Abstract
Capsule Shags move between breeding and non-breeding areas and this is associated with a significant change in diet.
Aims To determine whether the diet of Shags nesting on islets off the Croatian coast is the same as their diet after the post-breeding move to the Gulf of Trieste.
Methods Diet was determined by the analysis of 611 regurgitated food pellets.
Results A total of 23 988 prey items were identified in the sample of pellets. Post-breeding Shags in the Gulf of Trieste focused on demersal and relatively immobile Gobiidae (81.5% by number, 87.1% by biomass). The most frequent prey species was Gobius niger (70.8% by number). In the breeding season at Oruda island, Croatia, the diet was more varied. Breeding Shags fed on bentho-pelagic, mobile prey such as Atherina boyeri (28.4% in frequency), Serranus hepatus (16.1%) and Crenilabrus tinca (12.0%), while Gobiidae had a dietary frequency of only 18.1%. With respect to biomass the most important prey were Crenilabrus tinca (19.0%) and Serranus hepatus (18.4%).
Conclusion We suggest that the movement of Shags within the Adriatic Sea is driven by dietary requirements. Most previous studies of Shag diet have shown that Shags tend to have a more specialized diet during the breeding season, concentrating upon demersal prey species. However, we have found that birds breeding at the Croatian study colony show dietary diversity. We suggest that lack of dietary specialization is a facultative response to local prey abundance, and is probably the result of over-fishing of demersal species in the areas around the breeding locations in which the birds find suitable sites and are little disturbed by human activity. Shags may move immediately after breeding to the Gulf of Trieste because demersal species are likely to be more abundant there. As a consequence, the diet becomes more specialized and is then more similar to the diet of other populations of Shags.
Seabirds are key players at the top of marine food chains and respond predictably to alterations in prey abundance. They have therefore been used as reliable indicators of changes in fish populations (Hatch & Sanger Citation1992, Montevecchi Citation1993, Furness & Camphuysen Citation1997, Barrett Citation2002). Changes in a variety of seabird foraging parameters have been successfully used to detect alterations in prey age–class structure (Davoren & Montevecchi Citation2003), responses of prey populations to climate change (Miller & Sydeman Citation2004), and variations in the energetic value of prey (Wanless et al. Citation2005). Furthermore, seabirds' dietary adjustments may indicate shifts in pelagic food webs (Montevecchi & Myers Citation1996).
European Shags Phalacrocorax aristotelis are foot-propelled diving seabirds (Ashmole Citation1971), whose diet has been used to assess the recruitment and abundance of fish populations (Barrett et al. Citation1990, Barrett Citation1991). In Iceland the diet of this species was used as a measurement of the recruitment in commercially important fish species such as Saithe Pollachius virens and Plaice Pleuronectes platessa (Lilliendahl & Solmundsson Citation2006). Shag diet varies depending on annual changes in prey availability (Carss Citation1993) and, to a lesser extent, on location. The species feeds on a wide range of benthic, demersal and schooling pelagic fish, and for this reason it is classified as opportunistic in its feeding habits (Barrett Citation1991, Grémillet et al. Citation1998, Velando & Freire Citation1999). Foraging areas have depths ranging between 7 and 80 m (Guyot Citation1988, Barrett & Furness Citation1990, Wanless et al. Citation1991a, Citation1993a), with a mean of 30 m (Wanless et al. Citation1997a), and are usually between 7 and 17 km around the breeding colonies (Wanless et al. Citation1991b). The average daily food consumption of a Shag is between 16 and 24% of body mass (Barrett et al. Citation1990). During incubation, Shags are estimated to have an average daily requirement of 389 g of fish, while a bird with three chicks requires about 920 g (Wanless et al. Citation1993b). The diet of the species has been studied as early as the first half of the last century (Steven Citation1933), mainly in north-western Europe. In this area the Atlantic subspecies P. aristotelis aristotelis is distributed from Iceland and Northern Scandinavia to the Iberian Peninsula (Nelson Citation2005). The analysis of dispersal patterns suggests that Iberian Shags are isolated from northern populations (Velando Citation1997, Velando & Freire Citation1999). Sandeels Ammodytes spp. dominate the diet of P. aristotelis aristotelis, both in summer (Barrett et al. Citation1986, Grémillet et al. Citation1998, Furness & Tasker Citation2000) and in other seasons (Harris & Wanless Citation1991). Exceptions to Sandeel dominance were found in Norway, where Gadoids (Gadidae) were the most important prey (Barrett et al. Citation1990). In Iceland Shags rely heavily on Lesser Sandeels Ammodytes marinus during the breeding season, whereas Bull-rout Myoxocephalus scorpius and Gadoids become increasingly important in autumn and winter (Lilliendahl & Solmundsson Citation2006). Sandeels are also the dominant prey on the Galician coast of north-west Spain (Velando & Freire Citation1999). In this area some seasonal differences in diet have been found; in February and March Shags foraged on Gobiidae and Sand Smelts Atherina presbyter and this dietary change may be due to the seasonal changes in the abundance of Sandeel schools. In northern Spain breeding Shags fed on Labridae and Atherinidae (Alvarez Citation1998).
Even though the Atlantic subspecies is well studied, very little is known of the diet, general biology and population of the Mediterranean subspecies P. aristotelis desmarestii, which is endemic to the Mediterranean and Black Seas. The breeding range includes most States along the Mediterranean coast (Croatia, Albania, Bulgaria, Ukraine, Turkey, Cyprus, Egypt, Libya, Tunisia and Algeria). The total population has been estimated at less than 10 000 pairs, half of them breeding within the eastern coast of Spain, Baleares, Corsica, Sardinia, the Tuscany archipelago, Lampedusa, Crete and the islets of the Ionian Sea. Substantial year-to-year fluctuations in breeding numbers have been reported in several colonies, and there appears to be an overall decrease (Aguilar & Fernandez Citation2002). This occurs mainly because Mediterranean Shags are severely affected by a number of limiting factors, including human disturbance (Guyot Citation1993), chemical pollution (Lambertini & Leonzio Citation1986), oil spills (Velando et al. Citation2005) and accidental catch (Aguilar Citation1991, Velando & Freire Citation2002). The subspecies is consequently listed in Annex I of the Birds Directive 147/2009 and is the focus of an Action Plan (Aguilar & Fernandez Citation2002). To our knowledge, the available data for the diet of the species in the whole Mediterranean rely on the study of Guyot Citation(1988) on the Corsican population, which suggested that Labridae and Ammodytidae were the most important prey. However, a small sample of chick regurgitations from some Sardinian breeding colonies showed the presence of a wider range of species in the diet, including Crenilabrus sp., Mullus surmuletus, Coris julis, Mugil sp., Serranus sp., Sardina pilchardus and Boops salpa (Brichetti et al. Citation1992).
