mechanisms by which the associated strains of the influenza A virus have emerged in the human population. Among the competing hypotheses (CitationOxford 2000), current evidence suggests that avian influenza viruses (influenza viruses for which birds are the natural reservoir) might play a pivotal role in the evolutionary process. According to this mechanism, a new pandemic strain emerges when an influenza A virus, possessing novel viral genes from an avian source, appears in the human population. Should the new virus have the capacity to cause disease, and to spread from human to human in an efficient and sustained manner, an influenza pandemic may ensue (CitationHorimoto and Kawaoka 2001; CitationCapua and Alexander 2004; Citationde Jong and Hien 2006).
The Current Pandemic Alert: Avian Influenza (H5N1)
On Thursday, 15 May 1997, a three-year-old boy was admitted to a Hong Kong hospital with fever, sore throat, and cough of six days' duration. The child's condition deteriorated rapidly, with acute respiratory distress, multiorgan failure, and death on the twelfth day of illness. An atypical influenza virus, recovered from the child's upper respiratory tract, was typed as avian influenza A (H5N1)—a novel and highly pathogenic virus of poultry that had first been identified in China the previous year (CitationCenters for Disease Control and Prevention 1997, Citation1998). The international community had received its first warning that the H5N1 virus, then known to be circulating in the poultry stock of Hong Kong, had the ability to cross the species barrier and cause severe disease and death in humans.
Further “dead-end” jumps of H5N1 from birds to humans were recorded in Hong Kong in late 1997 and again in early 2003 (CitationClaas, de Jong, et al. 1998; CitationPeiris et al. 2004; see ), but it was the unprecedented events of the winter of 2003–2004 that raised international concerns over H5N1 to a new level. Beginning in the latter part of 2003, poultry-based outbreaks of avian influenza, caused by genetic variants of the H5N1 virus, erupted in geographically disseminated form in east Asia. From this early epicenter, the virus has spread westward to Siberia, Europe, the Middle East, and Africa (). As of 18 May 2006, poultry-based outbreaks of avian influenza (H5N1) had been confirmed in a total of thirty-two countries, with a further twenty-one countries having documented the virus in wild bird species (see Appendix).
Figure 1 Countries in which avian influenza A (H5N1) was reported in wild birds and poultry, December 2003 to May 2006. Shading categories identify World Organisation for Animal Health (OIE) member states in which outbreaks were first confirmed in the period 1 January–18 May 2006 (dark shading) and in prior time periods (light shading). Isochrones are modified from evidence presented by the Emergency Preparedness and Response Branch, U.N. World Food Programme, and show the approximate position of the panzootic wave front at six-month intervals. Vectors indicate the inferred corridors of panzootic diffusion. Summary details of the first reported outbreak(s) of H5N1 in OIE member states are given in the Appendix. Source: Based on information in Disease Information (Paris: OIE, 12 December 2003–18 May 2006).
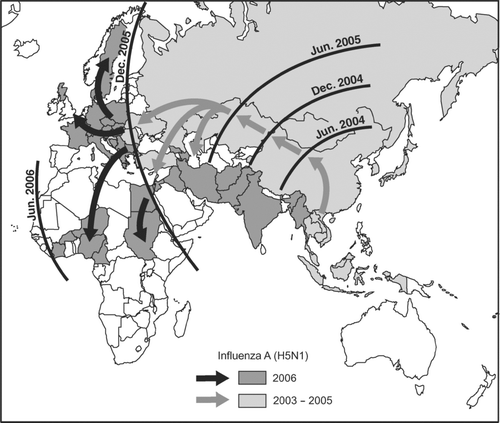
Table 2 Documented human infections with avian influenza A viruses, 1959–2005
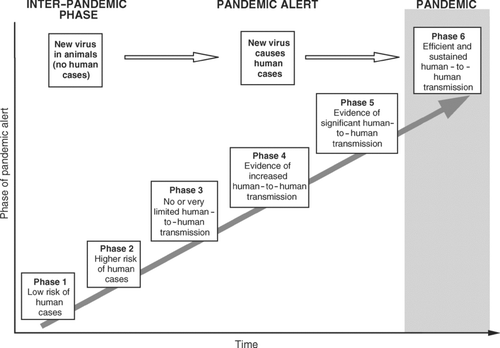
Although still primarily an infection of avian species, the H5N1 virus has demonstrated a disturbing propensity to extend its host range to include several mammalian species;Footnote 2 cases of human infection confirmed by the World Health Organization (WHO) exceeded 300 by May 2007 (WHO 2007). This capacity to cross the species barrier and cause severe disease and death in humans has raised grave concerns over the pandemic potential of the virus. In the words of the late Dr. Lee Jong-wook, former Director-General of the WHO, the advent of the H5N1 virus has moved the world “closer to a further [influenza] pandemic than … at any time since 1968” (CitationWHO 2005a, 3). In recognition of the heightened concern, WHO currently classifies the world at Phase 3 of the operative six-phase system of global pandemic alert for influenza ():
Phase 3. Human infection(s) with a new [influenza virus] subtype, but no human-to-human spread, or at most rare instances of spread to a close contact. (CitationWHO 2005d, 2)
Figure 2 Phases of global pandemic alert for influenza. The Global Influenza Preparedness Plan of the World Health Organization (WHO) identifies six phases of pandemic alert. Each phase of alert is associated with a series of recommended responses and activities to be implemented by WHO, the international community, governments, and industry. The epidemiological behavior of the disease and the characteristics of circulating viruses, among other factors, determine changes from one phase to another. Source: Based on information in CitationWHO (2005d).
Although the pandemic transmission of human influenza has attracted a substantial geographical literature, little is known of the antecedent spread of the avian influenza viruses from which many human pandemic strains—including the Spanish H1N1 (1918–1919), Asian H2N2 (1957–1958), and Hong Kong H3N2 (1968–1969) viruses ()—are believed to have evolved (CitationHorimoto and Kawaoka 2001; Citationde Jong and Hien 2006). It is too early to say whether the first human influenza pandemic of the twenty-first century will be triggered by the H5N1 virus, although the weight of international scientific opinion identifies it as a very likely candidate (CitationWHO 2005a, Citation2005c, Citation2005d). Accordingly, this article presents an international perspective on the first thirty months of the currently recognized outbreak of highly pathogenic avian influenza A (H5N1) that began in east Asia in late 2003 and has subsequently spread to more than fifty countries on three continents. Our study focuses on the panzootic transmission of the virus in birds, and the attendant occurrence of the disease in humans.
Context and Layout of Article
Through the years, the marchland of two very ancient subjects—geography and medicine—has been explored from various directions. Occasionally, scientists and practitioners from the powerful medical state have travelled confidently into geographical terrain. Less often and less confidently, researchers from geography have wandered into medical country. This article is an essay by geographers on a subject appropriately dominated by veterinary scientists and epidemiologists. We make no apology for this. The nineteenth century showed a wide range of approaches to what was variously called medical geography or geomedicine in France and Germany, reviewed in CitationCliff and Haggett (2003). During the last century, medical geographical research has followed two main strands: mapping disease distributions and describing and modeling the processes by which mapped disease distributions arise. This study of avian influenza A (H5N1) straddles both strands, as do many other geographical forays into medical territory (see, for example, CitationPyle 1969; CitationGilg 1973; CitationCliff et al. 1981; CitationShannon and Pyle 1989). More particularly, this study is framed by an ongoing geographical concern with the spatial diffusion of human influenza, as illustrated by the studies of CitationHunter and Young (1971), K. D. Patterson and Pyle (Citation1983, Citation1991), Cliff, Haggett, and Ord (1986), K. D. CitationPatterson (1986), CitationPyle (1986) and Smallman-Raynor, Johnson, and Cliff (2002).
Our examination of avian influenza A (H5N1) begins with an overview of the nature of avian influenza, the clinical and epidemiological dimensions of the highly pathogenic H5N1 virus, and the data sources available to examine the geographical spread of H5N1 infection in birds and humans. The principal published sources on the international occurrence of the H5N1 virus include: (1) the World Organisation for Animal Health's (OIE) weekly Disease Information (infections in wild birds and poultry); and (2) the WHO's quasi-daily Situation Updates—Avian Influenza (infections in humans). In subsequent sections of the article, we use the information included in Disease Information and Situation Updates to trace the international spread of H5N1 in birds and humans in the thirty months to May 2006. We conclude with a review of the economic costs of H5N1, current global plans for pandemic aversion and mitigation, and prospects for the future geographical spread of the virus. Areas in which geographers can make an effective contribution to knowledge about H5N1 infection and disease are considered.
A Note on Terminology
This article uses a number of standard terms from the animal health sciences that are formally defined elsewhere (see, for example, Boden 2005). In general, an epizootic (equivalent to an epidemic in humans) is defined as an unusually high incidence of a specified disease in an animal population. A panzootic (equivalent to a pandemic in humans) is defined as a geographically widespread epizootic, and epizootiology (equivalent to epidemiology in humans) is the term applied to the study of the processes that govern the dynamics of diseases in animal populations. Finally, the term reservoir denotes a host organism or population that maintains a disease agent in nature and that provides a source of infection for susceptible hosts. Other terms are defined at their point of use in the text.
The Nature of Highly Pathogenic Avian Influenza A (H5N1)
The Ecology of Avian Influenza
Avian influenza is a disease of birds and, occasionally, certain terrestrial and marine mammals (including horses, humans, pigs, seals, and whales) caused by infection with type A strains of avian influenza virus. Although the majority of wild and domestic bird species are known to be susceptible to infection with influenza A viruses, and the viruses are widespread in nature, particular interest attaches to wild aquatic birds as natural reservoirs of infection and to domestic poultry as birds of economic significance.
Wild Aquatic Birds
Wild aquatic birds (ducks, gulls, and shorebirds) are the principal reservoir of influenza A viruses in nature. Infection in these species is usually asymptomatic and is indicative of the optimal adaptation of the viruses to their reservoir hosts. Studies of wild ducks have revealed that influenza A viruses replicate in both the respiratory systems and intestines of infected birds; virus is shed in large quantities in feces, with the fecal contamination of surrounding waters serving as an efficient route for the onward transmission of the virus. Virus is also shed in the respiratory secretions of infected birds and this might serve as an additional source of environmental contamination (CitationTollis and Di Trani 2002; CitationWHO 2005a, Citation2005b).
Domestic Poultry
Domestic poultry (including chickens, turkeys, ducks, and geese)Footnote 3 can become infected with influenza A viruses through direct contact with infected wild waterfowl or other wild birds, other infected poultry, or surfaces and materials (including water) that have been contaminated with virus. Two principal forms of clinical disease, distinguished on the basis of severity, are recognized:
-
Low pathogenic avian influenza (LPAI), a mild disease associated with influenza viruses of low virulence and characterized by minor respiratory disorders, depression, ruffled feathers, and a drop in egg production in laying birds. This mild form of the disease can go undetected in affected birds.
-
Highly pathogenic avian influenza (HPAI), a severe disease associated with influenza viruses of high virulence and characterized by depression, loss of appetite, cessation of egg laying, disturbances of the nervous system, swelling and discoloration of combs and wattles, coughing, sneezing, and diarrhea. The disease can affect many internal organs and has a mortality rate for infected flocks that can approach 100 percent within forty-eight hours.
Subtypes of Avian Influenza Viruses
Avian influenza viruses are classified as members of the Influenzavirus A genus of the Orthomyxoviridae virus family. Virus subtypes are defined by the expression of the surface proteins, hemagglutinin (H), governing the ability of the virus to bind to, and enter, host cells; and neuraminidase (N), governing the release of newly formed virus particles from host cells. Wild birds demonstrate infection with the largest variety of subtypes of influenza A virus in nature, with the majority of possible combinations of the currently recognized sixteen H subtypes (H1–H16) and nine N subtypes (N1–N9) having been identified in avian species. On the basis of current evidence, however, viruses that cause HPAI are restricted to the H5 and H7 subtypes, although not all H5 and H7 subtypes are highly pathogenic (CitationTollis and Di Trani 2002; CitationCapua and Alexander 2004).
