Abstract
Capsule Crèches formed early in the season lasted longer than those formed later, but a longer crèching period did not appear to confer higher chick survival.
Aims To investigate the ecological factors influencing the benefit to parents of crèching behaviour by measuring chick survival.
Methods Mark–recapture was used to model apparent daily survival of 505 chicks during the crèching period in three different crèches. We contrasted models with different tipping points to assess possible differences across crèches in chick survival during the first week and in the moment at which chick departures began.
Results We did not find a clear difference across crèches on daily chick survival during the first stages of the crèche. By modelling chick apparent survival as a linear function of time we showed that the latest formed crèche dispersed more rapidly.
Conclusions The two crèches formed early in the season lasted longer than the one formed later but chicks did not appear to have a higher survival over the first week of crèching. We suggest that a longer period at the crèche should result in a higher survival in the period soon after fledging because chicks leave the crèche 4–7 days older than other chicks. Furthermore, early crèches are synchronous with those of other species breeding in the same area, thus perhaps diluting predation. We discuss the limitations of our analysis and the possible implications for the community of waterbirds breeding at our study site.
Birds exhibit a wide range of parental behaviours related to the mode of development of the young (Campbell et al. Citation1985). At the extremes of this range there are altricial species, e.g. passerines, in which newly hatched remain in the nest until fully grown, and precocial species, e.g. magapodes, in which the young leave the nest a few days after hatching and are independent from their parents. Within this spectrum, a particular parenting behaviour is the formation of a crèche, in which chicks of different pairs spend part of their development together. The formation of a crèche is observed in seven of the 14 colonial waterbird families (Evans Citation1980, Besnard Citation2001) and it is likely to have evolved as a response to different selective pressures mediated by habitat instability (Besnard Citation2001). The most commonly reported of these pressures is predation (Pettingill Citation1960, Schaller Citation1964, Carter & Hobson Citation1988, Besnard et al. Citation2002). The predation risk is higher for isolated chicks compared with those in the crèche (Tourenq et al. Citation1995, Le Bohec et al. Citation2005). For gulls, Besnard Citation(2001) reported that all crèching species occupy unstable habitats such as coastal lagoon or salt marshes. These habitats might change throughout the breeding season and those that were initially inaccessible to terrestrial predators might become connected to the mainland (Besnard et al. Citation2002). Crèching behaviour allows chicks to leave the breeding site while maintaining the colony structure and its ability to defend against predation (Besnard et al. Citation2002).
The present study aimed to describe the ecological framework in which this behaviour evolved and investigate possible differences across crèches of Slender-billed Gulls Larus genei. Slender-billed Gulls are the only Larid in Europe that exhibits crèching behaviour (del Hoyo et al. Citation1996). The species breeds in large, dense colonies (del Hoyo et al. Citation1996) in isolated localities from West Africa to western India (Cramp & Simmons Citation1983, Oro Citation2002, Isenmann et al. Citation2005, Chokri et al. Citation2008). It colonized the western part of the Mediterranean only after the second half of the 20th century (Sadoul et al. Citation1996). Data for the southern coast of the Mediterranean are scarce, but since 1982 the species has been breeding annually on the Sfax salina, in central-eastern Tunisia (34°39′N, 10°42′E), a site listed as a ‘wetland of international importance’ under the RAMSAR convention since 2007 (). The Sfax salina is at present the only site on the northern coast of Africa where Slender-billed Gulls breed regularly (Isenmann et al. Citation2005, Chokri et al. Citation2008) and it is at present the largest known population in the western Mediterranean (Chokri Citation2008, Chokri et al. Citation2008). We investigated whether chick apparent survival at the crèche changed across colonies. We predicted that chick mortality would be lower in larger crèches due to a dilution effect of predation (Williams Citation1996) and that it would be higher in late-formed crèches, as previous studies have shown that fledging success in Larids decreases as the breeding season progresses (Parsons Citation1975, Boersma & Ryder Citation1983). We investigated possible differences across crèches in (1) the apparent survival during the first week of crèche formation, when chicks are too young to leave the group, and (2) whether the rate at which survival decreased was consistent with a gradual, i.e. a constant rate of decline, or a synchronous departure of chicks.