In the Adriatic Sea, Mediterranean Shags breed in Croatia (2000 pairs) and Albania (5–10 pairs) (Aguilar & Fernandez Citation2002). From the 1980s onward, the species has become a regular summer visitor in the Gulf of Trieste, north east Italy, with increasing numbers (Utmar Citation1999). The maximum size of the population approached 2500 individuals in July 2007. This population comes mainly from the Croatian breeding colonies (Sponza et al. Citation2010). Because of the high sensitivity of Shags to human disturbance during breeding (Guyot Citation1993), the Gulf of Trieste does not offer suitable and low disturbed places for nesting, but these characteristics are to be found within the Croatian islets. Given Mediterranean Shags' conservation status and the very few studies on this subspecies, we have determined the diet during the breeding season in Croatia and the post-breeding period in the Gulf of Trieste. We use this dietary information to suggest reasons why Shags breed in Croatia but spend the post-breeding period in another area of the Adriatic Sea.
METHODS
Study area
We studied the diet of Mediterranean Shags during the breeding season (January–April) at Oruda island (Lošinj archipelago) (44°33′N, 14°30′E), one of the most important Croatian colonies in the upper Adriatic Sea. In this rocky, low-vegetated island, covering an area of 36 ha, about 100 breeding pairs nest inside bushes of Paliurus spina-Christi and Crataegus monogyna near the sea. The diet of the species in the post-breeding period (May–October) was studied in the Gulf of Trieste (45°39′N, 13°46′E). The two study areas have very different sea bottom depth profiles. The Gulf of Trieste is 20 m deep within 1 km of the coastline and deepest at 25 m. The Lošinj archipelago is characterized by a steep, sloping sea bed. The eastern part of the archipelago is 40–45 m deep within a few metres of the coastline and reaches a maximum depth of 80 m. Conversely, the western side is characterized by shallower depths (20–25 m).
Diet analysis
Diet was characterized by pellet analysis, a non-invasive method that can provide large samples over time. Dietary data were described in a preliminary way by Sponza et al. Citation(2010), in which the key prey were identified by pellet analysis and linked with diving strategies, and this in turn served to characterize the different foraging habitats and benefits of dives. In this paper the entire data set is used for a comprehensive analysis of the diet of Mediterranean Shags during the breeding and post-breeding seasons.
Pellet collection involves little or no disturbance to birds and analyses require minimal laboratory facilities (Carss et al. Citation1997). This method has been widely used to characterize the diet composition of Cormorants, Shags, Gulls, Terns and Skuas (reviewed in Barrett et al. Citation2007) and to estimate body sizes and biomasses of the prey eaten (Duffy & Jackson Citation1986, Dirksen et al. Citation1995, Velando & Freire Citation1999, Eschbaum et al. Citation2003, Gagliardi et al. Citation2007, Barquete et al. Citation2008). However, the analysis of pellets has to consider the partial erosion of otoliths caused by the digestive process, so fish lengths derived from otoliths could be under-estimates (Johnstone et al. Citation1990, Carss et al. 1997). Moreover, the smallest otoliths (i.e. belonging to very small fish) could be completely digested (Johnstone et al. Citation1990) and otolith wear can vary from bird to bird or from time to time, so the validity of the technique will vary with the species. For example, it was shown that Gadidae, unlike Ammodytidae, have very robust otoliths (Barrett et al. Citation1990). However, the potential degree of otolith erosion for most of the North Adriatic fish species has not yet been characterized. Unlike some invasive method (Duffy & Jackson Citation1986, Carss et al. 1997), pellet analysis has been recognized as an appropriate method for the temporal and spatial comparison of the diet of Shags (Duffy & Laurenson Citation1983, Barrett et al. Citation1990, Barrett Citation1991), and it is to this purpose that we use data based upon these techniques in this article.
In the Gulf of Trieste, during the 2005 post-breeding period (May–October), we collected 23–30 pellets per month at each of the three roosts, except for May at the beginning of the season (14–15 pellets) (). The three roosts are: Isonzo river mouth (45°43′N, 13°33′E), Filtri (45°44′N, 13°39′E) and Lazzaretto (45°36′N, 13°42′E). At the first site Shags roost on beached, dead trees and sand banks, while in the latter two sites, birds rest on floating platforms used for mussel farming. We used a canoe to reach sand banks, beached trunks, and floating platforms, minimizing disturbance to birds. We analysed a total of 486 items. In Croatia, during the 2006 breeding season (January–April) we analysed a total of 125 pellets ( ), collected monthly near the nests at the Oruda colony. During the monthly visits we observed adult birds in breeding plumage only. All samples were fresh and showed no signs of deterioration. Upon collection, all specimens were individually sealed in a plastic bag and tagged with an identification number and then stored and frozen until processing. The probability of analysing pellets regurgitated by the same individual was negligible, given the high numbers of Shags present in the two study areas, and given that each collection was carried out in different sites and periods.
Table 1. Dietary composition of Mediterranean Shags Phalacrocorax aristotelis desmarestii in the Gulf of Trieste during the 2005 post-breeding season, expressed as numerical frequency (%N), biomass (%B) and frequency of occurrence (%O) percentage values.
Table 2. Dietary composition of Mediterranean Shags Phalacrocorax aristotelis desmarestii at the breeding colony at Oruda, Croatia, during the 2006 breeding season, expressed as numerical frequency (%N), biomass (%B) and frequency of occurrence (%O) percentage values.