Geographical Spread and Epizootic Control
Avian influenza A viruses (of low and high pathogenicity) are readily transmitted from farm to farm by the movement of live poultry and by people and equipment contaminated with virus. Until recently, the detection of HPAI viruses in wild birds was a rare event, with cases usually limited to small numbers of birds found dead or moribund within the flight range of a poultry outbreak. This observation has been interpreted as evidence that wild waterfowl are not agents for the onward transmission of influenza viruses in their highly pathogenic form. Recent events, however, have indicated that some migratory birds do have the capacity to spread the highly pathogenic H5N1 virus over extended distances (CitationWHO 2005b).
Effective measures for the control of HPAI in domestic poultry include the rapid culling of exposed birds, quarantine, disinfection, and movement restrictions. In general, these methods are best suited to the control of large-scale outbreaks on commercial farms, with outbreaks in backyard flocks proving especially difficult to control. Although vaccines against specific subtypes of avian influenza virus are available, and their use may be advocated under certain circumstances, a number of scientific, technical, economic, and social considerations have dictated against their routine use in many OIE member states (CitationTollis and Di Trani 2002; CitationCapua and Alexander 2004).
Avian Influenza A (H5N1)
Although descriptions of poultry-based outbreaks of HPAI can be traced to the latter part of the nineteenth century,Footnote 4 the first confirmed report of the disease dates to 1959 and an outbreak of a highly pathogenic H5N1 virus (designated A/chicken/Scotland/59) in two flocks of chickens in Aberdeen, Scotland (CitationPereira, Tu°mová, and Law 1965). During the next thirty years, reports of the disease were relatively uncommon and were limited to Europe, North America, and Oceania (CitationCapua and Alexander 2004). Many of these early outbreaks were geographically localized (occasionally restricted to a single farm or flock), with no evidence of transmission across international borders. Beginning in the 1990s, however, there was a surge in the global record of HPAI activity, with outbreaks noted for the first time in Africa, Asia, and Latin America.
The Food and Agriculture Organization (Citation2005b, 5–11) attribute the global increase in HPAI activity since the 1990s to a combination of factors, including (1) enhanced disease surveillance, (2) changes in the nature of circulating influenza viruses, and (3) increases in poultry populations in the absence of appropriate developments in biosecurity. Prominent among these factors, the global trend toward the intensification of poultry production, with the rapid growth of industrial-scale farming and a concomitant increase in the concentration of susceptible birds, has provided ideal conditions for the spread of LPAI viruses, a precondition for the emergence of HPAI viruses. Outbreaks of HPAI in Pennsylvania in 1983 (H5N2), Australia in 1992 (H7N3), and Canada in 2004 (H7N3), among others, are known or suspected to have been attributable to these developments in farming methods. At the same time, an associated increase in the international movement of poultry and poultry products has raised the specter of the long-distance transfer of avian influenza viruses, as illustrated by the suspected role of imported turkey meat in the introduction of the H5N1 virus to a poultry plant in Suffolk, England, in January 2007 (Department for Environment, Food and Rural Affairs 2007).Footnote 5
Origins: The Emergence of the Influenza A (H5N1) Virus in Asia
A prominent feature of the recent surge in HPAI is the occurrence of outbreaks due to H5N1 in east Asia. The known evolution of highly pathogenic H5N1 influenza viruses in the region can be traced to an outbreak of HPAI on a goose farm in Guangdong Province, southern China, in the summer and early autumn of 1996 (CitationXu et al. 1999). The following year, outbreaks of severe disease due to an H5N1 virus were recorded in chickens, and—for the first time—among humans, in Hong Kong (CitationClaas, Osterhaus, et al. 1998; CitationHorimoto and Kawaoka 2001; CitationTam 2002), with the causative agent identified as a genetic reassortment of the Guangdong and other avian influenza viruses (CitationXu et al. 1999). Multiple genotypes of the H5N1 virus continued to be detected in ducks and geese from neighboring areas of southern China in subsequent years (CitationSims et al. 2005; Citationde Jong and Hien 2006). By 2003, the so-called Z genotype of the H5N1 virus had emerged as the dominant genotype in the region, with an antigenic variant of this genotype having been identified as the principal agent of the current panzootic of avian influenza A (H5N1) (CitationSims et al. 2005; CitationWebster et al. 2005).Footnote 6
The evolutionary linkages between the strains of H5N1 that emerged in east Asia in the 1990s and those that were formerly isolated in Europe (Scotland in 1959 and England in 1991) have yet to be elucidated, although clear differences in the pathogenicity of the Asian and European viruses have been documented (see, for example, CitationDybing et al. 2000). In summarizing the evidence for the geographical origins of the current H5N1 panzootic, CitationChen, Li, Smith, et al. (2006) identify southern China as the most likely (immediate) source on account of (1) the original detection of the precursor H5N1 virus in 1996 and (2) the high degree of genetic diversity of H5N1 viruses in the region. The evidence, Chen and colleagues observe, is consistent with the influenza epicenter hypothesis—a hypothesis that identifies southern China as the source of new pandemic strains of influenza.Footnote 7
Human Infection with the Influenza A (H5N1) Virus
Avian influenza viruses are highly species-specific. Transmission of the viruses to nonavian species is relatively uncommon, although infections with viruses of avian origin have been detected in both marine and terrestrial mammals (see CitationTollis and Di Trani 2002). As shows, reports of human infection with avian influenza viruses are few in number and have been limited to just four virus subtypes (H5N1, H7N3, H7N7, and H9N2). Clinically, these infections have usually manifested as mild respiratory illnesses and viral conjunctivitis, although infection with the H5N1 subtype has been associated with severe illness and a high fatality rate.
Clinical Course
Current knowledge of the clinical spectrum of human infection with avian influenza A (H5N1) virus is based on the surveillance of hospital patients with moderate and severe disease. The frequency of milder illness and subclinical infection has yet to be determined, although epidemiological studies suggest that both occur (CitationThorson et al. 2006). In confirmed cases, an incubation period of two to eight days gives way to high fever (> 38°C) and a typical influenza-like illness, sometimes accompanied by diarrhea, vomiting, abdominal pain, and bleeding from the nose and gums. Breathing difficulties usually begin on the fifth or sixth day of illness, with the development of clinically apparent pneumonia in the majority of cases. Multiorgan failure with signs of renal dysfunction and, occasionally, cardiac compromise have been reported in some patients, with death (primarily due to progressive respiratory failure) typically occurring nine to ten days (range = two to thirty-one days) after onset of the clinical illness. The observed case fatality rate is greater than 50 percent (Writing Committee of the WHO Consultation on Human Influenza A/H5 2005; CitationWHO 2006b).
Transmission Routes
As judged by the exposure histories of confirmed human cases of avian influenza A (H5N1), present evidence is consistent with bird-to-human, environment-to-human and limited, nonsustained, human-to-human transmission. Direct contact with sick and dead birds or with surfaces and objects contaminated by poultry feces, is believed to represent the primary route of human exposure. The slaughter, plucking, and preparation of poultry for consumption has been identified by WHO as a particular risk factor, and environmental exposure to poultry-contaminated land and water bodies is another potential source of infection (CitationWHO 2006a). Limited human-to-human transmission has been implicated in some family clusters (see, for example, CitationUngchusak et al. 2005).
Pandemic Potential of the Influenza A (H5N1) Virus
As Alvarado de la Barrera and Reyes-Terán (2005) observe, the occurrence of a pandemic of human influenza is dependent on three conditions: (1) a new influenza virus emerges; (2) the new virus has the ability to cause severe disease in humans; and (3) the new virus can spread from human to human in an efficient and sustained manner. The H5N1 virus currently meets conditions 1 and 2, but not the transmissibility condition 3. Two principal processes are recognized by which increased transmissibility among humans may arise:
-
Reassortment events, in which genetic material is exchanged between avian influenza viruses and more readily transmissible human influenza A viruses during co-infection in reassortment vessels (such as pigs or humans). This process is believed to have underpinned the emergence of the fully transmissible virus strains associated with the Asian (H2N2) pandemic of 1957–1958 and the Hong Kong (H3N2) pandemic of 1968–1969.
-
Adaptive mutation, whereby an avian influenza virus develops, through a gradual process of genetic adaptation, an enhanced ability to bind to human cells. This process is believed to have underpinned the emergence of the fully transmissible virus strain associated with the Spanish (H1N1) pandemic of 1918–1919. In particular, a recent study by CitationTumpey et al. (2007) indicates that minor adaptations in the hemagglutinin of the H1N1 virus, resulting in a predilection for virus receptors in human airways rather than in bird intestines, may have served to trigger the transmissibility of the virus between humans.
Global Data Sources and Data Matrices
To examine the international spread of highly pathogenic H5N1, we draw on geocoded information gathered by the bodies responsible for the global surveillance of influenza A (H5N1) in avian species (OIE) and humans (WHO). Here, we summarize our principal sources of data and the associated data matrices on which our analysis is based.
Data Matrices 1: Avian Species
Global surveillance for influenza A (H5N1) in avian and other animal species is undertaken by OIE, Paris. The disease is subject to urgent notification (within twenty-four hours of outbreak identification), with summary details of reported outbreaks in OIE member states included in the weekly editions of Disease Information. Beginning with the first reported outbreak of avian influenza A (H5N1) associated with the currently recognized panzootic (report dated 12 December 2003), information on H5N1 outbreaks in OIE member states was abstracted from Disease Information to form an 889 (day of report) × n (reporting country) matrix of outbreak counts from 12 December 2003 to 18 May 2006. Further matrices of outbreak counts were then formed by dividing the original (889 × n) matrix into the constituent countries of the six standard WHO world regions (Africa, Americas, Eastern Mediterranean, Europe, South-East Asia, and Western Pacific). Finally, for each of the matrices, the daily counts were combined by seven-day period to yield 128-week world regional and global time series of outbreak counts.Footnote 8
Data Matrices 2: Humans
In response to the public health risks posed by the epizootic spread of H5N1, a framework for the enhanced global surveillance of H5 viral infections in humans was established by WHO in February 2004 (WHO 2004b). All WHO member states are requested to submit notifications of probable and confirmed human cases to WHO headquarters, with details of confirmed cases summarized in Situation Updates—Avian Influenza.Footnote 9 To parallel the avian data matrices, information on WHO-confirmed human cases of H5N1 infection was abstracted from the Situation Updates to build 889 (day) × n (reporting country) matrices of case counts by day of clinical onset and day of report to WHO, from 12 December 2003 to 18 May 2006. The original matrices were then converted, in the manner described for the avian matrices, to yield 128-week national and global time series of case counts.
Unless stated otherwise, the set of data matrices 1 and 2 form the basis of analysis in subsequent sections. We supplement this quantitative information with qualitative evidence on disease activity included in OIE's Disease Information and WHO's Situation Updates.
Data Quality
Although WHO and OIE have made substantial investments in capacity for the national and international surveillance of influenza viruses, the available data must be treated with due caution. As regards human cases of avian influenza A (H5N1), the CitationWHO (2006b) notes that the completeness, reliability, and format of epidemiological information varies in time and space. Information on key epidemiological facets of the disease (including exposure histories and dates of clinical onset) is unavailable for some patients, and multiple selection biases affect the spatial and temporal completeness of case reporting in an unknown manner. Most data relate to patients with moderate and severe disease and for whom infection with the H5N1 virus has been confirmed by laboratory tests. Geographical variations in the sensitivity of disease surveillance systems and levels of diagnostic expertise (CitationWorld Health Organization 2006b), the number of mildly symptomatic patients who do not seek medical care (CitationThorson et al. 2006), and the reticence of national authorities to disclose sensitive epidemiological information (CitationEnserink 2006) all affect the accuracy of the available data.
Global surveillance for the H5N1 virus in animals is even less satisfactory. As CitationFerguson et al. (2004) observe, outbreak reports collated by OIE are dependent on the surveillance operations of government veterinary services in the individual member states. Many poorer countries lack the necessary veterinary infrastructure to undertake effective surveillance for H5N1 (CitationEuropean Centre for Disease Surveillance and Control 2006), with the majority of outbreak reports arising from the detection of unusual levels of morbidity and mortality in animals. Systematic surveillance for H5N1 in wild birds is lacking in many countries. As for human cases, political sensitivities might serve to limit the availability of information (CitationFerguson et al. 2004).
