Abstract
Capsule The breeding success of Lesser Spotted Woodpeckers Dendrocopos minor is now lower in England than previously reported and also lower than found in studies elsewhere in Europe.
Aims To quantify the breeding success and identify the causes of nest failure. To test the hypotheses that breeding success is related to aspects of food limitation and parental care, and inclement weather during the nesting period, or to interactions with Great Spotted Woodpeckers.
Methods Nests were monitored in three regions of England, recording survival and causes of failure. We measured aspects of food limitation and parental care, rainfall and Great Spotted Woodpecker interactions at nests, to explore whether there was any evidence that these factors were related to breeding success. We compared results to other studies from the UK and continental Europe.
Results Nest survival was 52%. The average number of chicks produced from successful nests was 2.8. Chick-stage daily nest survival was positively related to provisioning rates, indicating that food supply may be limiting. The most common cause of nest failure was presumed starvation of chicks after the disappearance of an adult. Some females ceased visiting nests, leaving provisioning solely to the male. This behaviour has been reported elsewhere in Europe, but in the present study males were unable to compensate fully by increasing their provisioning rates, leading to poor nest survival. Provisioning rates and chick-stage daily nest survival were negatively associated with rainfall. Nest predation by Great Spotted Woodpeckers occurred but was a less frequent cause of failure. Aggressive interactions were recorded between the two woodpecker species but these were unrelated to breeding parameters.
Conclusions Low breeding success is most probably related to food shortages in the breeding period. Simple population modelling using parameters from the present study and from published work shows that if the low productivity that we have observed is replicated throughout Britain, it would be sufficient to account for the observed population decline. However, the possibility that survival rates are also low cannot be ruled out.
INTRODUCTION
Lesser Spotted Woodpeckers Dendrocopos minor have declined significantly in the UK since the early 1980s (Amar et al. Citation2006, Hewson et al. Citation2007) and consequently it is a UK Biodiversity Action Plan priority species and was added to the UK Red List of birds of highest conservation concern in 2009 (Eaton et al. Citation2009). The causes of the decline are still unknown and this has prevented the development and implementation of a clear set of practical measures aimed at reversing the decline. Many of the hypotheses about the cause of decline relate to the effects on survival and productivity of changes in the abundance or availability of food resources, which are in turn linked to changes the composition and structure of woodlands at the local and landscape scale (Charman et al. Citation2010). It has also been suggested that nest predation and nest-site competition from the increasing UK population of Great Spotted Woodpeckers Dendrocopos major may be a significant factor (Charman et al. Citation2010, Fuller et al. Citation2005).
Pilot work by the Royal Society for the Protection of Birds (RSPB) in 2005 and 2006 found evidence of low breeding success for Lesser Spotted Woodpeckers (RSPB unpubl. data). Of six nests, three failed because of desertion of eggs or young and the estimated overall nest survival was 66.8%, considerably lower than rates reported from Sweden (80%; n = 124 [Wiktander et al. 2001]), Germany (74%; n = 31 [Rossmanith Citation2005, Rossmanith, Höntsch et al. 2007]), or UK up to the late 1980s (83%; n = 129 [Glue & Boswell 1994]).
In the present article we describe a study of Lesser Spotted Woodpecker breeding ecology in England designed to quantify breeding success and identify the causes of low productivity. Specifically, we wished to test three non-exclusive hypotheses.
Hypothesis 1: low breeding success is related to food limitation and aspects of parental care
Food supply is a key factor which can regulate bird populations (Newton Citation1998, Amar et al. Citation2003), either directly through starvation or though low breeding rates in response to food shortage. This hypothesis proposes that food supply may limit the breeding success of Lesser Spotted Woodpeckers and this may be particularly critical because of unusual aspects of parental care and the breeding system in this species. In Sweden and Germany it has been found that male Lesser Spotted Woodpeckers usually take a larger share of chick feeding than females, who often stop feeding large chicks (Wiktander et al. Citation2000, Rossmanith et al. Citation2009). Abandonment of the nest by the female in the late-chick period is commonplace but the males are generally able to compensate by increasing provisioning rates with usually no adverse impact on productivity (Wiktander et al. Citation2000). Therefore, a secondary aim of our work was to establish if abandonment by the female occurs regularly in England and the degree to which the male is able to compensate. In Sweden and Germany, polyandry was reported for a significant number of breeding attempts and appeared to be linked to the apparent abandonment of some nests (Sweden: 16% [Wiktander et al. 2000]; Germany: 19% [Rossmanith et al. 2009]). We were not successful in individually marking many birds, but we attempted to identify the potential for polyandry in the population studied here.