Table 3. Descriptive analysis of prey size (fish length, cm) in the diet of Mediterranean Shags Phalacrocorax aristotelis desmarestii in the Gulf of Trieste and at the breeding colony at Oruda, Croatia.
We processed all pellets in the laboratory following a dissolution method described by Privileggi Citation(2003). Each pellet was individually stored in a beaker (50 ml), soaked in a detergent solution, and then left to settle for about 20 hours. After the mucous had been dissolved, Isopod parasites and Nematode worms were removed and, after decanting, otoliths and any skeletal structures useful for prey identification were separated out. We used a filter in order not to lose the smallest otoliths during the outflow. To remove any mucous residue and to bleach bones, each sample was treated with a solution of sodium hydroxide (NaOH, 10%) and sodium hypochlorite (NaClO, 50%). This method ensured the conservation of the smallest bony fragments during the mucous dissolution. We then rinsed all samples with water and air dried them at room temperature before storage and examination. Where possible, we paired otoliths. We also accurately separated and identified (according to our own reference collection and to Härkönen Citation1986, Keller Citation1993, Veldkamp Citation1995) otoliths, scales, opercula and vertebrae to the lowest taxonomic level, by using a stereomicroscope with an ocular micrometer (6–32× zoom lens). The reference collection of diagnostic bones (Privileggi Citation2003) includes 79 marine (1107 individuals) and 45 freshwater fish species (1465 individuals) of different size classes. We evaluated the total length and mass of each prey by means of both original (Privileggi Citation2003) and published (Keller Citation1993, Volponi Citation1994, Veldkamp Citation1995) equations that relate otoliths length to the body size and the mass of fish. We produced an equation for each fish species, except Cepola rubescens, Belone belone, Gobius bucchichii and Sphyraena sphyraena, which were excluded from this analysis. While analysing eroded otoliths, particular attention was paid to the comparison of the morphological characters of outline, sulcus, rostrum and ostium with the reference collection, in order to estimate and adjust the size of the otoliths.
The level of taxonomic resolution for identification was different for some prey types. In this paper we treated the family Mugilidae as one category, as the species that make up this family are difficult to identify because of the extreme similarity of the otoliths. Moreover, the family Maenidae is represented essentially by Maena maena and Maena chryselis, whereas many families are represented by one species only (i.e. Atherinidae by Atherina boyeri, Labridae by Crenilabrus tinca, Serranidae by Serranus hepatus) ( & 2).
Each prey type in the diet was estimated as numerical frequency (N), biomass (B), and frequency of occurrence (O, number of samples containing each prey type). Contingency tables tested by chi-square test (χ 2) were used to compare the numerical frequencies (not percentages) of different prey types between the two study areas and the monthly differences within each area. For this purpose, we grouped the different prey species by family. Families representing less than 1% at both study areas were categorized as ‘Other species’. Other statistical differences were assessed with Mann–Whitney U test and Spearman's rank correlation.
As regards prey size, we report the descriptive analysis only. Consequently we do not compare the two study areas and the different months within each study area, in order not to go beyond the limits of the pellet analysis method.
The significance threshold was set at P < 0.05 and the analysis was performed using spss 13.0 and statistica 7.1 software.
RESULTS
Analysis of 611 pellets revealed 23 988 identified prey. Of these, 20 716 belonged to 31 taxa in the Gulf of Trieste, whereas 3272 belonged to 26 taxa in Croatia ( & 2). In the Gulf of Trieste, Gobiidae were the focal prey (81.5%N). The most captured species was the Black Goby Gobius niger (70.8%N), which occurred (%O) in 99.2% of the pellets analysed. The second in importance (11.9%N) were Atherinidae (i.e. Atherina boyeri) (). Conversely, in Croatia, Atherinidae were the most important prey (28.4%N), followed by Gobiidae (18.1%N), Serranidae (16.1%N) (i.e. Serranus hepatus), Labridae (12.0%N) (i.e. Crenilabrus tinca), and Sparidae (12.6%N) (, ).
Figure 1. Numerical frequency (%N) and biomass (%B) percentages of prey in the diet of Mediterranean Shags Phalacrocorax aristotelis desmarestii in the Gulf of Trieste and at Oruda breeding colony, Croatia. Fish families are listed in the key.
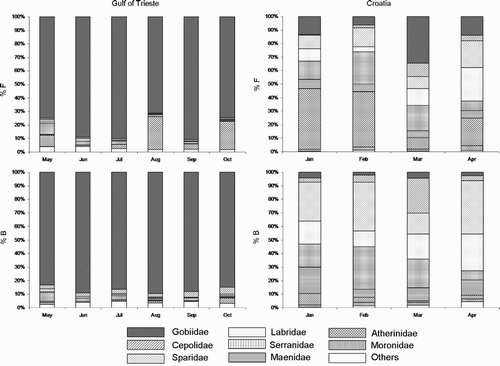
Diet composition was significantly different between the two study areas (χ 2 = 8894.5, df = 8, P < 0.0001) (). The main contributions to the total Chi-square were given by 5 (Sparidae, Gobiidae, Serranidae, Labridae, Maenidae) over nine categories. However, the main contributions were first due to the high consumption of Sparidae (family contribution/total contribution = 1860.7/8894.5) and then to the low consumption of Gobiidae (family contribution/total contribution 1346.3/8894.5) in Croatia with respect to the Gulf of Trieste. In this latter area, the differences between months were significant (χ 2 = 2463.9, df = 40, P < 0.0001). In the context of a huge exploitation of Gobiidae, both by number and biomass (, ), the monthly differences in frequency were principally due to Atherinidae during the whole period (family contribution/total contribution = 1649.8/2463.9). Such differences were particularly evident in August, when we recorded at the same time the lowest frequencies (with respect to the expected values) for Gobiidae (monthly family contribution/monthly total contribution = 60.5/692.9) and the highest for Atherinidae (monthly family contribution/monthly total contribution = 595.5/692.9). A high consumption of Serranidae was recorded in May (monthly family contribution/monthly total contribution = 263.2/385.9).