METHODS
Data collection
The Sfax salina covers an area of about 1600 ha, formed by more than 200 ponds, separated by human-managed dykes, where salinity ranges from 39 to 300 g/l. The breeding population of Slender-billed Gulls is divided into several colonies on islets, vestiges of old dykes, and dykes (Chokri Citation2008). The breeding population varies slightly from year to year and may reach up to over 6000 pairs, with a maximum recorded size of 7924 breeding pairs in 2010. Breeding pairs lay clutches of two to three eggs synchronously during two main peaks of nesting activity: a first peak of early breeders in late April and a second peak in mid-May made of birds that had failed to lay or have lost their nests during the first peak (Chokri Citation2008). About 10 days after hatching, chicks group into a crèche which moves to safe places around the salina system (Chokri Citation2008). Older chicks begin to leave the crèche progressively and the group dissolves about 4–5 weeks after crèche formation. We marked chicks at three crèches about 12 days after crèche formation with a coloured PVC ring with a unique alphanumeric code. Two crèches, one in 2009 and one in 2010, were formed from early breeding birds (hereafter named ‘early crèches’) and one in 2010 was formed from late breeders (hereafter named ‘late crèche’). After marking, each crèche was surveyed every 3–4 days until the fledging of all chicks or until the crèche dissolved. Observations were made using a telescope early in the morning or in the evening (06:00–08:00 or 17:00–19:00 local time). Field work was carried out by the same observer for all three crèches.
Capture–recapture framework
Observations of each marked chick were coded into individual capture histories (Lebreton et al. Citation1992) in which, at each observation session, a ‘1’ code for detection and a ‘0’ for non-detection of each bird was recorded. Observation sessions were conducted at a different date for each crèche and with a different time interval between consecutive occasions within each crèche. We combined data into a single analysis to be able to model and compare survival across crèches by model selection procedures. To do this, data were coded into individual capture histories made of 17 occasions, in which the first six were filled with observations of birds in the first crèche, the subsequent six by those in the second crèche and the last five by those in the third crèche. Crèches were then treated as separate groups. In this way we were able to consider different parameters for each crèche and to account for the different length of the interval between consecutive occasions. The 16 unequal time-intervals between each pair of occasions were of 6, 3, 3, 4, 3, 3, 7, 4, 3, 3, 3, 3, 6, 4, 4, and 3 days, respectively. The two numbers in bold indicate the dummy intervals that were introduced to separate the different sets of occasions. On these occasions survival and recapture were fixed to 0. Data were analysed using a capture–recapture model for individual capture-histories using program mark (White & Burnham Citation1999). Model selection procedure was based on AICc values (Burnham & Anderson Citation1998) and the model with the lowest AICc value was considered the best compromise between model complexity and deviance explained. Survival, φ, and recapture probabilities, p, were modelled on a logit scale and estimated by maximum likelihood from the retained model. We modelled data by contrasting models with a crèche effect (3 levels, A, B, and C for the two early and the late crèche, respectively), an effect of occasion noted ‘t’ when used as a factor (5 levels) or ‘T’ when we assessed a linear trend (see below). Due to the small number of crèches compared (n = 3) we were not able to test these effects simultaneously (see Discussion) and we used model information theory to assess the importance of each effect separately. We adopted a notation similar to the one used by Tavecchia et al. Citation(2005) in which each type of parameter in the model is separated by the symbol ‘/’, a subscript indicates the colony, and the effects considered are noted in brackets. Hence, the model from which selection began that assumed survival and detection probabilities dependent on time and crèche will be notated φA(t)/φB(t)/φC(t)/pA(t)/pB(t)/pC(t). The goodness of fit of this model was assessed using the software u_care (Choquet et al. Citation2002). Finally, short-term survival tends to be close to the upper boundary value of 1 and parameter variance cannot be routinely estimated; when this happened the lower 95% confidence limit was calculated by profile likelihood, i.e. by fixing the parameter to progressively lower values until model deviance changed significantly (Tavecchia et al. Citation2009).