Hypothesis 2: inclement weather during the nesting period can have an adverse impact on breeding success
Climatic extremes can have major effects on birds (Newton Citation1998). It is possible that heavy rainfall during the nesting period may have a negative impact on provisioning rates through changes in prey availability, foraging efficiency or parental care (e.g. time spent brooding). For Lesser Spotted Woodpeckers this may be particularly critical at nests where only males are feeding the young. Wiktander et al. Citation(1994) found low nest survival (33%) in one year of their study when there was an exceptionally cold, wet spring with low temperatures throughout and particularly heavy rainfall during the chick-rearing period. In that year they attributed six out of seven failures to a shortage of food for the young or adults.
Hypothesis 3: interactions with Great Spotted Woodpeckers negatively affect Lesser Spotted Woodpecker breeding success
Great Spotted Woodpeckers are known to predate nests of Lesser Spotted Woodpeckers (Wiktander et al. Citation2001) and the population of Great Spotted Woodpeckers increased in England by 120% between 1995 and 2009 (Risely et al. Citation2011). Despite this increase there is little evidence for Great Spotted Woodpeckers being linked to population declines of other woodland species. For example, Lewis et al. Citation(2007) compared numbers of Great Spotted Woodpeckers in woods where Willow Tits Poecile montanus had persisted or gone extinct and found no significant differences. Charman et al. Citation(2010) found no relationships between wood occupancy by Lesser Spotted Woodpeckers and the abundance of Great Spotted Woodpeckers. However, given the large increases of Great Spotted Woodpecker and the anecdotal evidence of interactions between the two woodpecker species, this is a plausible hypothesis to test.
MATERIALS AND METHODS
The present study was carried out in three areas; South Yorkshire (SY) and the Hampshire/Wiltshire border (HA) in 2007, and Worcestershire (WO) from 2007 to 2009. We selected study areas that supported good populations of Lesser Spotted Woodpeckers based on the 1988–91 breeding atlas (Gibbons et al. Citation1993), the RSPB/Forestry Commission/Natural England/British Trust for Ornithology Bird Conservation Targeting project (www.rspb.org.uk/targeting) and local knowledge of county bird recorders. The WO study area focused on the Wyre Forest and satellite woods. The HA study area incorporated areas of the New Forest and some woodlands just outside the forest boundary. Woods in the SY study area centred on Sheffield.
Within each area, woodlands > 20 ha but < 85 ha in area were selected for intensive fieldwork. They were surveyed systematically for the presence of Lesser Spotted Woodpeckers from 1 March until 20 April each year (see Charman et al. [2010] for a full account of these methods). Those woods that were found to be occupied during this period were then searched intensively for nests throughout the rest of the breeding season.
Nest searches concentrated on areas where we had seen or heard birds during the occupancy surveys. We searched the focal area, checking all possible nesting locations on live and dead trees. Nests were located by observing adult behaviour, monitoring likely holes, listening for an excavating bird or by finding fresh wood chippings directly under a nest-hole. Although every effort was made to find nests as early as possible in the nesting cycle, a small number (n = 3) were found later in the season when chicks were noisy and almost ready to fledge.
Nests were checked from the ground every two to three days until fledging or failure. Presence of adults entering the cavity to incubate or bring food to chicks confirmed a nest was still active. Nests up to 15 m from the ground (n = 17) in WO and HA were inspected using a video camera mounted on a pole (adapted from Smith et al. [Citation2006]), usually three or four times through the nesting cycle. The camera images allowed the determination of clutch and brood sizes and provided a rough approximation of chick age based on the state of their development (this information was used to estimate fledging date for fieldwork planning and to classify chicks into early and late stages). Clutch size at the onset of incubation was assumed to be the maximum number of eggs observed during the incubation period for those nests found at that stage. It was not feasible to measure the exact brood size at hatching so we used maximum brood size recorded in our analyses. A successful nest was confirmed by the presence of chicks sufficiently well developed to fledge at the previous visit and by their absence from the cavity on the next visit, with no signs of nest predation. The number of chicks fledged from successful nests was measured using the number counted at the last visit prior to fledging. After fledging or failure, the camera was used to inspect the cavity for un-hatched eggs or dead chicks. Nest failure was assumed when there was no activity by the adults at the nest-hole but the stage of nest was not sufficiently advanced for the chicks to have fledged. In cases of suspected nest failure, signs left at the nest-hole, such as a secondary excavation indicating predation, were recorded.