In Croatia, the differences between months were significant (χ2 = 879.8, df = 24, P < 0.0001). Atherinidae (family contribution/total contribution = 280.9/879.8), Gobiidae (family contribution/total contribution = 223.2/879.8), and Labridae (family contribution/total contribution = 135.5/879.8) contributed the most to the differences. The highest contribution was due to March, when we observed an increase in the consumption of Gobiidae (monthly family contribution/monthly total contribution = 146.3/371.1) and a concurrent decrease of Atherinidae (monthly family contribution/monthly total contribution = 137.8/371.1).
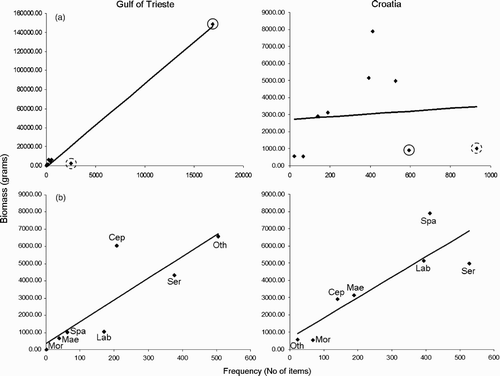
In order to assess a possible relationship between frequency and biomass values, we correlated the overall data for the two study areas (). We found a significant correlation in the Gulf of Trieste only (Spearman's rank correlation: N Gulf of Trieste = 9, r s = 0.88, P < 0.01; N Croatia = 9, r s = 0.35, P = 0.36). However, at both sites, we showed two atypical points of the two more abundant taxa (circles in , that are Gobiidae and Atherinidae. In Croatia, the effect of both values lead to high frequency and low biomass values. In the Gulf of Trieste this trend was confirmed by Atherinidae, while a high frequency of Gobiidae corresponded to high biomass values. If we removed these exceptions, the two regressions became similar and overlapping (Spearman's rank correlation: N Gulf of Trieste = 7, r s = 0.96, P < 0.001; N Croatia = 9, r s = 0.86, P < 0.02) (.
Figure 2. (a) Frequency/biomass correlations in the two study areas. Black points indicate values for each one of the nine fish families. Black circles indicate the value for Gobiidae, while dashed circles indicate Atherinidae. (b) Frequency/biomass correlations with Gobiidae and Atherinidae values removed (Cep, Cepolidae; Lab, Labridae; Mae, Maenidae; Mor, Moronidae; Oth, Other species; Ser, Serranidae; Spa, Sparidae).
Considering the ecology of prey (benthic, bentho-pelagic and pelagic), we found significant differences between the two areas (χ 2 = 7213.2, df = 2, P < 0.0001). In the Gulf of Trieste, Shags strongly preferred benthic fishes, both in terms of frequency (83.0%) and biomass (91.2%), whereas in Croatia there was a higher consumption of bentho-pelagic prey (frequency 46.4%, biomass 78.0%). In fact the main contributions to the total Chi-square were due to the low frequency of benthic (category contribution/total contribution = 1194.1/7213.2) and a high consumption of bentho-pelagic prey (category contribution/total contribution = 4526.6/7213.2) in Croatia.
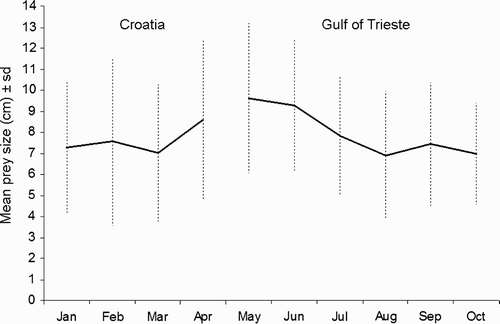
We determined a prey size mode of 6 cm in the Gulf of Trieste, whereas in Croatia we recorded two modal sizes, at 4 and 8 cm. The larger prey sizes are recorded in May and June. Moreover, prey lengths decreased significantly within the season (Spearman's rank correlation: n = 6, r s = – 0.83, P < 0.05) ().
DISCUSSION
European Shags are opportunistic predators, and the variability in composition of their diet in different locations is related to geographical differences in the available potential prey (Barrett Citation1991, Velando & Freire Citation1999). Moreover, the species has a high flexibility of feeding grounds, both in terms of diving depths and bottom sediment characteristics (Guyot Citation1988, Wanless et al. Citation1991b, Velando & Freire Citation1999). Our study supports this evidence. Recognizing the limitations of the pellet analyses used, we highlight a wide prey spectrum, mostly composed of fish species that are found at a diversity of depths and in different habitats, including pelagic prey (such as Atherina boyeri), bentho-pelagic fishes from sandy and rocky bottoms (such as Pagellus erythrinus and Serranus hepatus) and demersal species from sandy-mud bottoms (such as the family Gobiidae). We never observed Shags following fishing trawlers nor feeding on by-catch discards. As also reported by Oro & Ruiz Citation(1997) and Arcos et al. Citation(2002), we consider this behaviour as very occasional in our study population.
Are Shags really opportunistic predators?