Modelling chick departure using a linear trend of survival
After the first week, the apparent survival of chicks is expected to increase as a result of birds progressively leaving the crèche. If a constant fraction of chicks leave the crèche on each occasion, the apparent survival after the first week is expected to be constant over time, of the form:
RESULTS
We marked 505 chicks from three crèches, 247 from the early crèche marked in 2010 (crèche A), 127 from the early crèche captured in 2010 (crèche B) and 131 from the 2010 late crèche (crèche C). The three crèches were formed by 1250, 168 and 283 chicks, respectively. The goodness-of-fit tests indicated that the general model φA(t)/φB(t)/φC(t)/pA(t)/pB(t)/pC(t) described the data adequately (χ2 7 = 11.53, p = 0.117). The average recapture probabilities for crèche A, B and C were 0.21, 0.44, and 0.49, respectively (from model 2), but they changed over time due to the different effort and the different temperature conditions during observation (model 2 versus model 1). A consequence of the sampling design was that each detection parameter corresponded to a different date and a different crèche (see Methods). Therefore, a model without the interaction between crèche and time in the probability of recapture was unrealistic. In contrast, we were able to fully model the probability of survival because, for example, the first parameter after marking corresponded to the first week in all groups. The daily survival probability over the first week from the general model φA(t)/φB(t)/φC(t)/pA(t)/pB(t)/pC(t) was 0.998, 0.987, and 0.999, for the two early and the late crèches (A, B, and C, respectively). In all crèches this value decreases over time for the progressive departure of chicks (). Indeed a model in which survival was changing as in equation 2 was preferred in each crèche, but the tipping point appeared to change across crèches (model 4–10). Although the change in deviance was small, the data were more consistent with τ = 1 with the exception of crèche A where τ = 3 ( & ). These results suggested that departures were synchronous after the first week from marking (about 3 weeks of age), but began a week later in the largest crèche, crèche A. An alternative hypothesis is that the decrease in survival in crèches B and C reflected a real mortality pattern rather than early chick departures. Finally, a further reduction in AICc value was obtained by setting the first survival in all crèches equal (model 14). This is equivalent to setting a communal intercept but a different slope for each crèche in equation 2. The communal daily survival estimated from model 14 was 0.988 (95% confidence limits: 0.981–0.995).
Figure 2. Estimates of survival for each recapture occasion according to crèche from a time-dependent model (model 1). Symbols: ▪ = crèche A (large and early formed colony), ▪ = crèche B (small and early formed colony), x = crèche C (small and late-formed colony).
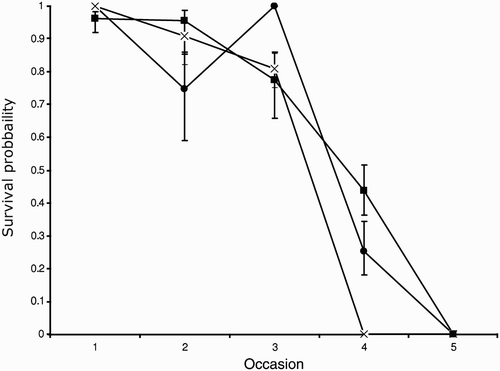
Figure 3. Change in model deviance as a function of the tipping point, τ. In these models, survival is constant until the occasion τ after which it is assumed to abate linearly. The tipping point, τ is >1 only in crèche A where τ = 3. Crèches A, B and C are indicated by symbols.
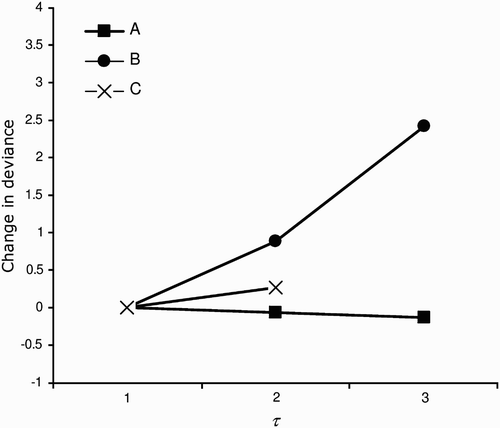
Figure 4. Apparent survival of chicks according to crèche in Sfax salinas. Crèches. A and B are the early crèches. Estimates are from the consensual model (model 11, ). Crèches A, B and C are indicated by symbols.