Only two birds in our study area were individually marked, so we were not able to follow individual birds within or between seasons. As well as monitoring breeding success, we collected additional data to allow us to test the three aforementioned hypotheses.
Hypothesis 1: food limitation and parental care
To test the hypothesis that Lesser Spotted Woodpecker breeding success is related to aspects of parental care, provisioning and the relative contributions of the male and female, we carried out regular watches (every two to three days) at nests from the time they were located until chicks fledged or the nest failed. Nests were watched using a telescope (20 × magnification) from a distance of 50–75 m for a period of at least 120 minutes with start times randomized in blocks (early morning 06:00–09:00; late morning 09:00–12:00; early afternoon 12:00–15:00; late afternoon 15:00–18:00) through the day. Data were collected after an initial settling period (15 minutes) to ensure the birds' behaviour was not affected by the presence of the observer. Each time an adult visited the nest-hole we recorded the sex (Lesser Spotted Woodpeckers can be sexed in the field using plumage differences), time inside the hole, whether it was a provisioning event, the number of Lepidoptera larvae brought to the chicks (these are an important chick food source [Olsson 1998] and were readily identifiable compared to other prey items) and where the chicks were fed (inside the cavity or at the hole, to aid ageing). We carried out 191 watches (mean duration: 120.7 minutes, range: 120–150 minutes, total: 23 046 minutes) at 27 nests (54 watches pre-hatching, 77 at the early-chick stage and 60 at the late-chick stage). For each watch, we calculated the total number of visits, provisioning visits and time in the nest-hole by the female, male and both sexes combined. Provisioning was also calculated as the observed numbers of caterpillars brought to the chicks per hour.
Hypothesis 2: inclement weather during the nesting period
We used data on daily rainfall from the metrological stations nearest to the centre of each study area (WO: Bridgnorth, 20 km north; SY: Sheffield, centre of study area; HA: Hurn, 15 km southwest), calculated mean daily rainfall (mm) during the relevant periods for each nest (chick stages and whole breeding period) and tested for correlations with various measures of breeding performance.
Hypothesis 3: interactions with Great Spotted Woodpeckers
Great Spotted Woodpeckers were counted in 2007 as part of wood occupancy surveys and these data were used to produce a relative Great Spotted Woodpecker index for each site: mean Great Spotted Woodpecker encounters per hour per ha (Charman et al. Citation2010). In all years, inter-specific interactions at Lesser Spotted Woodpecker nest-holes during observation sessions were recorded and the mean number of Great Spotted Woodpecker interactions per hour calculated for each nest-hole. Interactions included displays between the species and pursuits between them as well as incidents of Great Spotted Woodpeckers visiting active Lesser Spotted Woodpecker nest-sites.
Statistical analysis
We used a nesting period of 35 days: 15 days pre-hatching (4 laying plus 11 incubating) and 20 days between hatching and fledging (Winkler et al. Citation1995). We refer to broods between one and seven days old as early-chick stage and over seven days old as late-chick stage. We report mean values ± 1 sd for clutch size, brood size, and chicks fledged per nesting attempt and from all successful nests. We define breeding success as the proportion of nests successfully fledging one or more chicks. Daily nest survival rates were calculated following the Mayfield Citation(1975) method with 95% CIs calculated following Johnson Citation(1979). Estimates were calculated separately for the egg and chick stages, with their product giving an overall estimate of nest survival throughout the whole nesting period. All tests were univariate, as appropriate for the small sample size. Percentage data was arc-sine transformed before analysis where necessary. Analyses were carried out using r version 2.12.1.
Hypothesis 1: food limitation and parental care
At each nest we calculated the following explanatory variables: the proportion of time the male, female and both adults combined spent in the nest-hole; the rate of chick provisioning per hour in the early- and late-chick period and throughout by the male and female separately and combined; and number of Lepidoptera larvae brought to chicks per hour in the early- and late-chick period and throughout by the male and female separately and combined. Differences between males and females in their contributions to parental care and provisioning were tested using t-tests. We then related parental care and provisioning rate variables to daily nest survival at the relevant stage. Daily failure probability of nests was modelled as a function of each explanatory variable separately using binomial logistic regression (Aebischer Citation1999). The dependent variable was the binary variable failure or non-failure, and each day on which the nest was monitored (exposure day) was taken to be a binomial trial so the exposure period in days was entered as the binomial denominator. Nest failure was assumed to occur half-way between the last visits. Significance was assessed using deletion likelihood-ratio tests and by examining the change in deviance using chi-squared or F-tests, depending on whether the model was over-dispersed. We examined the relationship between the number of chicks fledged from successful nests and provisioning variables using linear regression.