Although Shags have flexible feeding habits that allow them to exploit both benthic and pelagic resources (Grémillet et al. Citation1998), they should be considered as predominantly benthic feeders (Cramp & Simmons Citation1977, Barrett et al. Citation1990, Wanless et al. Citation1991a, Citation1993a, Citation1998, Watanuki et al. Citation2008). Diet analyses in most of Europe actually show that Shags rely on Sandeels (Ammodytes spp.) (Barrett et al. Citation1986, Harris & Wanless Citation1991, Citation1993, Grémillet et al. Citation1998, Velando & Freire Citation1999, Furness & Tasker Citation2000, Lilliendahl & Solmundsson Citation2006). Even if Sandeels sometimes form schools within the water column (Jonsson Citation1992), Shags catch these prey on or near the bottom (Harris & Wanless Citation1991, Wanless et al. Citation1991a, Citation1997b). We confirm the importance of the sea bed for Mediterranean Shags, by showing that these birds exploit prey in the Gulf of Trieste which are essentially benthic. Similarly, in Croatia, Shags forage mostly (69.0% by number) on benthic and bentho-pelagic fishes. Moreover, we emphasize that, from May to October, the period with the larger presence of Shags (Sponza et al. Citation2010), the Gulf of Trieste is one of the most important Adriatic areas for the abundance of pelagic ephemeral species such as Engraulis encrasicolus, Sardina pilchardus and Scomber scomber. In late spring, these species leave the central Adriatic areas and reach the Gulf of Trieste's shallow waters for spawning (Skrivanic & Zavodnik Citation1973, Orel & Zamboni Citation2004). While they represent 74% of the north Adriatic fish landings (Prestamburgo et al. Citation2005), these fishes represent less than 1% and 0.5% by number of Shags' diet in the Gulf of Trieste and Croatia, respectively. We therefore suggest that Shags aim at diving towards the sea bed to exploit mainly benthic, but also bentho-pelagic, food resources, and do not take advantage of abundant pelagic prey.
Diet specialization
Sandeels play a key role in the diet of Shags in most European waters and are the basis of a feeding specialization during the chick rearing period (Harris & Wanless Citation1993, Velando & Freire Citation1999). This specialization is probably linked to the high energetic value and the abundance of these fishes, which also represent the focal prey of many seabird species (Rindorf et al. Citation2000). Contrary to what has been found in most studies, we did not find any prey specialization during the breeding season. Rather, in Croatia, the prey spectrum is particularly wide, as we recorded the prevailing importance of five families (Sparidae, Gobiidae, Serranidae, Labridae, Maenidae).
The role of Gobiidae
Feeding specialization is conversely recorded during the post-breeding period. In the Gulf of Trieste, Shags indeed focused on demersal Gobiidae, both in terms of frequency (81.5%) and biomass (87.1%). We report slight exceptions to such dominance in August and October, when there was a decrease of Gobiidae and an increase in Atherinidae. The species belonging to both families are sedentary and perform short movements (Riedl Citation1991). Gobiidae are strictly demersal and poor swimmers (Louisy Citation2005), whereas Atherinidae are confined in estuaries and coastal lagoons (Louisy Citation2005). They aggregate in schools which lay at different depths (Riedl Citation1991). Given the role of Gobiidae, the possible temporary differences in the availability of these demersal prey could lead Shags to forage in the upper layers of the water column as well, thus exploiting other fish. Similarly, Lilliendahl & Solmundsson Citation(2006) noted momentary changes in Shags' diet as a consequence of possible variations in the availability of their focal prey (Sandeels). Shags also shift between Gobiidae and Atherinidae in Croatia during March. The significant increase of Gobiidae could be linked to the Shags' efforts to focus on these demersal prey. Since the April data do not substantiate a further increment, this pattern could be simply linked to a temporary accessibility of these fishes. The likely non-prevalence of a fish species over another could lead Croatian Shags to exploit the currently available prey, which would bring about an increase in dietary diversity. This is particularly evident in March and April, which is at the peak of chick rearing (Sponza et al. Citation2010). It is important to stress that Lilliendahl & Solmundsson Citation(2006) recorded a high dietary overlap between nestlings and adult Shags in Iceland. Moreover, Velando & Freire Citation(1999), even if suggesting some differences in diet between nestlings and adults in Spain, showed that chicks were fed almost exclusively with Sandeels. In May and June, the main chick rearing period in Spain, Sandeels represented more than 80% by number of adults' diet. We hence maintain that Shags' dietary variability in Croatia reflected nestlings' diet.
The importance of Gobiidae can also be inferred from the analysis of frequency/biomass ratio, which highlights how these prey are a discriminating factor at both study areas. If we remove Gobiidae values, the diet indeed tends to a similar frequency/biomass ratio in the two areas. On the contrary, we record a positive effect of Gobiidae in the Gulf of Trieste only. This is due to the larger size of Gobies in the Gulf of Trieste with respect to Croatia.
Prey size
Prey sizes are slightly shorter by comparison with the prey of Shags breeding in Scotland (9.7 cm) (Wanless et al. Citation1993a) and Spain (9.8 cm) (Velando & Freire Citation1999). The most frequent prey length in the Gulf of Trieste is 6 cm, whereas in Croatia we detected two modes (4 and 8 cm length), which are similar to the modal lengths (6 and 8 cm) recorded by Wanless et al. Citation(1993b).
Nonetheless, the most interesting aspect is the trend of the monthly mean size of prey between the two areas. We suggest that there is an increase in prey length at the end of the breeding season (April) which is probably linked to chick rearing. Conversely, larger prey are taken in May and June in the Gulf of Trieste. Thereafter, the prey size decreases. We could assume that this trend is probably related to an abundance of adult Gobies in May and June due to spawning (Patzner Citation2007).
Ecological perspectives
Shags summering in the Gulf of Trieste come from the Croatian breeding colonies (Sponza et al. Citation2010). These post-breeding movements are linked to a more profitable foraging activity in the Gulf of Trieste with respect to Croatian waters, due to shallower depths (maximum 25 m versus 80 m in Croatia) and reduced physiological stress during diving (Sponza et al. Citation2010). These movements have not been always a habitual pattern, since they increased consistently from the 1980s to become a real migratory movement at the present time (Sponza et al. Citation2010). During the 1980s and 1990s, there was a substantial decrease (67%) of demersal fisheries landings along the eastern Adriatic Sea coastline (Slovenia, Croatia, Bosnia-Herzegovina, Montenegro and Albania) (Mannini et al. Citation2005). We suggest that there has been over-fishing in Croatian waters and the intensive use of unselective bottom trawls have lead to a shortage of demersal fish, thus reducing the probability of Shags showing feeding specialization during breeding. The consequent necessity to exploit bentho-pelagic resources possibly forced Shags to a broader and more variable diet. Conversely, although feeding specialization is attainable in the Gulf of Trieste, Shags cannot nest there because of human disturbance and a lack of suitable sites. Nonetheless, the specialization on demersal Gobiidae allows Shags to efficiently recover from the costs of the breeding season in Croatia (Sponza et al. Citation2010).