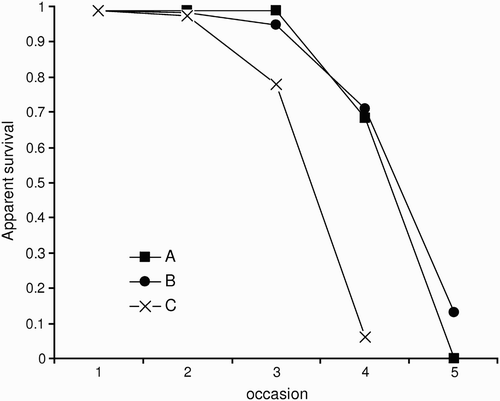
Table 1. Modelling survival, Φ, and recapture probability, p, of Slender-billed Gull chicks at three crèches. Model notation: t = time, Tτ = linear trend from occasion τ to k (τ = 1 corresponds to a progressive departure of chicks from week 1, τ > 1 corresponds to models for a synchronous departure).
DISCUSSION
In crèching species, such as Slender-billed Gulls, parental behaviour is thought to have evolved as a response to predatory pressure and habitat instability (Cramp & Simmons Citation1983, del Hoyo et al. Citation1996, Besnard Citation2001, Chokri Citation2008). In Sfax salina, we observed several predation attempts by stray dogs and Yellow-legged Gulls Larus michahellis on Slender-billed Gull chicks at the crèche. When a predator approached, chicks moved quickly to water and grouped to form a high density crèche. The same pattern was observed in crèches of Greater Flamingo Phoenicopterus roseus (Swift Citation1960, Salathé Citation1983, Tourenq et al. Citation1995). Because increasing numbers in the group allows earlier detection of approaching predators (Powell Citation1974, Lazarus Citation1979), large crèches should increase the probability that chicks will avoid predation. A direct prediction of the predation hypothesis is that chick survival should be higher in larger crèches. However, much of the information on Slender-billed Gull ecology comes from the small populations at the margin of the geographical distribution of this species (in France: Isenmann Citation1976, Sadoul Citation1996, Besnard Citation2001 and in Spain: Dies & Dies Citation2000, Oro Citation2002) and a potential effect of crèche size can distort results on chick survival. Sfax salina is the only site in the western Mediterranean region where the population size of Slender-billed Gulls can exceed 6000 breeding pairs divided between three or four colonies of different sizes and with different laying dates (Chokri Citation2008). Our study is based only on three crèches and at present we are unable to contrast fully the effect of colony size on chick survival. However, although crèches differed markedly in size, we did not find any difference in chick survival across crèches during the first week. In contrast, we found that crèches differed in their duration and in the way that they dissolved. Chicks appeared to stay longer in the largest crèche from which they departed synchronously after approximately 2 weeks; survival began to abate earlier in the two smaller crèches. Additionally, the rate at which chicks departed was higher for the late-formed crèche, crèche C ( & 4). However, the small sample size (three crèches) is obviously not sufficient to fully test a size-dependent effect or an effect of the laying date. Nevertheless, this result is in agreement with previous studies that have shown a benefit of large versus small colonies in breeding success (Chokri Citation2008). Indeed, chicks fledge only from colonies which contained at least 100 nests and chick productivity increases with colony size (Chokri Citation2008). Moreover, earlier colonies were characterized by larger clutch size (modal clutch size = 3 eggs in early colonies and 2 in late colonies; Chokri Citation2008). The benefit of breeding in large colonies is not reflected in the overall survival of chicks in the crèche during the first week, which is independent of colony size, but it seems to be reflected in the longer time that chicks stay in the crèche. It is likely that chicks in long-lasting and larger crèches will have a higher survival after fledging, since they leave the protection of the crèche older and larger than chicks in smaller or late-formed crèches (Tourenq et al. Citation1995, Le Bohec et al. Citation2005). In our case this is only a speculation, but early colonies of Slender-billed Gulls are larger than late ones and begin breeding synchronously with other ground-nesting colonial waterbird species present in Sfax salina, including Pied Avocets Recurvirostra avocetta, Little Terns Sterna albifrons, Common Terns Sterna hirundo, and Gull-billed Terns Sterna nilotica (Chokri Citation2008). Early colonies and early-formed crèches might benefit from the dilution of predation across all species. In this respect we think that the study of the costs and benefits of the crèching strategy could also be approached at the community level rather than within a single species. This is in agreement with the hypothesis that crèching behaviour has been shaped by the selective pressure of predation in unpredictable habitats (Besnard Citation2001) which is common to all crèching species.