Geographical Corridors of Panzootic Transmission
In this section, we use the data matrices to trace the international spread of the panzootic of highly pathogenic H5N1 in wild birds and poultry from December 2003 to May 2006. To assist our discussion, shades the fifty-three OIE member states in which avian outbreaks of H5N1 had been confirmed in the thirty-month observation period; the isochrones give the approximate position of the panzootic wave front at six-month intervals; and the vectors depict the inferred spatial corridors of H5N1 transmission. For reference, the Appendix gives summary details of the timing, location, and wild or domestic bird species associated with the first confirmed H5N1 outbreak(s) in each of the fifty-three countries.
Global and World Regional Time Series
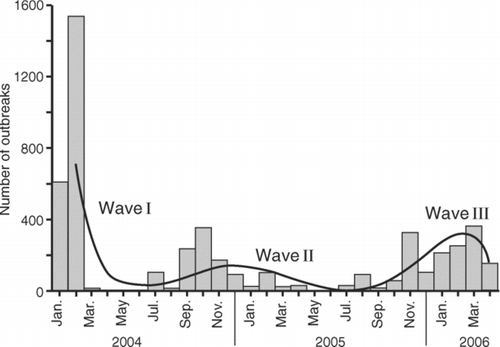
As shows, the H5N1 panzootic diffused as a spatially contagious wave of infection, pushing outward from an apparent source in east Asia and moving progressively westward across the Eurasian landmass to Africa. This diffusion process was underpinned by three distinct phases of outbreak activity. By way of illustration, the bar chart in is based on information included in the OIE's Disease Information and plots, by month of report, the global count of H5N1 outbreaks in wild birds and poultry from January 2004 to April 2006. A polynomial regression line, fitted to the monthly series of reported outbreaks by ordinary least squares, is shown for reference. Inspection of the graph reveals a brief and intense primary wave of outbreak activity in January and February 2004 (denoted Wave I), followed by two extended and less intense secondary waves in July 2004–April 2005 (Wave II) and July 2005–April 2006 (Wave III). We examine the spread of each of these waves in turn.
Figure 3 Global time series of outbreaks of avian influenza A (H5N1) in wild birds and poultry, January 2004–April 2006. The bar chart plots the global count of outbreaks, by month of report, to World Organisation for Animal Health. A polynomial regression line, fitted to the monthly series of reported outbreaks by ordinary least squares, is shown for reference. Source: Based on information in Disease Information (Paris: OIE, 12 December 2003–18 May 2006).
Wave I: Epizootic Onset in East Asia (January–February 2004)
Although genotypes of H5N1 are known to have been circulating in waterfowl and terrestrial poultry in east Asia since the mid-1990s, occasional detections of the virus gave way to an epizootic surge in the latter part of 2003. The first indication of the upsurge can be traced to the Korean Peninsula. On Friday, 12 December 2003, Dr. Chang-Seob Kim, Chief Veterinary Officer for the Republic of Korea, issued an emergency report to OIE of a suspected outbreak of highly pathogenic avian influenza on a commercial chicken farm in the central province of Chungcheong-buk (OIE 2003). The outbreak had first come to the attention of local authorities on the evening of 10 December, with H5N1 subsequently confirmed as the cause of the rapid and high mortality (OIE 2004d). The source of the virus that sparked this and several subsequent farm-based outbreaks in the Republic of Korea is unknown, although some connection with the seasonal appearance of migratory birds in late October and November 2003 is suspected (OIE 2003; CitationWee et al. 2006).
Although measures to stamp out the disease were swiftly implemented by the Korean authorities (CitationWee et al. 2006), the new year presented evidence that H5N1 was already widely distributed in other parts of the region. plots the monthly count of outbreaks of avian influenza A (H5N1) as reported to OIE from the countries of Asia from December 2003 to February 2004. Emergency reports of poultry-based outbreaks were issued in rapid succession by Vietnam (8 January), Japan (12 January), Thailand (23 January), Cambodia (24 January), Lao PDR (27 January), Indonesia (2 February), and China (4 February),Footnote 10 with Thailand and Vietnam forming the epicenter of outbreak activity (). The early and widespread dissemination of the virus in the region appears to have been fueled by wild birds, silently infected domestic waterfowl, and the large-scale movement and trade of poultry, especially at live bird markets (CitationFood and Agriculture Organization 2005b).Footnote 11
Figure 4 Wave I of the panzootic transmission of avian influenza A (H5N1). Proportional circles are based on information included in World Organisation for Animal Health's Disease Information and plot, by country, the count of reported outbreaks of H5N1 in wild birds and poultry in January (B) and February (C) 2004. The initial outbreaks in December 2003 are shown for reference (A). The date of report of the first outbreak of H5N1 in each affected country is given in parentheses.
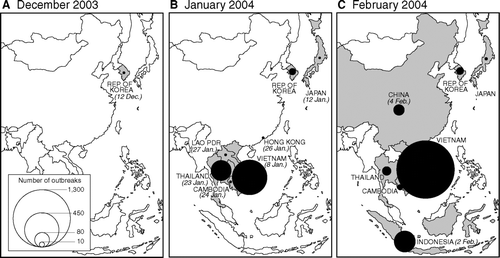
Whatever the mechanisms involved in the seeding of the virus, H5N1 rapidly colonized the poultry populations of some countries. In the week up to 30 January 2004, for example, Thailand recorded 156 outbreaks in thirty-two provinces, with reports of the disease extending to include chickens, ducks, geese, turkeys, ostriches, quail, and peacocks in commercial and backyard settings (CitationOIE 2004b; ). An even more extreme situation emerged in Vietnam where, between 24 January and 19 February, 1,282 separate poultry-based outbreaks—resulting in the death and destruction of some 6.62 million birds—were recorded across the country (CitationOIE 2004g; , ).
Wave II: Epizootic Consolidation (July 2004–April 2005)
In some countries, the early outbreaks of H5N1 were quickly contained in commercial poultry flocks and the virus eliminated. In Japan, for example, the final outbreak was recorded on 5 March 2004; on 12 July, the Ministry of Agriculture, Forestry and Fisheries informed the OIE that the disease had been “completely controlled and eradicated” (CitationOIE 2004b, 196). Likewise, the final outbreak in the Republic of Korea was confirmed on 21 March and, after the monitoring of sentinel birdsFootnote 12 for several months, the Korean authorities declared that the country was free of H5N1 (OIE 2004c).
Elsewhere in the region, the summer of 2004 heralded fresh poultry-based outbreaks of H5N1 in Cambodia, China, Indonesia, Thailand, and Vietnam, and in mid-August, Malaysia issued its first report of the disease. As shows, Thailand was the epicenter of reported outbreaks in this second wave, with provinces of central and northern Thailand forming the principal foci of the epizootic. Such were the developments that, by January 2005, WHO could conclude that H5N1 was enzootic in some parts of the region, the virus having “established a permanent ecological niche” in Asian poultry (CitationWHO 2005a, 16).
Figure 5 Waves II and III (part) of the panzootic transmission of avian influenza A (H5N1), July 2004–December 2005. Proportional circles are based on information included in World Organisation for Animal Health's Disease Information and plot, by country, the count of reported outbreaks of H5N1 in wild birds and poultry. (A) Wave II (July 2004–April 2005); (B) Wave III (part) (July–December 2005). Vectors for Wave III (B) plot the postulated routes of transmission of H5N1 from a primary focus of infection in east Asia, with the date of report of the first documented outbreak in a given country indicated (see Appendix). Infected countries are shaded.
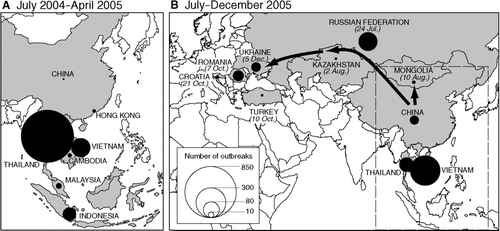
Wave III: Panzootic Expansion (July 2005–April 2006)
A two-month lull in reported outbreak activity gave way, in July 2005, to the fastest and geographically most extensive spread of highly pathogenic avian influenza ever recorded. In a period of 10 months, the virus expanded beyond its initial focus in Asia, sweeping westward across large tracts of Eurasia and Africa, affecting more than fifty countries and resulting in the loss from disease and culling activities of more than 200 million birds. The sequence of extension of the panzootic, first to include lands east of the Urals, and then the eastern Mediterranean, Europe, and West Africa, is tracked in and . Again, summary details of the first reported outbreak(s) in the newly infected countries are given in the Appendix.
Precursors to Expansion: The Qinghai Lake DieOff
The rapid spatial expansion of the panzootic was linked to a rarely observed phenomenon in the epizootiology of avian influenza: the apparent ability of migratory wildfowl to carry H5N1 in highly pathogenic form over extended distances, and to seed the virus along principal flyways. The first substantial evidence of the phenomenon came in late April 2005, when a mass die-off of some 6,000 migratory birds was recorded at the Qinghai Lake nature reserve (a major rendezvous and breeding site for birds on Asia–Siberia migratory routes) in central China. The source of H5N1 that sparked the die-off is unknown, although an importation with bar-headed geese (Anser indicus) arriving from areas of enzootic infection via one of the Asian flyways is suspected (CitationLiu et al. 2005; CitationChen, Li, Li, et al. 2006).
Initial Expansion (July–December 2005)
Beginning in late July 2005, H5N1 viruses—virtually identical to genotypes implicated in the Qinghai Lake die-off—began to appear in wild birds and poultry in countries to the north and northwest of China (). In Siberia, poultry-based outbreaks of Qinghai-like viruses were reported in adjacent regions of the Russian Federation (Novosibirsk) in late July and Kazakhstan (Pavlodar) in early August (CitationOIE 2005a, Citation2005d), with wild waterfowl in local open water reservoirs identified as the likely sources of infection (see Appendix). At about the same time, Mongolian authorities reported deaths among migratory waterfowl at lakes in the north of the country, with Qinghai-like genotypes again implicated as the cause of the outbreaks (CitationOIE 2005b; CitationChen, Li, Li, et al. 2006).
Cognizant of developments in Siberia, a special issue of the Food and Agriculture Organization's (FAO) FAO AIDE News, dated 1 September 2005, alerted readers to the potentially severe implications for Europe and elsewhere:
it is plausible that HPAI H5N1 virus could spread from Siberia to the Caspian and Black Sea areas in the foreseeable future. Some birds are currently nesting in the newly HPAI affected areas … in Russia and will migrate to the above-mentioned areas for upcoming winter or land to rest … on their way to Africa and Europe. (CitationFAO 2005a, 1)
Accelerated Expansion (January–May 2006)
From the newly established bridgehead in the Black Sea region, available evidence suggests that migratory waterfowl served to carry H5N1 across the countries of southern, central, and northern Europe, the Eastern Mediterranean, and west Africa in the early months of 2006. Countries in which highly pathogenic H5N1 was recorded for the first time in the period between 1 January and 18 May 2006 are identified by the dark shading in . We consider the evidence for the spread of H5N1 in Europe, Eastern Mediterranean, and Africa in turn.
Europe
Beginning with Azerbaijan, Greece, and Bulgaria in late January, a total of twenty-one countries of the WHO European Region were reported as newly infected with H5N1 in the five months to May 2006. As indicated in the Appendix, many of the initial outbreaks were recorded in dead and moribund swans, with the clustering of detections in February and March linked to the westward movement of migratory birds in response to adverse weather conditions in the Black Sea region (Department for Environment, Food and Rural Affairs 2006). With a few noteworthy exceptions (Albania, Azerbaijan, France, Germany, and Israel), reports of outbreaks in domestic poultry were lacking in the period to May 2006.Footnote 13
Eastern Mediterranean Region
Coincident with the westward spread of H5N1 in Europe, OIE began to receive reports of the disease in countries to the south of Turkey and the Black Sea (see Appendix). Commencing with Iraq and Iran in early and mid-February, many countries of the WHO Eastern Mediterranean region, both to the east (Afghanistan and Pakistan) and the west (Egypt, Jordan, Palestinian Autonomous Territories, and Sudan), had reported outbreaks of H5N1 virus subtypes among wild birds, poultry, or both by May 2006.