This study suggests that the diet of Mediterranean Shags can be used to index the availability of fish in marine ecosystems (Thayer & Sydeman Citation2007, Mills et al. Citation2007) and to provide useful information about the fish resource conditions that can be incorporated in fisheries management models (Hislop & Harris Citation1985, Hatch & Sanger Citation1992, Barrett Citation2002, Roth et al. Citation2007).
ACKNOWLEDGEMENTS
We thank C. Trani for help in collecting pellets. Particular thanks are due to J. Kralj and M. Kulieri'c of the Institute of Ornithology of Zagreb (Croatia) for permissions and logistic support. At Veli Lošinj (Croatia) we thank Blue World staff, in particular N. Rako, P. Makelworth and Karlo, for bringing us to and from Oruda island. A PhD scholarship was granted to B. Cimador by the University of Trieste. We finally thank E.A. Ferrero for valuable criticism and we are also indebted to the anonymous reviewers for constructive suggestions and comments on a previous version.
REFERENCES
- Aguilar , J. S. 1991 . Resum de l'Atlas d'ocells marins de les Balears, 1991 . Anuari Ornitológic de les Baleares , 6 : 17 – 28 .
- Aguilar , J. S. and Fernandez , G. 2002 . “ Species Action Plan for the Mediterranean Shag (Phalacrocorax aristotelis desmarestii) ” . Convention on the Conservation of European Wildlife and Natural Habitats, Strasbourg, December 2002. BirdLife International, Cambridge, UK
- Alvarez , D. 1998 . The diet of shags (Phalacrocorax aristotelis L.) in the Cantabrian Sea (North of Spain) during the breeding season . Seabird , 20 : 22 – 30 .
- Arcos , J. M. , Ruiz , X. , Bearhop , S. and Furness , R. W. 2002 . Mercury levels in seabirds and their fish prey at the Ebro Delta (NW Mediterranean): the role of trawler discards as a source of contamination . Mar. Ecol. Prog. Ser. , 232 : 281 – 290 .
- Ashmole , N. P. 1971 . “ Seabird ecology and the marine environment ” . In Pages in Avian Biology Edited by: Farner , D. S. and King , J. R. Vol. 1, pp. 224–271, Academic Press, New York
- Barquete , V. , Bugoni , L. and Vooren , C. M. 2008 . Diet of Neotropic cormorant (Phalacrocorax brasilianus) in an estuarine environment . Mar. Biol. , 153 : 431 – 443 .
- Barrett , R. T. 1991 . Shags (Phalacrocorax aristotelis L.) as potential samplers of juvenile Saithe (Pollachius virens L.) stocks in Northern Norway . Sarsia , 76 : 153 – 156 .
- Barrett , R. T. 2002 . Atlantic Puffin Fratercula arctica and Common Guillemot Uria aalge chick diet and growth as indicators of fish stocks in the Barents Sea . Mar. Ecol. Prog. Ser. , 230 : 275 – 287 .
- Barrett , R. T. and Furness , R. W. 1990 . The prey and diving depths of seabirds on Hørnoy, North Norway, after a decrease in the Barents Sea Capelin stocks . Ornis Scand , 21 : 179 – 186 .
- Barrett , R. T. , Strann , K.–B. and Vader , W. 1986 . Notes on the eggs and chicks of North Norwegian Shags . Phalacrocorax aristotelis. Seabird , 9 : 3 – 10 .
- Barrett , R. T. , Røv , N. , Loen , J. and Montevecchi , W. A. 1990 . Diets of Shags Phalacrocorax aristotelis and Cormorants P. carbo in Norway and possible implications for gadoid stock recruitment . Mar. Ecol. Prog. Ser. , 66 : 205 – 218 .
- Barrett , R. T. , Camphuysen , C. J. , Anker–nilssen , T. , Chardine , J. W. , Furness , R. W. , Garthe , S. , Hüppop , O. and Leopold , M. F. 2007 . Diet studies of seabirds: a review and recommendations . ICES J. Mar. Sci. , 26 : 1675 – 1691 .
- Brichetti , P. , De Franceschi , P. and Baccetti , N. 1992 . Fauna d'Italia. Aves I, Gaviidae–Phasianidae , 99 – 120 . Bologna : Edizioni Calderini .
- Carss , D. N. 1993 . Shags Phalacrocorax aristotelis at cage fish farms in Argyll, Western Scotland . Bird Study , 40 : 203 – 211 .
- Carss, D.N. & The Diet Assessment and Food Intake Working Group . 1997 . Techniques for assessing Cormorant diet and food intake: towards a consensus view . Supplemento Ricerche di Biologia della Selvaggina , 26 : 197 – 230 .
- Cramp , S. and Simmons , K. L.S. 1977 . The Birds of the Western Palaearctic. Vol. 1: Ostrich to Ducks , Oxford : Oxford University Press .
- Davoren , G. K. and Montevecchi , W. A. 2003 . Signals from seabirds indicate changing biology of capelin stocks . Mar. Ecol. Prog. Ser. , 258 : 253 – 261 .
- Dirksen , S. , Boudewijn , T. J. , Noordhuis , R. and Marteijn , E. C.L. 1995 . Cormorants Phalacrocorax carbo sinensis in shallow eutrophic freshwater lakes: prey choice and fish consumption in the non-breeding period and effects of large-scale fish removal . Ardea , 83 : 167 – 184 .
- Duffy , D. C. and Jackson , S. 1986 . Diet studies of seabirds: a review of methods . Colon. Waterbirds , 9 : 11 – 17 .
- Duffy , D. C. and Laurenson , I. J.R. 1983 . Pellets of Cape Cormorants as indicators of diet . Condor , 85 : 305 – 307 .
- Eschbaum , R. , Veber , T. , Vetemaa , M. and Saat , T. 2003 . “ Do cormorants and fishermen compete for fish resources in the Väinameri (eastern Baltic) area? ” . In Interactions Between Birds and Fish: Implications for Management , Edited by: Cowx , I. G. 72 – 83 . Oxford : Blackwell Science .