Limitations of the analysis
In capture–recapture models survival probability is confounded by permanent emigration, so that the progressive disappearance of chicks from the crèche would generate an increase in apparent mortality. Here we assumed that mortality during the first week, when chicks are too young to leave the crèche, can be interpreted as real mortality, while after this period, it should be viewed as an indication of the rate at which crèches dissolve. We believe this is a realistic assumption as chicks were marked at about 2 weeks from hatching and fledging is not expected before weeks 3 to 4. A first limitation in our analysis is that survival refers to a short interval (3 days). In this case, the estimates will be close to 1.0 and might generate problems in calculating parameter variance and comparing estimates across groups. However, when we detected a parameter at the boundary, the lower 95% confidence limit was calculated by profile likelihood, i.e. by fixing the parameter to progressively lower values until model deviance changed significantly (Tavecchia et al. Citation2009). A second limitation of our analysis is that we collected data from three crèches only. As a consequence, a possible relationship between colony size and chick survival cannot be fully tested. Model information theory suggested a possible positive effect of colony size and/or a negative effect of the laying date on the duration of the crèche, but results are not conclusive. Long-term data are required to investigate further how colony size and laying date influence post-fledging survival rather survival during the crèche period only. In addition, further research should consider quantifying mortality rate before the crèching stage and the pattern of crèche formation.
ACKNOWLEDGEMENTS
We are grateful to Alejandro Martinez-Abrain, Albert Fernandez, Ana Sanz Aguilar (from IMEDEA, CSIC-UIB, Esporles, Spain), Lassad Dassi (from Gabès University, Tunisia), Thomas Blanchon and Yves Kayser (from The laboratory of Tour du Valat, Camargue, France) for their help in the field. Aurelien Besnard provided very useful comments on the manuscript. The study has been funded by the A.E.C.I.D. (project number: A/019893/09) and supported by project BFU2009-09359 of the Spanish Minister of Research.
REFERENCES
- Besnard , A. 2001 . Evolution de l'élevage des poussins en crèche chez les laridés [Evolution of crèching behaviour in larids] , Montpellier : University of Montpellier II .
- Besnard , A. , Gimenez , O. and Lebreton , J. D. 2002 . A model for the evolution of crèching behaviour in gulls . Evol. Ecol. , 16 : 489 – 503 .
- Boersma , D. and Ryder , J. P. 1983 . Reproductive performance and body condition of earlier and later nesting Ring-billed Gulls . J. Field Ornithol. , 54 : 374 – 380 .
- Burnham , K. P. and Anderson , D. R. 1998 . Model Selection and Inference: A Practical Information-Theoretic Approach , New York, NY : Springer .
- Campbell , B. , Lack , E. and British Ornithologists' Union . 1985 . A Dictionary of Birds , Shipman, VA : Published for the British Ornithologists' Union by Buteo Books .
- Carter , H. R. and Hobson , K. A. 1988 . Creching behavior of Brandt's Cormorant chicks . Condor , 90 : 395 – 400 .
- Chokri, M.A. 2008. Importance de l'Environnement du Salin de Sfax, Tunisie, pour la Reproduction des Oiseaux d'Eau Coloniaux. PhD thesis, Carthage University, Faculté des Sciences de Bizerte.
- Chokri , M. A. , Sadoul , N. , Medhioub , K. and Bechet , A. 2008 . Analyse comparée de la richesse avifaunistique du salin de Sfax dans le contexte tunisien et méditerranéen . Rev. Ecol-Terre Vie , 63 : 53 – 72 .
- Choquet , R. , Reboulet , A. , Lebreton , J. D. , Gimenez , O. and Pradel , R. 2002 . U_Care2.2: User's Manual . Available online at: http://ftp.cefe.cnrs.fr/biom/Soft-CR/. CEFE-CNRS
- Cramp, S. & Simmons, K.L. (eds). 1983. The Birds of the Western Paleartic. Vol. III. Oxford University Press, Oxford.
- del Hoyo , J. , Elliot , A. and Argatal , J. 1996 . Handbook of the Birds of the World , Barcelona : Lynx Edicions .
- Dies , J. I. and Dies , B. 2000 . Breeding parameters of the Slender-billed Gull (Larus genei) in a new colony located at l'Albufera de Valencia (E Spain) . Ardeola , 47 : 255 – 258 .