Hypothesis 2: inclement weather during the nesting period
The influence of rainfall on daily nest survival and the number of chicks fledged from successful nests were examined with binomial and linear glms, respectively. We used Pearson's correlation to establish relationships between parental care and provisioning variables with rainfall.
Hypothesis 3: interactions with Great Spotted Woodpeckers
Daily nest survival at the egg and chick stage were analysed in relation to: (1) mean Great Spotted Woodpecker encounters per hour at the nest-hole; (2) the incidence of Great Spotted Woodpecker encounters (two-level factor, 1 = Great Spotted Woodpeckers observed at the nest-hole; 0 = no Great Spotted Woodpeckers observed at the nest-hole); and (3) the relative abundance of Great Spotted Woodpeckers in each wood. For the latter test, to account for more than one nest per wood and multiple years, mean daily nest survival per wood was used rather than daily nest survival per nest.
RESULTS
Overall breeding parameters
Twenty-seven nests were found during the 3 years of the project – 15 in 2007; 6 in 2008; and 6 in 2009; 9 during cavity excavation, 10 during laying and incubation and 8 at the chick stage. The mean first-egg date (nests found during the excavation or egg stage only) was 29 April (range: 22 April–6 May) (mean date within years: 30 April in 2007; 1 May in 2008; 24 April in 2009).
The mean clutch size was 5.2 ± 1.2 (n = 12; range: 3–6), mean maximum brood size 4.0 ± 1.4 (n = 17; range: 2–6) and the mean number of young fledged from successful nests was 2.8 ± 1.4 (n = 12; range: 1–5). Overall productivity (chicks fledged over all nesting attempts) was 1.4 ± 1.7 (n = 23; range: 0–5). There were no significant differences in clutch size or maximum brood size between years or study areas (no clutch or brood size data from SY) (years: F 2,9 = 0.26, P = 0.77; F 2,14 = 0.01, P = 0.96; study areas: F 1,10 = 0.07, P = 0.80; F 1,15 = 0.30, P = 0.59).
Of the 27 nests, 16 (59%) successfully fledged one or more chicks. The Mayfield estimate of the daily nest survival during egg laying and incubation was 0.985 (95% CI: 0.968–1.000), giving a mean overall nest survival during this phase of 81% (95% CI: 62–100%). During the chick stage the mean daily nest survival was 0.978 (95% CI: 0.963–0.993) giving an overall nest survival at this stage of 64% (95% CI: 46–87%). Across the whole nesting period, daily nest survival rate was 0.963 (95% CI: 0.931–0.995), corresponding to an overall nest survival of 52% (95% CI: 28–90%).
The causes of the nest failures were loss of one or more adults attending the nest (n = 2 during incubation and n = 5 during chick stage), predation (n = 3 during the chick stage) and flooding of the nest cavity (n = 1 during egg stage).
Hypothesis 1: food limitation and parental care
During the egg stage, the cavity was occupied by an adult for a mean of 67.5% ± 28.2 of the time. There were no differences in the proportion of time the male and female spent in the nest cavity (males: 32.7% ± 14.9; females: 34.8% ± 23.4; t 30 = –0.34, P = 0.73). The similarity of the contributions of males and females during the egg stage suggests that either our nests were the primary nests of polyandrous females or polyandry was infrequent. At only 2 nests out of 19 that were under observation during the laying and incubation phases was there any evidence of an extremely low contribution by the female, which could be indicative of polyandry. At one nest in WO in 2008, the female was never recorded near the nest after it was found at the late-egg stage (this nest failed). At another nest in WO in 2007, we saw low contribution by the female, who disappeared after five days with the male continuing to incubate until abandonment at Day 14. It is possible that at these nests low attendance by the female was because of polyandry, but that cannot be confirmed. Egg-stage nest survival was not related to mean incubation rates per nest (change in deviance = 1.12, df = 1, P = 0.29).