African Region
By May 2006, H5N1 had pushed southwestward, beyond the western-most territories of the Eastern Mediterranean, to form a focus of epizootic activity in west Africa (). Beginning with a report of an outbreak of H5N1 on a commercial poultry production layer unit in Kaduna Province, north-central Nigeria, in early February (OIE 2006c), outbreaks of the disease—variously in wild birds and poultry—had been reported in the proximal countries of Burkina Faso, Cameroon, Côte d'Ivoire, and Niger by the end of the observation period (see Appendix). Although some uncertainty surrounds the source of the virus in the region, available evidence from Nigeria is consistent with multiple introductions of the virus with migratory birds (CitationDucatez et al. 2006).
In addition to the European, Eastern Mediterranean, and African regions, shows that the early months of 2006 were associated with an extension of epizootic activity in the South-East Asian region, with first-time reports of poultry-based outbreaks in India and Myanmar (CitationOIE 2006a, Citation2006b).
Summary
Although the panzootic of H5N1 had spread to include fifty-three countries of Africa, Asia, and Europe in the thirty months to May 2006, the original heartland of east Asia continued to form the epicenter of virus activity. In the period to mid-2006, H5N1 spread repeatedly from its established source in southern China to nearby countries, with virus transmission fueled by migratory birds and the international trade in poultry and poultry products. At the same time, antigenically distinct sublineages of H5N1 had begun to emerge, suggestive of the long-term enzooticity of the virus in east Asian poultry (CitationChen, Li, Smith, et al. 2006). As Chen, Li, Smith, and colleagues (2006) observed, viral persistence and evolution in the region have served to exacerbate the pandemic risk of H5N1 in humans. It is to the occurrence of H5N1 in humans that we now turn.
Avian Influenza A (H5N1) in Humans: Space–Time Patterns
Beginning in late October 2003—coincident with the inferred time of onset of the epizootic spread of H5N1 in domestic poultry in Asia—sporadic human cases of severe respiratory illness began to present at hospitals in Hanoi and neighboring provinces of northern Vietnam. Among the series of fourteen suspicious cases, a twelve-year-old girl from Ha Nam Province, admitted to hospital in Hanoi on 27 December, was subsequently identified as the first WHO-confirmed human case of avian influenza A (H5N1) associated with the nascent panzootic (WHO 2004a).Footnote 14 From this beginning, a global total of 216 confirmed human cases of avian influenza A (H5N1), including 122 deaths, were documented by WHO in the thirty months to May 2006 ().
Table 3 Confirmed human cases of influenza A (H5N1), 2003–2006
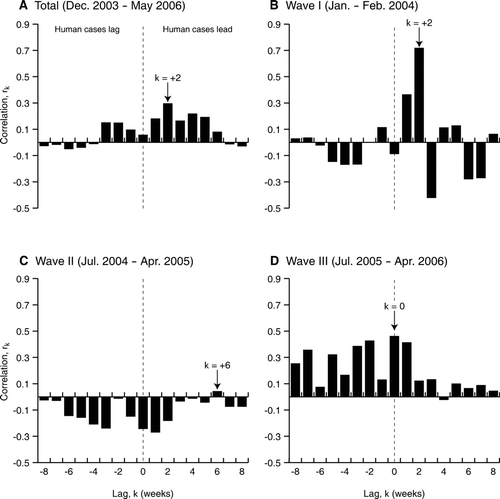
Consistent with an avian-to-human transmission route, the weekly time series of human case reports reveals pronounced periods of raised disease activity (January–March 2004, January–June 2005, and October 2005–May 2006), with each period coincident with upswings in outbreak activity associated with Waves I through III of the avian panzootic (). Cross-correlation analysis between the weekly time series of n = 6,651 avian outbreaks and n = 205 human casesFootnote 15 confirms the close temporal correspondence between the two disease patterns in the period from December 2003 to May 2006, with peaks in human cases leading peaks in avian outbreaks by two weeks (). The results of the cross-correlation analysis for individual waves are variable, but with a tendency for a slightly leading human case peak for Wave I (n = 2,150 avian outbreaks, n = 30 human cases; ) and an in-phase association between the avian and human series in Wave III (n = 2,567 avian outbreaks, n = 91 human cases; ). Finally, the apparent lack of temporal association in Wave II (n = 1,040 avian outbreaks, n = 61 human cases; ) is reflected in the extended period between the main phases of disease activity in birds (October–December 2004) and humans (January–June 2005).Footnote 16
Figure 6 Cross-correlation functions between weekly time series of avian outbreaks and human influenza cases due to H5N1, December 2003–May 2006. (A) Complete time series; (B) avian influenza Wave I (January–February 2004); (C) avian influenza Wave II (July 2004–April 2005); (D) avian influenza Wave III (July 2005–April 2006). The lag k associated with the maximum cross-correlation is indicated on each graph for reference.
Geographical Patterns
Although the H5N1 panzootic wave had spread to fifty-three countries by May 2006, WHO-confirmed human cases of the disease were limited to just ten countries (). plots the geographical incidence of these human cases in the periods December 2003–December 2004 (A), January–December 2005 (B), and January–May 2006 (C). Countries in which H5N1 had been confirmed in avian species by the end of each time period are shaded. For reference, the isochrones in and are replotted from and show the approximate position of the panzootic wave front at six-month intervals. Finally, the bar charts in plot the weekly time series of human cases by reporting country; the corresponding national series of outbreaks in wild birds and poultry are represented on each graph as the line traces.
Figure 7 Geographical distribution of human cases of avian influenza A (H5N1) confirmed by the World Health Organization, December 2003–May 2006. Circles show the distribution of the 216 human cases reported to 18 May 2006. (A) December 2003–December 2004; (B) January-December 2005; (C) January–May 2006. Countries in which avian influenza A (H5N1) had been confirmed in wild birds, poultry, or both by the end of each time period are shaded. Isochrones are replotted from and show the approximate position of the panzootic wave front at six-month intervals.
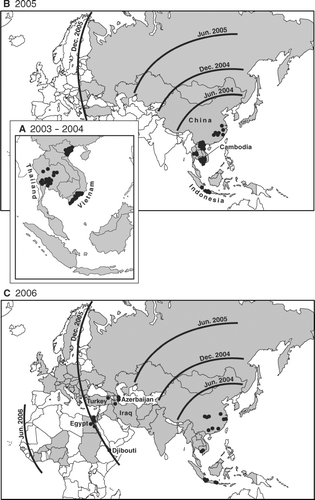
Figure 8 National time series of confirmed human cases of influenza A (H5N1) confirmed by the World Health Organization (WHO), December 2003–May 2006. The bar charts plot the number of confirmed human cases by week of report in WHO's Situation Updates—Avian Influenza. Countries are ordered according to the date of first report of cases (see Appendix). (A) Vietnam; (B) Thailand; (C) Cambodia; (D) Indonesia; (E) China; (F) other countries. Counts of human cases are given by country in . For reference, the line traces plot the national count of confirmed outbreaks of H5N1 in wild birds and poultry by week of report in the World Organisation for Animal Health's Disease Information.
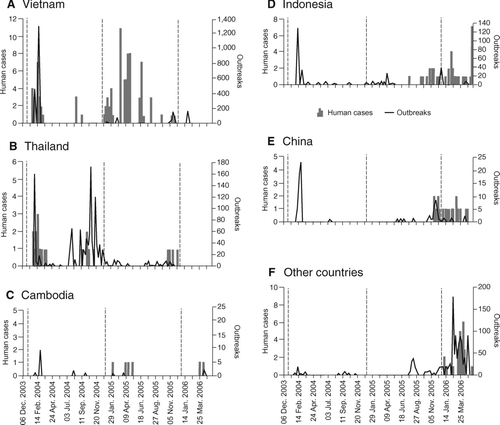
WHO-confirmed human cases of avian influenza A (H5N1) were confined to east Asia in the period to December 2004 (), with major clusters of disease activity in northern and southern Vietnam and central Thailand associated with strongly defined peaks of epizootic activity (, ). Notwithstanding the rapid westward movement of the panzootic wave front, reported human cases of the disease remained concentrated in east Asia in 2005 (), with Cambodia, Indonesia, and, by the end of the year, China reporting their first cases (). Finally, trailing the panzootic wave front, human cases of the disease were reported for the first time in Europe (Azerbaijan and Turkey) and the Eastern Mediterranean (Djibouti, Egypt, and Iraq) in the period from January to May 2006 ().
In deciphering the evolution of cases in , we note that the spatial pattern of human cases has tracked—albeit imperfectly—the spatial pattern of poultry-based H5N1 outbreaks as recorded in the final column of (see Appendix). Evidence of human infection due to contact with migratory waterfowl and other wild birds has been reported on only rare occasions,Footnote 17 and no human cases of avian influenza A (H5N1) have been documented in countries for which outbreaks have been limited to wild bird species. Thus the pattern highlights the pivotal role of poultry in the pancontinental extension of human infection with H5N1.
Epidemiological Facets
To date, confirmed human cases of avian influenza A (H5N1) have been relatively few in number, and many epidemiological aspects of the disease in humans are still poorly understood (CitationWorld Health Organization 2006b). In this section, we examine three prominent epidemiological facets of the disease identified thus far: (1) the age bias of cases toward children and young adults; (2) the seasonal bias of cases toward the winter and spring months; and (3) the occurrence of cases in family clusters.
Age Distribution
One noteworthy epidemiological feature of WHO-confirmed human cases of avian influenza (H5N1) is the skewed distribution toward children and young adults, with relatively few cases in older age categories (CitationWHO 2006b). To illustrate the phenomenon, the box-and-whisker plots in are based on a sample of 169 cases (seventy-seven males and ninety-two females) in and show the age distribution of patients by gender (A), year of report (B), patient outcome (C), and country (D).Footnote 18 The mean age of the 169 sample cases was 19.8 years (median = 18.0; range = 0.3–75.0), with estimated age-specific case rates per million population of 0.15 (0–9 years), 0.15 (10–19 years), 0.13 (20–29 years), 0.08 (30–39 years), and 0.02 (≥40 years).Footnote 19 The skewed age distribution is reflected in each field of , with the third quartiles of the plots (Q 3, defined by the box tops) demarcating an age band (30–35 years) above which proportionally very few cases (≤10 percent overall) occurred.
Figure 9 Age distribution of confirmed human cases of avian influenza A (H5N1), December 2003–May 2006. Box-and-whisker plots show the age distribution of cases by (A) gender, (B) year of report, (C) patient outcome, and (D) country. The horizontal line and bullet mark in each box give, respectively, the median and mean age of cases. The variability in age is shown by plotting, as the outer limits of the shaded box, the first and third quartiles, Q 1 and Q 3, of the ages. Whiskers encompass all ages that satisfy the criteria Q 1 − 1.5(Q 3 − Q 1) (lower limit) and Q 3 + 1.5(Q 3 − Q 1) (upper limit). Points beyond the whiskers denote outliers. Information in (C) is based on the recorded status of patients according to World Health Organization (WHO) sources, with the category “alive” formed to include patients who were last reported as hospitalized (alive) or discharged (recovered). The age band 30–35 years (diagonal shading) is marked on each graph for reference. Source: Redrawn from Smallman-Raynor and Cliff (2007, 511).