- Evans , R. M. 1980 . “ Development of behavior: an ecological perspective ” . In Behavior of Marine Animals, Vol. 4: Marine Birds , Edited by: Burger , B. L.O. and Winn , H. E. New York, NY : Plenum Press .
- Isenmann , P. 1976 . Contribution a l'étude de la biologie de la reproduction et de l'etho-ecologie du Goéland railleur . Larus genei. Ardea , 64 : 48 – 61 .
- Isenmann , P. , Thierry , G. , Hili , A. , Azafzaf , H. , Dlensi , H. and Smart , M. 2005 . Oiseaux de Tunisie – Birds of Tunisia , Paris : Société d'Études Ornithologiques de France .
- Lazarus , J. 1979 . The early warning function of flocking in birds: an experimental study with captive Quelea . Anim. Behav. , 27 : 855 – 865 .
- Le Bohec , C. , Gauthier-Clerc , M. and Le Maho , Y. 2005 . The adaptive significance of crèches in the king penguin . Anim. Behav. , 70 : 527 – 538 .
- Lebreton , J. D. , Burnham , K. P. , Clobert , J. and Anderson , D. R. 1992 . Modeling survival and testing biological hypotheses using marked animals: case studies and recent advances . Ecol. Monog. , 62 : 67 – 118 .
- Oro , D. 2002 . Breeding biology and population dynamics of Slender-billed Gulls at the Ebro delta (north-western Mediterranean) . Waterbird , 25 : 67 – 77 .
- Parsons , J. 1975 . Seasonal variation in the breeding success of the Herring Gull: an experimental approach to prefledging success . J. Anim. Ecol. , 44 : 553 – 573 .
- Pettingill , J. O.S. 1960 . Crèche behavior and individual recognition in colony of Rockhopper penguins . Wilson Bull. , 72 : 213 – 221 .
- Powell , G. V.N. 1974 . Experimental analysis of the social value of flocking by starlings (Sturnus vulgaris) in relation to predation and foraging . Anim. Behav. , 22 : 501 – 505 .
- Sadoul , N. 1996 . Dynamique spatiale et temporelle des colonies de charadriiformes dans les salins de Camargue: implication pour la conservation , Montpellier : Université Montpellier II .
- Sadoul , N. , Johnson , A. , Walmsley , J. G. and Leveque , R. 1996 . Changes in the numbers and the distribution of colonial Charadriiformes breeding in the Camargue, Southern France . Colon. Waterbird , 19 : 46 – 58 .
- Salathé , T. 1983 . La predation du Flamant rose, Phoenicopterus ruber roseus, par le Goéland leucophee, Larus cachinnans, en Camargue . Rev. Ecol-Terre Vie , 37 : 87 – 115 .
- Schaller , G. B. 1964 . Breeding behavior of the White Pelican at Yellowstone Lake, Wyoming . Condor , 66 : 2 – 23 .
- Swift , J. J. 1960 . Densité des nids et notion de territoire chez le flamant de Camargue . Alauda , 28 : 1 – 14 .
- Tavecchia , G. , Coulson , T. , Morgan , B. J.T. , Pemberton , J. M. , Pilkington , J. C. , Gulland , F. M.D. and Clutton-Brock , T. H. 2005 . Predictors of reproductive cost in female Soay sheep . J. Ecol. , 74 : 201 – 213 .
- Tavecchia , G. , Viedma , C. , Martínez-Abraín , A. , Bartolomé , M. , Gómez , J. A. and Oro , D. 2009 . Maximizing re-introduction success: assessing the immediate cost of release in a threatened waterfowl . Biol. Conserv. , 142 : 3005 – 3012 .
- Tourenq , C. , Johnson , A. R. and Gallo , A. 1995 . Adult aggressiveness and crèching behavior in the greater flamingo . Phoenicopterus ruber roseus. Colon. Waterbird , 18 : 216 – 221 .
- White , G. C. and Burnham , K. P. 1999 . Program MARK: survival estimation from populations of marked animals . Bird Study , 46 : 120 – 129 .
- Williams , G. C. 1996 . Adaptation and Natural Selection: A Critique of some Current Evolutionary Thought , Princeton, NJ : Princeton University Press .