- Furness , R. W. and Camphuysen , C. J. 1997 . Seabirds as monitors of the marine environment . ICES J. Mar. Sci. , 54 : 726 – 737 .
- Furness , R. W. and Tasker , M. L. 2000 . Seabird–fishery interactions: quantifying the sensitivity of seabirds to reductions in sandeel abundance, and identification of key areas for sensitive seabirds in the North Sea . Mar. Ecol. Prog. Ser. , 202 : 253 – 264 .
- Gagliardi , A. , Martinoli , A. , Preatoni , D. , Wauters , L. A. and Tosi , G. 2007 . From mass to body elements to fish biomass: a direct method to quantify food intake of fish eating birds . Hydrobiologia , 583 : 213 – 222 .
- Grémillet , D. , Argentin , G. , Schulte , B. and Culik , B. M. 1998 . Flexible foraging techniques in breeding Cormorants Phalacrocorax carbo and Shags Phalacrocorax aristotelis: benthic or pelagic feeding? . Ibis , 140 : 113 – 119 .
- Guyot , I. 1988 . “ Relationships between shag feeding areas and human fishing activities in Corsica (Mediterranean Sea) ” . In Seabird Food and Feeding Ecology: 22–23 Edited by: Tasker , M. L. Proceedings of the 3rd International Conference of the Seabird Group, Glasgow
- Guyot , I. 1993 . “ Breeding distribution and number of Shag (Phalacrocorax aristotelis desmarestii) in the Mediterranean ” . In Estatus y Conservación de Aves Marinas Edited by: Aguilar , J. S. , Monabailliu , X. and Paterson , A. M. 37 – 46 . Actas del II Simposio MEDMARAVIS, SEO, Madrid
- Härkönen , T. 1986 . Guide to the Otoliths of the Bony Fishes of the Northeast Atlantic Danbiu, Aps Hellerup, Denmark
- Harris , M. P. and Wanless , S. 1991 . The importance of the lesser sandeel Ammodytes marinus in the diet of the Shag . Phalacrocorax aristotelis. Ornis Scand. , 22 : 375 – 383 .
- Harris , M. P. and Wanless , S. 1993 . The diet of Shags Phalacrocorax aristotelis during the chick-rearing period assessed by three methods . Bird Study , 40 : 135 – 139 .
- Hatch , S. A. and Sanger , G. A. 1992 . Puffins as samplers of juvenile Pollock and other forage fish in the Gulf of Alaska . Mar. Ecol. Prog. Ser. , 80 : 1 – 14 .
- Hislop , J. R.G. and Harris , M. P. 1985 . Recent changes in the food of young Puffins Fratercula arctica on the Isle of May in relation to fish stocks . Ibis , 127 : 234 – 239 .
- Johnstone , I. G. , Harris , M. P. , Wanless , S. and Graves , J. A. 1990 . The usefulness of pellets for assessing the diet of adult shags . Phalacrocorax aristotelis. Bird Study , 37 : 5 – 11 .
- Jonsson , G. 1992 . Icelandic Fishes 2nd ed. Fjolvi, Reykjavik
- Keller , T. 1993 . Untersuchungen zur Nahrungsökologie von in Bayern überwinternden Kormoranen . Phalacrocorax carbo sinensis. Orn. Verh. , 25 : 811 – 828 .
- Lambertini , M. and Leonzio , C. 1986 . “ Pollutant levels and their effects on Mediterranean seabirds ” . In: MEDMARAVIS & Monabailliu, X. (eds). Mediterranean Marine Avifaun, pp. 359–378. Springer-Verlag, Berlin–Heidelberg
- Lilliendahl , K. and Solmundsson , J. 2006 . Feeding ecology of sympatric European Shags Phalacrocorax aristotelis and Great Cormorants P. carbo in Iceland . Mar. Biol. , 149 : 979 – 990 .
- Louisy , P. 2005 . Guida all'identificazione dei Pesci Marini d'Europa e del Mediterraneo Il Castello, Milano, Italy
- Mannini , P. , Massa , F. and Milone , N. 2005 . “ Adriatic Sea Fisheries: outline of some main facts ” . In Interactions Between Aquaculture and Capture Fisheries: A Methodological Perspective Edited by: Cataudella , S. , Massa , F. and Crosetti , D. General Fisheries Commission for the Mediterranean, Rome
- Miller , A. and Sydeman , W. J. 2004 . Rockfish response to low frequency ocean climate change as revealed by the diet of a marine bird over multiple time scales . Mar. Ecol. Prog. Ser. , 281 : 207 – 216 .
- Mills , K. L. , Laidig , T. , Ralston , S. and Sydeman , W. 2007 . Diets of top predators indicate pelagic juvenile rockfish (Sebastes spp.) abundance in the California Current System . Fish. Oceanogr. , 16 : 273 – 283 .
- Montevecchi , W. A. 1993 . “ Birds as indicators of change in marine prey stocks ” . In Birds as Monitors of Environmental Change , Edited by: Furness , R. W. and Greenwood , J. J.J. 219 – 266 . London : Chapman and Hall .
- Montevecchi , W. A. and Myers , R. A. 1996 . Dietary changes of seabirds indicate shifts in pelagic food webs . Sarsia , 80 : 313 – 322 .
- Nelson , B. J. 2005 . Pelicans, Cormorants, and their Relatives. The Pelecaniformes , Oxford : Oxford University Press .
- Orel , G. and Zamboni , R. 2004 . Proposte per un piano pluriennale di gestione della fascia costiera del Golfo di Trieste. II Edizione riveduta ed ampliata ARIES—Progetto Pesca, SFOP 2000–2003
- Oro , D. and Ruiz , X. 1997 . Exploitation of trawler discards by breeding seabirds in the north-western Mediterranean: differences between the Ebro Delta and the Balearic Islands areas . ICES J. Mar. Sci. , 54 : 695 – 707 .