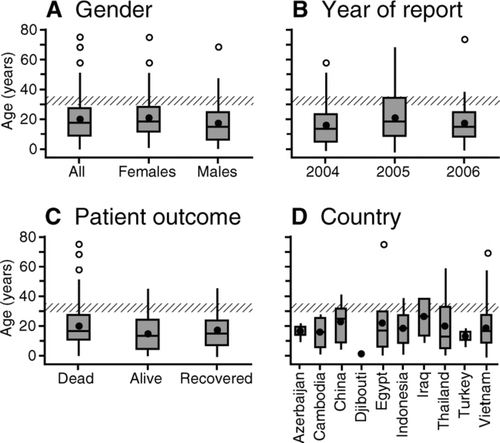
Behavioral factors that increase the risk of H5N1 exposure in younger persons, including the engagement of children and young adults in the slaughter, defeathering, and cooking of poultry, have been proposed by WHO as one determinant of the skewed age distribution in (CitationWHO 2006b). Biological mechanisms, too, might account for the apparent selective demographic targeting of H5N1. A recent experimental study by CitationKash et al. (2006) suggests that the 1918 (H1N1) pandemic influenza virus provoked a greatly enhanced immune response, resulting in massive damage to lung tissue. The immune response was most potent in young people with healthy immune systems, giving rise to an excess mortality among those in early adulthood (M. M. Patterson 2005). A similar hyperactive immune response has been reported from studies of the pathogenesis of H5N1 in Vietnamese patients (Citationde Jong et al. 2006), and again might account for the apparently higher levels of severe disease in younger subjects. Finally, we note that a biological model of geographically widespread immunity in persons born prior to 1969 (that is, about thirty-five years prior to the onset of the currently recognized panzootic in domestic poultry) might also account for some of the demographic pattern in (CitationSmallman-Raynor and Cliff 2007).
Seasonality
A long-recognized epidemiological feature of human influenza is the marked seasonality of the disease, usually manifesting as a sharply defined peak of activity in the winter hemisphere (CitationGlezen and Couch 1997). Although the cause of this phenomenon is not fully understood, a combination of both human host- and environment-related factors is generally suspected (CitationCliff, Haggett, and Ord 1986). To check for a seasonal dimension to the human disease pattern, the average count of WHO-confirmed human cases of avian influenza A (H5N1) for a given calendar month (January–December) was computed over corresponding months in the period from December 2003 to May 2006. The average monthly count is plotted as the cobweb chart in . For reference, the corresponding average monthly count of avian outbreaks of H5N1 is plotted in . To facilitate interpretation of the cobweb charts, all monthly values are expressed in standard normal (z) score form, with months of above-average disease activity (z > 0) identified by the shaded sectors.
Figure 10 Seasonal distribution of avian influenza A (H5N1). Cobweb charts plot, as an average for each calendar month, the reported global occurrence of avian influenza A (H5N1), January 2004–May 2006. Monthly averages are expressed in standard normal (z) score form. Periods with above-average levels of disease activity (z > 0) are shaded. (A) Human cases confirmed by the World Health Organization; (B) avian outbreaks.
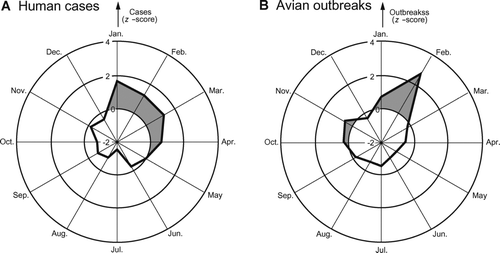
Recognizing that WHO-confirmed human cases of avian influenza A (H5N1) were restricted to the Northern Hemisphere in the period to May 2006 (), shows that H5N1 follows a typical seasonal pattern for influenza, with peak levels of human activity in the winter and early spring months (January–April). The summer and autumn months (July–October), by contrast, are associated with a marked reduction in reported disease activity. As judged by , the winter and spring increase in human cases is coincident with (or presaged by) a seasonal upswing in the epizootic activity of H5N1 in autumn and winter (October–February). As most confirmed human infections have arisen through contact with infected poultry, implies the existence of some as yet undetermined seasonal environmental control (possibility related to the enhanced survival and viability of influenza A virus at lower temperatures) on H5N1 activity in birds (CitationLi et al. 2004).
Family Clusters: Local Transmission Chains
As shows, the pandemic potential of H5N1 is contingent on the development of the capacity—through genetic reassortment or adaptive mutation—to spread between humans in an efficient and sustained manner. In monitoring for this development, particular interest attaches to family clusters of H5N1 illness as these may provide the first indication of the viral or epidemiological change associated with enhanced person-to-person transmission. In a review of the evidence to July 2005, CitationOlsen et al. (2005) identified a total of fifteen family clusters of avian influenza A (H5N1) in four east Asian countries: Vietnam (eleven clusters), Thailand (two clusters), Cambodia (one cluster), and Indonesia (one cluster). Clusters varied in size from two to five persons, and involved forty-one (37.6 percent) of the first 109 WHO-confirmed human cases of H5N1 infection. With the exception of one family cluster in Thailand, where limited person-to-person transmission was suspected on epidemiological grounds (CitationUngchusak et al. 2005), insufficient evidence was available to determine whether the clusters had resulted from person-to-person transmission or a common source of exposure (CitationOlsen et al. 2005).
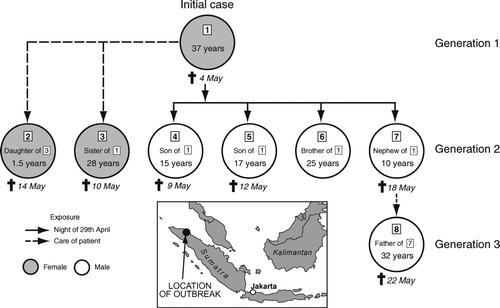
More recently, additional family clusters of H5N1 illness have been documented in Iraq (January–February 2006), Azerbaijan (March–April 2006), Egypt (April 2006), and Indonesia (April–May 2006).Footnote 20 The epidemiological associations involved in the latter cluster, formed to include members of an extended family in the North Sumatran villages of Kubu Simbelang and Kabanjahe, are shown in . The cluster consisted of an initial (suspected) case and seven confirmed cases, including adult siblings and their children in four separate households. As indicates, the case cluster can be divided into three generations, with each generation representing a particular time–space association in the infection pattern.
-
Generation 1 (one case). The initial case in the cluster was a thirty-seven-year-old female who developed symptoms of respiratory disease on 24 April and died ten days later on 4 May 2006. Although the patient died before H5N1 infection could be confirmed, epidemiological evidence indicates that she was exposed to sick and dying household poultry in the days preceding the onset of her illness.
-
Generation 2 (six cases). Between 3 May and 5 May, six members of the initial case's extended family (one sister, one brother, and four children) developed symptoms of an illness that was subsequently confirmed as avian influenza A (H5N1). Epidemiological investigations revealed that on 29 April—at a time when the initial case was severely ill and coughing heavily—a number of family members had spent the night in the same room as the index patient, while her sister had provided ongoing care. Five of the six cases in Generation 2 had died by mid-May.
-
Generation 3 (one case). The final case in the cluster was the thirty-two-year-old father of a ten-year-old boy in Generation 2. The father had provided care for his son while in hospital; father and son died within four days of each other.
Figure 11 Extended family cluster of avian influenza A (H5N1) in the villages of Kubu Simbelang and Kabanjahe, North Sumatra, Indonesia, April–May 2006. Confirmed (n = 7) and probable (n = 1) cases are represented by circles (coded 1–8). The vectors indicate inferred routes of virus exposure. The age, date of death, and relationship of cases are indicated. The inset map gives the location of the cluster. Source: Based on information included in Situation Updates—Avian Influenza (Geneva: WHO, 18–23 May), after CitationButler (2006a).
Discussion
International concern over the global threat of emerging infectious diseases has grown in recent decades (CitationLederberg, Shope, and Oaks 1992; Greenwood and De Cock 1998; Krause 1998; Smith et al. 2001). Broadly defined by Morse (1995, 7) as infections that have “newly appeared in the population, or have existed but are rapidly increasing in incidence or geographic range” and by Krause (1998, 5) as “clinically distinct conditions whose incidence in humans has increased regionally or worldwide,” the long list of emerging diseases includes such viral conditions as acquired immunodeficiency syndrome (AIDS), hantavirus pulmonary syndrome (HPS), and severe acute respiratory syndrome (SARS). To this list can be added HPAI A due to H5 and H7 viruses (), of which the H5N1 variety is distinguished by its association with severe and frequently fatal respiratory disease in humans.
Factors that have facilitated the surge in infectious disease activity over the last several decades encompass a broad range of social, physical, and biological mechanisms, including international travel and commerce, human demographics and behavior, technology and industry, economic development and land use change, and microbial adaptation (CitationMcMichael 2004; CitationMorens, Folkers, and Fauci 2004). Many of these factors reflect the increasing connectedness, integration, and interdependence of economies and societies through the broader processes of globalization (CitationZessin 2006), a concept that has recently been developed in the context of H5N1 and other animal diseases by the FAO (CitationDomenech et al. 2006) and the OIE (CitationVallat 2007). As Domenech et al. (2006, 104) observe, H5N1 has become “an international crisis since all regions in the World can be considered under risk due to the globalization of exchanges, with movements of animals, products, and humans and because of the possible spread of the virus through the migration of wild birds.” Such is the situation, CitationVallat (2007) warns, that “a single country failing to control animal disease outbreaks could put the entire world at risk.” Should H5N1 acquire the ability to spread from human to human in an efficient and sustained manner, interrelated and interdependent systems of travel, trade, and commerce have the capacity to accelerate the pace, and magnify the amplitude of, any ensuing pandemic (CitationWHO 2005b).
The most effective way to prevent the emergence of an H5N1-related human influenza pandemic is to monitor and control the virus at its source in animals (CitationDomenech et al. 2006). To alert geographers to the nature and development of the problem now facing the global community, this article has attempted to track the spatial spread of H5N1 during the first thirty months of the currently recognized panzootic, December 2003 to May 2006. Subject to caveats over data quality, four principal features of the spread of H5N1 emerge from our examination.
-
From an apparent source in east Asia, the geographical transmission of H5N1 was associated with a broadly defined panzootic wave front that pushed progressively westward, in a contagious manner, across central Asia, Europe, Middle East, and Africa. As far as the evidence allows, this pattern of geographical expansion was driven by a rarely observed phenomenon in the epizootiology of HPAI: the spread of highly pathogenic H5N1 along the flyways of migratory wildfowl.
-
Panzootic transmission was underpinned by three pronounced waves of outbreak activity in domestic and wild birds. Each of the three waves displayed a distinct autumn and winter peak, with Wave III (July 2005–April 2006) marking the fastest and geographically most extensive spread of HPAI ever detected. The latter wave, in turn, was associated with the establishment of an H5N1 bridgehead with migratory birds in the Black Sea region, with continued transmission to eastern and western Europe, the Middle East, and Africa in the early months of 2006.
-
Recognized human infections with H5N1 tracked, albeit imperfectly, the intercontinental expansion of the panzootic in birds, with WHO-confirmed human cases of avian influenza A (H5N1) limited to countries in which outbreaks of the virus had been recorded in domestic poultry.
-
WHO-confirmed human cases of avian influenza A (H5N1) followed the same seasonal pattern as avian outbreaks, with very limited evidence of human-to-human transmission and with a distinct age-related bias to children and young adults aged younger than thirty-five years.
Further Considerations
A number of questions follow from our consideration of the spread of H5N1. What are the current and likely future economic costs of H5N1 in birds and humans? What are the prospects for the future geographical extension of the panzootic? What plans are in place for the aversion and mitigation of a human pandemic?
Economic Costs of Panzootic and Pandemic Transmission
Estimates of the incurred economic costs of H5N1 run into the billions of dollars. Globally, more than 200 million head of poultry were lost to H5N1-related disease and culling activities by mid-2006, with the most severely affected countries of east Asia (Thailand and Vietnam) experiencing a 15–20 percent decline in poultry stock. In Vietnam, alone, the costs of the disease were estimated at US$76–450 million (0.3–1.8 percent of gross domestic product [GDP]) in 2003–2004, with severe localized losses—equivalent to 10 percent or more of annual household income—among backyard farmers in some rural areas (CitationMcLeod et al. 2005). More generally, the World Bank (2006) places the global costs of H5N1 to the poultry industry at more than US$10 billion in the period to 2006. These figures, however, pale alongside the projected costs of an H5N1-associated influenza pandemic in humans. According to World Bank estimates, the direct and indirect costs of a severe pandemic (about 70 million deaths worldwideFootnote 21 ) could amount to some US$1.25–2.00 trillion, equivalent to 3.0 to 5.0 percent of global GDP (CitationBrahmbhatt 2006).