In the early-chick stage (up to seven days old), chicks were brooded for 52.4% ± 19.4 of the time and the contribution from the male tended to be higher (males: 30% ± 11.5; females: 22.5% ± 12.2), a difference which was close to significant (t 35 = 1.94, P = 0.06). After Day 7, brooding decreased to a mean of 7% ± 5.2 of the time and, although males also spent more time brooding than the females (males: 4.1% ± 5.2; females: 2.9% ± 5.4), the difference was not significant (t 29 = 0.65, P = 0.52). Chick-stage nest survival was not related to brooding rates (total early-chick stage: change in deviance = 0.02, df = 1, P = 0.89; males, early-chick stage: change in deviance = 0.64, df = 1, P = 0.43; females, early-chick stage: change in deviance = 0.251, df = 1, P = 0.62; insufficient data for late-chick stage analyses).
Up to Day 7, adults fed chicks on average 9.6 times per hour ± 4.8 sd. After Day 7, this increased significantly to 14.1 visits per hour ± 4.3 sd (t 31 = 3.06, P = 0.01). There was no significant difference in the male and female contribution to early provisioning (males: 5.3 ± 3.1; females: 4.3 ± 2.1; t 29 = 1.14, P = 0.27); however, after chicks were older than seven days the male made significantly more provisioning visits per hour compared with the female (males: 8.8 ± 2.8; females: 5.3 ± 3.2; t 29 = 3.39, P = 0.002). Over the whole chick-provisioning period, the male provided more food visits per hour compared with the female (males: 6.6 ± 3.0; females: 4.6 ± 2.4; t 42 = 2.48, P = 0.02).
The main food brought to chicks was Lepidoptera larvae (brought on 84% of the provisioning visits). There was a significant increase in the number of caterpillars per hour brought to chicks between early- and late-chick stages (early: 15.3 ± 11.3; late: 26.6 ± 17.4; t 23 = –2.14, P = 0.04).
At nine nests (one during incubation and eight at the chick stage) one of the adults (in eight cases the female) ceased to contribute to the incubation or chick-feeding while the nest was still active. Although the remaining adult continued to attend, six of these nests (67%) eventually failed. At the chick stage, five out of eight nests (62%) with a single adult feeding failed, apparently because of chick starvation, whereas only three out of eight nests (37%; difference not significant P > 0.05) with two adults in attendance failed and these failures were attributed to nest predation.
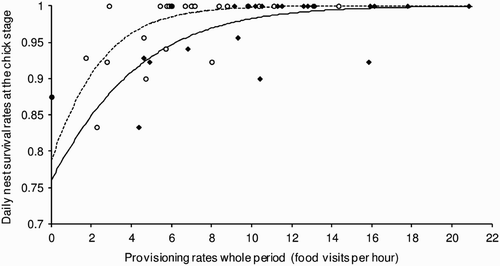
The number of chicks fledged from successful nests was not related to provisioning variables (total provisioning: F 1,10 = 0.74, P = 0.41; males only: F 1,10 = 0.16, P = 0.70; females only: F 1,10 = 3.42, P = 0.09). Chick-stage daily nest survival was positively related to the combined provisioning rate of both parents (; total provisioning: change in deviance = 6.12, df = 1, P = 0.01). There was also a strong positive relationship between chick-stage daily nest survival and the male provisioning rate alone, but not the female rate alone, suggesting that it is the male's contribution that drives this relationship (; males only: change in deviance = 7.64, df = 1, P = 0.01; females only: change in deviance = 1.03, df = 1, P = 0.31).
Figure 1. Relationship between daily nest survival rates of Lesser Spotted Woodpeckers at the chick stage and provisioning rates per hour during the chick stage.
— •, combined adults; ––– ○, males only.
Breeding birds were not individually marked so we have not been able to distinguish between desertion and death of adults. At 30% of the nests the female was lost (n = 8), including four that went on to be successful. The males appeared unable to compensate fully after this loss as the feeding rate at nests where the female was lost did not match the expected rate from nests where both sexes continued to feed (only 69% of that expected; ).
Figure 2. Mean (± se) male and female provisioning rates of Lesser Spotted Woodpeckers at the early- and late-chick stages. Comparisons are of nests with two parents present in the early-chick stage and late-chick stage and late-stage nests with male only.