- Patzner , R. A. 2007 . “ Mediterranean gobies ” . Availabel from: www.users.sbg.ac.at/~patzner/Gobiidae.htm [accessed 31 March 2011]
- Prestamburgo , M. , Cosmina , M. , Gallenti , G. , Mauro , L. and Girardini , C. 2005 . Progetto ADRI.FISH. Realizzazione della rete di monitoraggio dati ‘L'economia della pesca entro le tre miglia nell'area Adri.Fish’ PIC Interreg IIIB Cadses. Dipartimento di Economia e Tecnica aziendale, Università degli Studi di Trieste, Trieste
- Privileggi , N. 2003 . Great Cormorants Phalacrocorax carbo sinensis wintering in Friuli Venezia Giulia, Northern Adriatic: specific and quantitative diet composition . Vogelwelt , 124 : 237 – 243 .
- Riedl , R. 1991 . Fauna e Flora del Mediterraneo Franco Muzzio Editore, Padova, Italy
- Rindorf , A. , Wanless , S. and Harris , M. P. 2000 . Effects of changes in sandeel availability on the reproductive output of seabirds . Mar. Ecol. Prog. Ser. , 202 : 241 – 252 .
- Roth , J. E. , Mills , K. L. and Sydeman , W. J. 2007 . Chinook salmon (Oncorhynchus tshawytscha) – seabird covariation off central California and possible forecasting applications . Can. J. Fish. Aquat. Sci. , 64 : 1080 – 1090 .
- Skrivanic , A. and Zavodnik , D. 1973 . Migration of the sardine Sardina pilchardus in relation to hydrographical conditions of the Adriatic Sea . Neth. J. Sea Res. , 7 : 7 – 18 .
- Sponza , S. , Cimador , B. , Cosolo , M. and Ferrero , E. A. 2010 . Diving costs and benefits during post-breeding movements of the Mediterranean Shag in the North Adriatic Sea . Mar. Biol. , 157 : 1203 – 1213 .
- Steven , J. 1933 . The food consumed by shags and cormorants around shores of Cornwall (England) . J. Mar. Biol. Ass. UK , 19 : 277 – 292 .
- Thayer , J. A. and Sydeman , W. J. 2007 . Spatio-temporal variability in prey harvest and reproductive ecology of a piscivorous predator Cerorhinca monocerata in an upwelling system . Mar. Ecol. Prog. Ser. , 329 : 253 – 265 .
- Utmar , P. 1999 . “ Marangone dal ciuffo Phalacrocorax aristotelis ” . In Gli uccelli della provincia di Gorizia , Edited by: Parodi , R. Vol. 42 , 44 – 45 . Udine : Edizioni Museo Friulano di Storia Naturale .
- Velando , A. 1997 . “ Ecologia y comportamiento del cormoran monudo Phalacrocorax aristotelis ” . en las Islas Cies y Ons. PhD thesis. University of Vigo, Spain
- Velando , A. and Freire , J. 1999 . Intercolony and seasonal differences in the breeding diet of European Shags on the Galician coast (NW Spain) . Mar. Ecol. Prog. Ser. , 188 : 225 – 236 .
- Velando , A. and Freire , J. 2002 . Population modelling of European Shags (Phalacrocorax aristotelis) at their southern limit: conservation implications . Biol. Conserv. , 107 : 59 – 69 .
- Velando , A. , Alvarez , D. , Mouriño , J. , Arcos , F. and Barros , A. 2005 . Population trends and reproductive success of the European Shag Phalacrocorax aristotelis on the Iberian Peninsula following the Prestige oil spill . J. Ornithol. , 146 : 116 – 120 .
- Veldkamp , R. 1995 . The use of chewing pads for estimating the consumption of cyprinids by Cormorants Phalacrocorax carbo. Ardea . 83 : 135 – 138 .
- Volponi , S. 1994 . “ Ecologia del Cormorano Phalacrocorax carbo sinensis nel Delta del fiume Po ” . PhD Thesis, University of Ferrara, Italy
- Wanless , S. , Burger , A. E. and Harris , M. P. 1991a . Diving depths of shags Phalacrocorax aristotelis breeding on Isle of May . Ibis , 133 : 37 – 42 .
- Wanless , S. , Harris , M. P. and Morris , J. A. 1991b . Foraging range and feeding locations of Shags (Phalacrocorax aristotelis) during chick rearing . Ibis , 133 : 30 – 36 .
- Wanless , S. , Corfield , T. , Harris , M. P. , Buckland , S. T. and Morris , J. A. 1993a . Diving behaviour of the Shag Phalacrocorax aristotelis in relation to water depth and prey size . J. Zool. , 231 : 11 – 25 .
- Wanless , S. , Harris , M. P. and Russell , S. 1993b . Factors influencing food-load sizes brought in by Shags Phalacrocorax aristotelis during chick rearing . Ibis , 135 : 19 – 24 .
- Wanless , S. , Harris , M. P. , Burger , A. E. and Buckland , S. T. 1997a . Use of time-at-depth recorders for estimating depth and diving performance of European Shags . J. Field Ornithol. , 68 : 547 – 561 .
- Wanless , S. , Bacon , P. J. , Harris , M. P. , Webb , A. D. , Greenstreet , S. P.R. and Webb , A. 1997b . Modelling environmental and energetic effects on feeding performance and distribution of Shag (Phalacrocorax aristotelis): integrating telemetry, geographical information system, and modelling techniques . ICES J. Mar. Sci. , 54 : 524 – 544 .
- Wanless , S. , Grémillet , D. and Harris , M. P. 1998 . Foraging activity and performance of Shag Phalacrocorax aristotelis in relation to environmental characteristics . J. Avian Biol. , 29 : 49 – 54 .
- Wanless , S. , Harris , M. P. , Redman , P. and Speakman , J. R. 2005 . Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea . Mar. Ecol. Prog. Ser. , 294 : 1 – 8 .
- Watanuki , Y. , Daunt , F. , Takahashi , A. , Newell , M. , Wanless , S. , Satom , K. and Miyazaki , N. 2008 . Microhabitat use and prey capture of a bottom-feeding top predator, the European Shag, shown by camera loggers . Mar. Ecol. Prog. Ser. , 356 : 283 – 293 .