Future Spread of the Panzootic
Antigenically distinct regional sublineages of H5N1 have now become established among poultry in east Asia, indicative of the long-term enzooticity of the virus in the region (CitationChen, Li, Smith, et al. 2006). Notwithstanding the FAO's proposed program of H5N1 control and eradication (FAO 2006), transmission of the virus among birds—with the attendant risk of human infection—is likely to continue for the foreseeable future. Current evidence suggests that some countries of east Asia have been subject to multiple importations of H5N1 from the putative source region of southern China, leading CitationChen, Li, Smith, et al. (2006) to argue that successful control will ultimately depend on the containment of the epizootic in Chinese territory.
The apparent role of migratory birds in the rapid spread of highly pathogenic H5N1 over large tracts of Eurasia and Africa has raised concerns over the possible onward spread of the virus to the Western Hemisphere. CitationRappole and Hubálek (2006) observe that relatively few species of bird undertake regular interhemispheric migration—an observation that is reinforced by the genetically distinct lineages of avian influenza A viruses in the two hemispheres. Vagrancy (a biological phenomenon in which individual birds stray beyond their normal geographical range) could serve as a possible source of H5N1 in the Western Hemisphere, although an introduction with legally or illegally imported domestic or pet birds is viewed as a more likely prospect (CitationRappole and Hubálek 2006). On the basis of experimental studies, the high degree of susceptibility of certain species of North American duck and gull to highly pathogenic H5N1 suggests that, if imported into the Americas, the virus could be propagated in at least some native bird populations (CitationBrown et al. 2006).
Pandemic Preparedness and Response
Given the anticipated costs of a severe influenza pandemic, preparations for such an event have been identified as a global health priority by WHO. Working in cooperation with national governments, the operations of WHO's strategic Global Influenza Preparedness Plan are structured according to the six-phase system of pandemic alert in (CitationWHO 2005d). Each phase of alert is associated with a series of actions (including sensitive surveillance for human-to-human H5N1 transmission events, the development and manufacture of vaccines, and the production and stockpiling of antiviral drugs) that seek to contain, delay, and ultimately to minimize the impact of a pandemic virus. As described by CitationFerguson et al. (2006), mathematical simulations of alternative pandemic mitigation strategies in economically more developed countries have highlighted the potential effectiveness of combined control measures (notably household-based antiviral prophylaxis with reactive school closure) in reducing the influenza attack rate. The same models suggest that border and internal travel restrictions are unlikely to prove effective in halting or substantially delaying the geographical spread of a pandemic virus (CitationFerguson et al. 2006).
Concluding Comments: Opportunities for Geographical Research
Writing of the nascent AIDS pandemic in the 1980s, Shannon and Pyle (1989, 1) noted how “the complex of geographic factors necessary to its understanding require the interest and co-operation of a broad range of geographic specialities.” Echoing down a generation, the same sentiments apply today to the pandemic threat raised by H5N1. Although contributions to the geographical literature are still few in number (CitationKeil and Ali 2006), intrinsically spatial investigations of the epizootic transmission of H5N1 at local, national, and international scales have begun to appear in the medical literature (CitationGilbert, Chaitaweesub, et al. 2006; CitationGilbert, Xiao, et al. 2006). As these latter studies illustrate, there is enormous potential for the dynamic and cross-sectional mapping of H5N1 in avian species, investigations of the changing ecology of the virus, and geographical explorations of the environmental determinants of H5N1 infection and disease in birds and humans.
If the H5N1 virus does acquire the ability to spread in an efficient and sustained manner in the human population, geographers should be well placed to contribute time–space forecasts of the arrival, speed of growth, and ultimate size of an ensuing pandemic in the human population (CitationCliff, Haggett, and Ord 1986). Events surrounding the emergence and spread of SARS, in particular, have highlighted the rapidity with which a new respiratory disease agent can spread along the global airline network (CitationShannon and Willoughby 2004). Informed by the experience of SARS, measures of airline accessibility can be adapted to provide insights regarding the speed and direction of spread of a new pandemic influenza virus from n likely starting points (CitationBowen and Laroe 2006).
Beyond the traditional concerns of medical geography, H5N1 has had substantial social and economic impacts on some of the poorest and least developed rural communities of east Asia and elsewhere. What are the immediate and longer term implications for development in the affected communities? To what extent do political responses to H5N1 reflect the needs and circumstances of the affected communities, and in what ways are international agencies such as the World Bank and the FAO assisting in the process? With little prospect for the eradication of H5N1 in the foreseeable future, possibilities for effective and sustainable H5N1 control strategies, tailored to the circumstances of local populations, would seem to demand close attention.
Appendix: Avian Influenza A (H5N1) Outbreaks in Bird Species
Table A1 provides summary details of the timing and location of the first confirmed avian outbreaks of H5N1 in OIE member states from December 2003 to May 2006.
Table A1 First report of avian influenza A (H5N1) in bird species, by country (December 2003–May 2006)
Acknowledgments
The work described has been undertaken as part of a program of research entitled Historical Geography of Emerging and Re-Emerging Epidemics, 1850–2000, funded by the Wellcome Trust. We wish to express our thanks to the Wellcome Trust for their continuing support, and to the anonymous referees for their helpful comments on an earlier draft of this article.
Notes
aYears 1500–1875.
bExcludes “pandemics” listed by Hirsch (1883, 19) as exclusive to the Western Hemisphere (1647, 1737–1738, 1757–1758, 1761–1762, 1789–1790, 1798, 1807, 1815–1816, 1824–1826, 1843, and 1873).
cYears 1700–1977.
d“Probable” pandemic.
eK. D. CitationPatterson (1986) defines separate pandemics in the years 1830–1831 and 1833.
fSee Dowdle (1999) for a further consideration of the subtypes of influenza A virus associated with the events of 1889–1890 and 1899–1900.
aPneumonia and respiratory insufficiency in solitary fatal case.
a1 January–18 May 2006.
aTo 27 April 2006.
bDate of first recognition of event.
cA/H5 virus confirmed.
1. Estimates of the global mortality associated with the 1918–1919 pandemic vary widely. E. O. Jordan places the death toll at 21.6 million (CitationJordan 1927, 229–30). A revised estimate by K. D. Patterson and Pyle (Citation1991, 19) places the mortality at 30 million. More recently, CitationOxford et al. (1999) and CitationOxford (2000) place the mortality at 40 million. Other more extreme estimates have ranged up to 100 million (see, for example, CitationBurnet 1979, 203).
2. Outbreaks of H5N1 among big cats in zoos and rescue centers have been documented in Cambodia (2003) and Thailand (2003, 2004) (see, for example, Food and Agriculture Organization 2004; CitationKeawcharoen et al. 2004; OIE 2004f). The largest outbreak was recorded at a zoo in Si Racha District, Chon Buri Province, Thailand, in October 2004 and resulted in the death (through disease and slaughter) of 147 tigers (Panthera tigris). Feed, consisting of chicken carcasses, was identified as the most likely source of infection (OIE 2004f). Naturally occurring infections with H5N1 have also been reported in domestic cats, dogs, and stone martens, among other mammalian species. See, for example, CitationButler (2006b) and CitationSongserm et al. (2006).
3. The OIE's Terrestrial Animal Health Code 2005 defines poultry as “all birds reared or kept in captivity for the production of meat or eggs for consumption, for the production of other commercial products, for restocking supplies of game, or for breeding these categories of birds” (CitationOIE 2005g, Article 2.7.12.1).
4. The initial recognition of HPAI is attributed to Edoardo Perroncito and his description of a poultry-based outbreak of a contagious disease on farms near Turin, Italy, in 1878 (CitationPerroncito 1878).
5. Although an official report on the H5N1 outbreak in Suffolk failed to identify a proven specific source of the virus, an importation with turkey meat from Hungary (where outbreaks of H5N1 had previously been reported) was viewed as the most plausible route of introduction. See Department for Environment, Food and Rural Affairs (2007) for further details of the investigation.
6. Subsequent studies have traced the evolution of the Z genotype back to the viruses responsible for the outbreaks in China and Hong Kong in the mid-1990s (CitationLi et al. 2004; Citationde Jong and Hien 2006). It is assumed that these viruses, in turn, emerged from low pathogenic viruses of aquatic birds, although no such precursor viruses have yet been identified (CitationSims et al. 2005).
7. See Shortridge and Stuart-Harris (1982) for further details of the influenza epicenter hypothesis.
8. Weekly calendar periods (Sunday–Saturday) were formed by dividing the 889-day observation period into 128 seven-day units, beginning with the period Sunday 7–Saturday 13 December 2003 (week 1) and ending with the period Sunday 14–Saturday 20 May 2006 (week 128).
9. See WHO (2006c) for definitions of probable and confirmed human cases of avian influenza A (H5N1).
10. An additional report of H5N1 in a dead peregrine falcon was issued by Hong Kong S.A.R. on 26 January 2004 (OIE 2004a).
11. Illegal trade, in particular, has been identified by the Food and Agriculture Organization (Citation2005b, 19) as having played a potentially important role in the international transmission process: “Long land borders exist between many of the infected countries in the region and smuggling of poultry and poultry products across many of these is acknowledged. Movement of live poultry (including fighting cocks) across borders is considered to be the most likely source of infection in some places.”
12. Healthy domestic fowl that are susceptible to infection with avian influenza A (H5N1) virus and that are monitored for evidence of the circulation of the virus.
13. We note that the entries for Europe in the Appendix exclude two prior instances of the detection of H5N1 infection in imported birds: (1) two crested hawk-eagles, smuggled from Thailand and seized in hand luggage at Brussels International Airport, Belgium, on 18 October 2004 (CitationVan Borm et al. 2005); and (2) a consignment of mixed bird species, imported from Taiwan and transferred to a quarantine facility in Essex, southeast England, in late September 2005 (Department for Environment, Food and Rural Affairs 2005).
14. An earlier WHO-confirmed human case of avian influenza A (H5N1), with symptom onset on 25 November 2003, has been retrospectively identified by Chinese scientists. The patient (a twenty-four-year-old male in military service) was hospitalized with a severe respiratory illness in Beijing, China, and died on 3 December 2003. It was initially suspected that the patient was infected with the SARS virus. Stored specimens from the man tested positive for H5N1 (CitationZhu et al. 2006).
15. The analysis in was limited to 205 sample human cases for which the week of report to WHO could be ascertained.
16. In the interpretation of , we note that the majority of human cases in Wave II were recorded in Vietnam in the interval January–April 2005, and occurred in the absence of a corresponding increase in the reported number of avian outbreaks (see ). The extent to which the latter observation is the result of weaknesses in the surveillance infrastructure of Vietnam, or is the result of a real epidemiological process, requires further investigation.
17. The first recorded outbreak worldwide for which wild birds were the most likely source of human infection is described by CitationGilsdorf et al. (2006). The outbreak manifested in February and March 2006 as a cluster of seven laboratory-confirmed human cases of avian influenza A (H5N1), including six members of a single family, in the village of Daikyand, Salyan District, southeastern Azerbaijan. The outbreak was coincident with a massive die-off of swans, with the human cases having been involved in the defeathering of the dead birds.
18. Age-related information for an additional forty-seven cases in could not be ascertained from published WHO sources and have been omitted from the analysis in .
19. Case rates were derived from age-specific national population estimates for 2005 included in Population Division of the Department of Economic and Social Affairs of the United States Secretariat (2006).
20. See WHO's Situation Updates—Avian Influenza for further information.
21. Based on the assumption of a 35 percent global attack rate and a 3 percent case fatality rate. See Brahmbhatt (2006).
References
- Alvarado de la Barrera , C. and Reyes-Terán , G. 2005 . Influenza: Forecast for a pandemic . Archives of Medical Research , 36 ( 6 ) : 628 – 36 .
- Boden , E. , ed. 2005 . Black's veterinary dictionary, , 21st ed , London : A. & C. Black .
- Bowen , J. T. and Laroe , C. 2006 . Airline networks and the international diffusion of severe acute respiratory syndrome . Geographical Journal , 172 ( 2 ) : 130 – 44 .
- Brahmbhatt , M. 2006 . Economic impacts of avian influenza propagation , Washington, DC : World Bank . http://www.worldbank.org(last accessed 20 July 2007)
- Brown , J. D. , Stallknecht , D. E. , Beck , J. R. , Suarez , D. L. and Swayne , D. E. 2006 . Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses . Emerging Infectious Diseases , 12 ( 11 ) : 1663 – 70 .
- Burnet , F. M. 1979 . Portraits of viruses: Influenza virus A . Intervirology , 11 ( 4 ) : 201 – 14 .
- Butler , D. 2006a . Family tragedy spotlights flu mutations . Nature , 442 ( 7099 ) : 114 – 15 .
- Butler , D. 2006b . Thai dogs carry bird-flu virus, but will they spread it? . Nature , 439 ( 7078 ) : 773
- Capua , I. and Alexander , D. J. 2004 . Avian influenza: Recent developments . Avian Pathology , 33 ( 4 ) : 393 – 404 .
- Centers for Disease Control and Prevention . 1997 . Isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, May–December 1997 . Morbidity and Mortality Weekly Report , 46 ( 50 ) : 1204 – 07 .
- Centers for Disease Control and Prevention . 1998 . Update: Isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, 1997–1998 . Morbidity and Mortality Weekly Report , 45 ( 52–53 ) : 1245 – 47 .
- Chen , H. , Li , Y. , Li , Z. , Shi , J. , Shinya , K. , Deng , G. Qi , Q. 2006 . Properties and dissemination of H5N1 viruses isolated during an influenza outbreak in migratory waterfowl in western China . Journal of Virology , 80 ( 12 ) : 5976 – 83 .
- Chen , H. , Li , Y. , Smith , G. J. D. , Li , K. S. , Wang , J. , Fan , X. H. Rayner , J. M. 2006 . Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control . Proceedings of the National Academy of Sciences, USA , 103 ( 8 ) : 2845 – 50 .
- Claas , E. C. , de Jong , J. C. , van Beek , R. , Rimmelzwaan , G. F. and Osterhaus , A. D. M. E. 1998 . Human influenza virus A/Hong Kong/156/97 (H5N1) infection . Vaccine , 16 ( 9–10 ) : 977 – 78 .
- Claas , E. C. , Osterhaus , A. D. M. E. , van Beek , R. , de Jong , J. C. , Rimmelzwaan , G. F. , Senne , D. A. , Krauss , S. , Shortridge , K. F. and Webster , R. G. 1998 . Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus . Lancet , 351 ( 9101 ) : 472 – 77 .
- Cliff , A. D. and Haggett , P. 2003 . “ Geography and disease distributions ” . In A century of British geography , Edited by: Johnson , R. J. and Williams , M. 177 – 94 . Oxford, , U.K. : Oxford University Press for the British Academy .
- Cliff , A. D. , Haggett , P. and Ord , J. K. 1986 . Spatial aspects of influenza epidemics , London : Pion .
- Cliff , A. D. , Haggett , P. , Ord , J. K. and Versey , G. R. 1981 . Spatial diffusion: An historical geography of epidemics in an island community , Cambridge, , U.K. : Cambridge University Press .
- de Jong , M. D. and Hien , T. T. 2006 . Avian influenza A (H5N1) . Journal of Clinical Virology , 35 ( 1 ) : 2 – 13 .
- de Jong , M. D. , Simmons , C. P. , Thanh , T. T. , Hien , V. M. , Smith , G. J. D. , Chau , T. N. B. Hoang , D. M. 2006 . Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia . Nature Medicine , 12 ( 10 ) : 1203 – 07 .
- Department for Environment, Food and Rural Affairs . 2005 . Epidemiology report on avian influenza in a quarantine premises in Essex , London : DEFRA .
- Department for Environment, Food and Rural Affairs . 2006 . Situation analysis—Outbreaks of HPAI H5N1 virus in Europe during 2005/2006—An overview and commentary , Edited by: Sabirovic , M. , Wilesmith , J. , Hall , S. , Coulson , N. and Landeg , F. London : International Animal Health Division .
- Department for Environment, Food and Rural Affairs . 2007 . Outbreak of highly pathogenic H5N1 avian influenza in Suffolk in January 2007: A report of the epidemiological findings by the National Emergency Epidemiology Group, Defra, 5 April 2007 , London : DEFRA .
- Domenech , J. , Lubroth , J. , Eddi , C. , Martin , V. and Roger , F. 2006 . Regional and international approaches on prevention and control of animal transboundary and emerging diseases . Annals of the New York Academy of Sciences , 1081 : 90 – 107 .
- Dowdle , W. R. 1999 . Influenza A virus recycling revisited . Bulletin of the World Health Organization , 77 ( 10 ) : 820 – 28 .
- Ducatez , M. F. , Olinger , C. M. , Owoade , A. A. , De Landtsheer , S. , Ammerlaan , W. , Niesters , H. G. M. , Osterhaus , A. D. M. E. , Fouchier , R. A. M. and Muller , C. P. 2006 . Multiple introductions of H5N1 in Nigeria . Nature , 442 ( 7098 ) : 37
- Dybing , J. K. , Schultz-Cherry , S. , Swayne , D. E. , Suarez , D. L. and Perdue , M. L. 2000 . Distinct pathogenesis of Hong Kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses . Journal of Virology , 74 ( 3 ) : 1443 – 50 .
- Enserink , M. 2006 . Amid mayhem in Turkey, experts see new chances for research . Science , 311 ( 5759 ) : 314 – 15 .
- European Centre for Disease Surveillance and Control . 2006 . Highly pathogenic avian influenza A/H5N1—Update and overview of 2006 . Eurosurveillance , 11 ( 12 ) http://www.eurosurveillance.org E061221.1 (last accessed 20 July 2007)
- Ferguson , N. M. , Cummings , D. A. T. , Fraser , C. , Cajka , J. C. , Cooley , P. C. and Burke , D. S. 2006 . Strategies for mitigating an influenza pandemic . Nature , 442 ( 7107 ) : 448 – 52 .
- Ferguson , N. M. , Fraser , C. , Donelly , C. A. , Ghani , A. C. and Anderson , R. M. 2004 . Public health risk from the avian H5N1 influenza epidemic . Science , 304 ( 5673 ) : 968 – 69 .
- Food and Agriculture Organization . 2004 . Update on the avian influenza situation (as of 15/6/2004) . FAO AIDE News , 16 : 1 – 10 .
- Food and Agriculture Organization . 2005a . Potential risk of highly pathogenic avian influenza (HPAI) spreading through wild water bird migration . FAO AIDE News , 33 : 1 – 7 .
- Food and Agriculture Organization . 2005b . A review of highly pathogenic avian influenza in Asia and FAO's response to the disease . FAO AIDE News , 29 : 2 – 29 .
- Food and Agriculture Organization . 2006 . Avian influenza control and eradication: FAO's proposal for a global programme , Rome : FAO .
- Gilbert , M. , Chaitaweesub , P. , Parakamawongsa , T. , Premashthira , S. , Tiensin , T. , Kalpravidh , W. , Wagner , H. and Slingenbergh , J. 2006 . Free-grazing ducks and highly pathogenic avian influenza, Thailand . Emerging Infectious Diseases , 12 ( 2 ) : 227 – 34 .
- Gilbert , M. , Xiao , X. , Domenech , J. , Lubroth , J. , Martin , V. and Slingenbergh , J. 2006 . Anatidae migration in the Western Palearctic and spread of highly pathogenic avian influenza H5N1 virus . Emerging Infectious Diseases , 12 ( 11 ) : 1650 – 56 .
- Gilg , A. W. 1973 . A study in agricultural disease diffusion . Transactions of the Institute of British Geographers , 59 : 77 – 97 .
- Gilsdorf , A. , Boxall , N. , Gasimov , V. , Agayev , I. , Mammadzade , F. , Ursu , P. Gasimov , E. 2006 . Two clusters of human infection with influenza A/H5N1 virus in the Republic of Azerbaijan, February–March 2006 . Eurosurveillance , 11 ( 4–6 ) : 122 – 26 .
- Glezen , W. P. and Couch , R. B. 1997 . “ Influenza viruses ” . In Viral infections of humans: Epidemiology and control, , 4th ed. , Edited by: Evans , A. S. and Kaslow , R. A. 473 – 505 . New York : Plenum .
- Greenwood , B. and De Cock , K. 1998 . New and resurgent infections: Detection and management of tomorrow's infections , Chichester, , U.K. : Wiley .
- Gust , I. D. , Hampson , A. W. and Lavanchy , D. 2001 . Planning for the next pandemic of influenza . Reviews in Medical Virology , 11 ( 1 ) : 59 – 70 .
- Hirsch , A. 1883 . Acute infective diseases. Vol. 1 of Handbook of geographical and historical pathology , London : The New Sydenham Society .
- Horimoto , T. and Kawaoka , Y. 2001 . Pandemic threat posed by avian influenza viruses . Clinical Microbiology Reviews , 14 ( 1 ) : 129 – 49 .
- Hunter , J. M. and Young , J. C. 1971 . Diffusion of influenza in England and Wales . Annals of the Association of American Geographers , 61 ( 4 ) : 637 – 53 .
- Johnson , N. P. A. S. and Mueller , J. 2002 . Updating the accounts: Global mortality of the 1918–1920 “Spanish” influenza pandemic . Bulletin of the History of Medicine , 76 ( 1 ) : 105 – 15 .
- Jordan , E. O. 1927 . Epidemic influenza: A survey , Chicago : American Medical Association .
- Kash , J. C. , Tumpey , T. M. , Proll , S. C. , Carter , V. , Perwitasari , O. , Thomas , M. J. Basler , C. F. 2006 . Genomic analysis of increased host immune and cell death responses induced by the 1918 influenza virus . Nature , 443 ( 7111 ) : 578 – 81 .
- Keawcharoen , J. , Oraveerakul , K. , Kuiken , T. , Fouchier , R. A. M. , Amonsin , A. , Payungporn , S. Noppornpanth , S. 2004 . Avian H5N1 in tigers and leopards . Emerging Infectious Diseases , 10 ( 12 ) : 2189 – 91 .
- Keil , R. and Ali , S. H. 2006 . The avian flu: Some lessons learned from the 2003 SARS outbreak in Toronto . Area , 38 ( 1 ) : 107 – 09 .
- Krause , R. M. 1998 . Emerging infections , San Diego, CA : Academic Press .
- Lederberg , J. , Shope , R. E. and Oaks , S. C. 1992 . Emerging infections: Microbial threats to health in the United States , Washington, DC : National Academy Press .
- Li , K. S. , Guan , Y. , Wang , J. , Smith , G. J. D. , Xu , K. M. , Duan , L. Rahardjo , A. P. 2004 . Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia . Nature , 430 ( 6996 ) : 209 – 13 .
- Liu , J. , Xiao , H. , Lei , F. , Zhu , Q. , Qin , K. , Zhang , X.-W. Zhang , X.-L. 2005 . Highly pathogenic H5N1 influenza virus in migratory birds . Science , 309 ( 5738 ) : 1206
- McLeod , A. , Morgan , N. , Prakash , A. and Hinrichs , J. 2005 . Economic and social impacts of avian influenza , Rome : FAO Emergency Centre for Transboundary Animal Diseases Operations (ECTAD) . http://www.fao.org (last accessed 20 July 2007)
- McMichael , A. J. 2004 . Environmental and social influences on emerging infectious diseases: Past, present and future . Philosophical Transactions of the Royal Society B , 359 ( 1447 ) : 1049 – 58 .
- Morens , D. M. , Folkers , G. K. and Fauci , A. S. 2004 . The challenge of emerging and re-emerging infectious diseases . Nature , 430 ( 6996 ) : 242 – 49 .
- Morse , S. S. 1995 . Factors in the emergence of infectious diseases . Emerging Infectious Diseases , 1 ( 1 ) : 7 – 15 .
- OIE . 2003 . Highly pathogenic avian influenza in the Republic of Korea: Suspected outbreak . Disease Information , 16 ( 50 ) : 270 – 71 .
- OIE . 2004a . Avian influenza in Hong Kong, special administrative region of the People's Republic of China . Disease Information , 17 ( 5 ) : 18 – 19 .
- OIE . 2004b . Highly pathogenic avian influenza in Japan: Follow-up report no. 5 (final report) . Disease Information , 17 ( 29 ) : 196
- OIE . 2004c . Highly pathogenic avian influenza in Korea (Rep. of): Follow-up report no. 5 (final report) . Disease Information , 17 ( 39 ) : 277
- OIE . 2004d . Highly pathogenic avian influenza in the Republic of Korea: Follow-up report no. 4 . Disease Information , 17 ( 30 ) : 205
- OIE . 2004e . Highly pathogenic avian influenza in Thailand: Follow-up report no. 1 . Disease Information , 17 ( 6 ) : 27 – 28 .
- OIE . 2004f . Highly pathogenic avian influenza in Thailand in felines in a zoo . Disease Information , 17 ( 43 ) : 312 – 13 .
- OIE . 2004g . Highly pathogenic avian influenza in Vietnam: Follow-up report no. 2 . Disease Information , 17 ( 9 ) : 57 – 59 .
- OIE . 2005a . Highly pathogenic avian influenza in Kazakhstan . Disease Information , 18 ( 31 ) : 231
- OIE . 2005b . Highly pathogenic avian influenza in Mongolia in migratory birds . Disease Information , 18 ( 32 ) : 253
- OIE . 2005c . Highly pathogenic avian influenza in Romania . Disease Information , 18 ( 41 ) : 333 – 34 .
- OIE . 2005d . Highly pathogenic avian influenza in Russia . Disease Information , 18 ( 30 ) : 218 – 19 .
- OIE . 2005e . Highly pathogenic avian influenza in Turkey . Disease Information , 18 ( 41 ) : 336 – 38 .
- OIE . 2005f . Highly pathogenic avian influenza in Ukraine . Disease Information , 18 ( 49 ) : 485 – 86 .
- OIE . 2005g . Terrestrial animal health code 2005 , Paris : OIE .
- OIE . 2006a . Avian influenza in India . Disease Information , 19 ( 8 ) : 133 – 34 .
- OIE . 2006b . Avian influenza in Myanmar . Disease Information , 19 ( 11 ) : 223
- OIE . 2006c . Avian influenza in Nigeria . Disease Information , 19 ( 6 ) : 89 – 90 .
- Olsen , S. J. , Ungchusak , K. , Sovann , L. , Uyeki , T. M. , Dowell , S. F. , Cox , N. J. , Aldis , W. and Chunsuttiwat , S. 2005 . Family clustering of avian influenza A (H5N1) . Emerging Infectious Diseases , 11 ( 11 ) : 1799 – 801 .
- Oxford , J. S. 2000 . Influenza A pandemics of the 20th century with special reference to 1918: Virology, pathology and epidemiology . Reviews in Medical Virology , 10 ( 2 ) : 119 – 33 .
- Oxford , J. S. , Sefton , A. , Jackson , R. , Johnson , N. P. A. S. and Daniels , R. S. 1999 . Who's that lady . Nature Medicine , 5 ( 12 ) : 1351 – 52 .
- Patterson , K. D. 1986 . Pandemic influenza, 1700–1900: A study in historical epidemiology , Totowa, NJ : Rowman and Littlefield .
- Patterson , K. D. and Pyle , G. F. 1983 . The diffusion of influenza in sub-Saharan Africa during the 1918–1919 pandemic . Social Science and Medicine , 17 ( 17 ) : 1299 – 307 .
- Patterson , K. D. and Pyle , G. F. 1991 . The geography and mortality of the 1918 influenza pandemic . Bulletin of the History of Medicine , 65 ( 1 ) : 4 – 21 .
- Patterson , M. M. 2005 . The coming influenza pandemic: Lessons from the past for the future . Journal of the American Osteopathic Association , 105 ( 1 ) : 498 – 500 .
- Peiris , J. S. M. , Yu , W. C. , Leung , C. W. , Cheung , C. Y. , Ng , W. F. , Nicholls , J. M. Ng , T. K. 2004 . Re-emergence of fatal human influenza A subtype H5N1 disease . Lancet , 363 ( 9409 ) : 617 – 19 .
- Pereira , H. G. , Tu°mová , B. and Law , V. G. 1965 . Avian influenza A viruses . Bulletin of the World Health Organization , 32 ( 6 ) : 855 – 60 .
- Perroncito , E. 1878 . Epizoozia tifoide nei gallinacei [Typhoid epizootic in gallinaceous birds] . Annali della Academia d'agricoltora di Torino , 21 : 87 – 126 .
- Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat . 2006 . World population prospects: The 2004 revision and World urbanization prospects: The 2003 revision , New York : United Nations .
- Pyle , G. F. 1969 . Diffusion of cholera in the United States . Geographical Analysis , 1 ( 1 ) : 59 – 75 .
- Pyle , G. F. 1986 . The diffusion of influenza: Patterns and paradigms , Totowa, NJ : Rowman and Littlefield .
- Rappole , J. H. and Hubálek , Z. 2006 . Birds and influenza H5N1 virus movement to and within North America . Emerging Infectious Diseases , 12 ( 10 ) : 1486 – 92 .
- Shannon , G. W. and Pyle , G. F. 1989 . The origin and diffusion of AIDS: A view from medical geography . Annals of the Association of American Geographers , 79 ( 1 ) : 1 – 24 .
- Shannon , G. W. and Willoughby , J. 2004 . Severe acute respiratory syndrome (SARS) in Asia: A medical geographic perspective . Eurasian Geography and Economics , 45 ( 5 ) : 359 – 81 .
- Shortridge , K. F. and Stuart-Harris , C. H. 1982 . An influenza epicentre? . Lancet , 2 ( 8302 ) : 812 – 13 .
- Sims , L. D. , Domenech , J. , Benigno , C. , Kahn , S. , Kamata , A. , Lubroth , J. , Martin , V. and Roeder , P. 2005 . Origin and evolution of highly pathogenic H5N1 avian influenza in Asia . Veterinary Record , 157 ( 6 ) : 159 – 64 .
- Smallman-Raynor , M. R. and Cliff , A. D. 2007 . Avian influenza A (H5N1) age distribution in humans . Emerging Infectious Diseases , 13 ( 3 ) : 510 – 12 .
- Smallman-Raynor , M. R. , Johnson , N. and Cliff , A. D. 2002 . The spatial anatomy of an epidemic: Influenza in London and the county boroughs of England and Wales, 1918–19 . Transactions of the Institute of British Geographers New Series , 27 ( 4 ) : 452 – 70 .
- Smith , G. L. , Irving , W. L. , McCauley , J. W. and Rowlands , D. J. 2001 . New challenges to health: The threat of virus infections , Cambridge, , U.K. : Cambridge University Press .
- Songserm , T. , Amonsin , A. , Jam-on , R. , Sae-Heng , N. , Meemak , N. , Pariyothorn , N. , Payungporn , S. , Theamboonlers , A. and Poovorawan , Y. 2006 . Avian influenza H5N1 in naturally infected domestic cat . Emerging Infectious Diseases , 12 ( 4 ) : 681 – 83 .
- Tam , J. S. 2002 . Influenza A (H5N1) in Hong Kong: An overview . Vaccine , 20 ( suppl. 2 ) : S77 – S81 .
- Thorson , A. , Petzold , M. , Chuc , N. T. K. and Ekdahl , K. 2006 . Is exposure to sick or dead poultry associated with a flulike illness? . Archives of Internal Medicine , 166 ( 1 ) : 119 – 23 .
- Tollis , M. and Di Trani , L. 2002 . Recent developments in avian influenza research: Epidemiology and immunoprophylaxis . Veterinary Journal , 164 ( 3 ) : 202 – 15 .
- Tumpey , T. M. , Maines , T. R. , Hoeven , N. V. , Glaser , L. , Solórzano , A. , Pappas , C. Cox , N. J. 2007 . A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission . Science , 315 ( 5812 ) : 655 – 59 .
- Ungchusak , K. , Auewarakul , P. , Dowell , S. F. , Kitphati , R. , Auwanit , W. , Puthavathana , P. Uiprasertkul , M. 2005 . Probable person-to-person transmission of avian influenza A (H5N1) . New England Journal of Medicine , 352 ( 4 ) : 333 – 40 .
- Vallat , B. 2007 . Protecting the world from emerging diseases linked to globalisation , Paris : OIE . http://www.oie.int (last accessed 20 July 2007)
- Van Borm , S. , Thomas , I. , Hanquet , G. , Lambrecht , B. , Boschmans , M. , Dupont , G. , Decaestecker , M. , Snacken , R. and van den Berg , T. 2005 . Highly pathogenic H5N1 in smuggled Thai eagles, Belgium . Emerging Infectious Diseases , 11 ( 5 ) : 702 – 05 .
- Webster , R. G. , Guan , Y. , Poon , L. , Krauss , S. , Webby , R. , Govorkovai , E. and Peiris , M. 2005 . The spread of the H5N1 bird flu epidemic in Asia in 2004 . Archives of Virology Supplementum , 19 : 117 – 29 .
- Wee , S.-H. , Park , C.-H. , Nam , H.-M. , Kim , C.-H. , Yoon , H. , Kim , S.-J. , Lee , E.-S. , Lee , B.-Y. , Kim , J.-H. and Kim , C.-S. 2006 . Outbreaks of highly pathogenic avian influenza (H5N1) in the Republic of Korea in 2003/04 . Veterinary Record , 158 ( 10 ) : 341 – 44 .
- World Bank . 2006 . Economic impact of avian flu , Washington, DC : World Bank . http://www.worldbank.org (last accessed 20 July 2007)
- World Health Organization . 2004a . Avian influenza A(H5N1) in humans and poultry in Viet Nam , Geneva : WHO . http://www.who.int (last accessed 20 July 2007)
- World Health Organization . 2004b . WHO guidelines for global surveillance of influenza A/H5 , Geneva : WHO .
- World Health Organization . 2005a . Avian influenza: Assessing the pandemic threat , Geneva : WHO . WHO/CDS/2005:29
- World Health Organization . 2005b . Avian influenza: Frequently asked questions (updated on 19 October 2005) . Weekly Epidemological Record , 80 ( 44 ) : 377 – 84 .
- World Health Organization . 2005c . Responding to the avian influenza pandemic threat: Recommended strategic actions , Geneva : WHO . WHO/CDS/CSR/ GIP/2005.8
- World Health Organization . 2005d . WHO global influenza preparedness plan: The role of WHO and recommendations for national measures before and during pandemics , Geneva : WHO . WHO/CDS/CSR/GIP/2005.5
- World Health Organization . 2006a . Avian influenza fact sheet (April 2006) . Weekly Epidemiological Record , 81 ( 14 ) : 129 – 36 .
- World Health Organization . 2006b . Epidemiology of WHO-confirmed human cases of avian influenza A(H5N1) infection . Weekly Epidemiological Record , 81 ( 26 ) : 249 – 57 .
- World Health Organization . 2006c . WHO case definitions for human infections with influenza A(H5N1) virus , Geneva : WHO .
- World Health Organization . 2007 . Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO: 16 May 2007 , Geneva : WHO . http://www.who.int (last accessed 20 July 2007)
- Writing Committee of the WHO Consultation on Human Influenza A/H5 . 2005 . Avian influenza A (H5N1) infection in humans . New England Journal of Medicine , 353 ( 13 ) : 1374 – 85 .
- Xu , X. , Subbarao , K. , Cox , N. J. and Guo , Y. 1999 . Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong . Virology , 261 ( 1 ) : 15 – 19 .
- Zessin , K. H. 2006 . Emerging diseases: A global and biological perspective . Journal of Veterinary Medicine B: Infectious Diseases and Veterinary Public Health , 56 ( suppl. 1 ) : 7 – 10 .
- Zhu , Q. Y. , Qin , E. D. , Wang , W. , Yu , J. , Liu , B. H. , Hu , Y. , Hu , J. F. and Cao , W. C. 2006 . Fatal infection with influenza A (H5N1) virus in China . New England Journal of Medicine , 354 ( 25 ) : 2731 – 32 .