White bars represent data from the present study; dotted bars represent data from studies in Sweden showing that the male can compensate for the female being absent (reproduced from Wiktander et al. [2000] with permission of the author and John Wiley & Sons Ltd.); in the ‘Late, male-only nests’ columns, the observed data are presented alongside those expected from the males if they are fully compensating for the loss of a female (the sum of the late male and female values from each study).
![Figure 2. Mean (± se) male and female provisioning rates of Lesser Spotted Woodpeckers at the early- and late-chick stages. Comparisons are of nests with two parents present in the early-chick stage and late-chick stage and late-stage nests with male only. White bars represent data from the present study; dotted bars represent data from studies in Sweden showing that the male can compensate for the female being absent (reproduced from Wiktander et al. [2000] with permission of the author and John Wiley & Sons Ltd.); in the ‘Late, male-only nests’ columns, the observed data are presented alongside those expected from the males if they are fully compensating for the loss of a female (the sum of the late male and female values from each study).](/cms/asset/598f22fe-34fa-408a-8339-70dc3642621a/tbis_a_662941_o_f0002g.gif)
Hypothesis 2: inclement weather during the nesting period
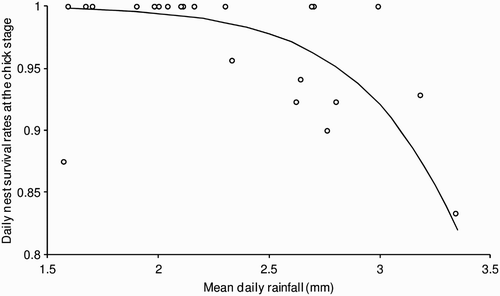
Chick-stage daily nest survival rates were negatively related to mean daily rainfall (; change in deviance = 23.66, df = 1, P < 0.001) but there was no relationship between the number of chicks fledged from successful nests and rainfall (F 1,10 = 1.39, P = 0.27).
Figure 3. The relationship between rainfall and chick-stage daily nest failure rates in Lesser Spotted Woodpeckers.
The solid line shows the modelled relationship, with open circles showing the actual data.
Chick provisioning rates per hour decreased with increasing rainfall (r = –0.48, n = 19, P = 0.02). The amount of brooding carried out in the early-chick period declined with increasing rainfall (r = –0.49, n = 19, P = 0.03), which may be associated with time being spent searching for food. This relationship was strongest and significant for the male contribution (r = –0.56, n = 19, P = 0.01) rather than that of the female (r = –0.25, n = 19, P = 0.30).
Comparison of rainfall data at nests where loss of the female led to nest failure (n = 4) with those where loss of the female did not lead to nest failure (n = 4) revealed that there was more rain at the former sites both during the whole nesting period and during the chick stage, although there were too few data to test this statistically (whole period mean daily rainfall, failed nests: 2.5 mm; successful nests: 2.1 mm; chick period mean daily rainfall, failed nests: 2.6 mm; successful nests: 2.5 mm).
Hypothesis 3: interactions with Great Spotted Woodpeckers
We attributed three nest failures during the chick stage to Great Spotted Woodpecker nest predation on the basis of the damage to the nest cavity. In addition, across all 3 years during our nest watches, we recorded 24 aggressive encounters with Great Spotted Woodpeckers at the nest-hole or tree of 9 pairs. Mean encounter rate was 0.07 Great Spotted Woodpeckers per hour at each nest-site but there was considerable variation (sd =± 0.18). There were no significant relationships between nest survival and Great Spotted Woodpecker encounters per hour (egg stage: change in deviance = 3.59, df = 1, P = 0.58; chick stage: change in deviance = 0.54, df = 1, P = 0.46) or the presence of Great Spotted Woodpecker encounters (egg stage: change in deviance = 0.08, df = 1, P = 0.78; chick stage: change in deviance = 0.10, df = 1, P = 0.75). Mean nest survival per wood was not correlated with the relative abundance of Great Spotted Woodpeckers in the woods (egg stage: r = –0.47, n = 14, P = 0.09; chick stage: r = –0.20, n = 20, P = 0.39).
DISCUSSION
The present study has shown that Lesser Spotted Woodpecker breeding success and the average number of young fledged per nesting attempt from successful nests are lower than previously reported from continental Europe. This indicates that there should be cause for concern over breeding success in the UK. summarizes the key comparable parameters from previous breeding studies and compares them with our own findings.
Table 1. Comparison of key breeding parameters of Lesser Spotted Woodpeckers between the present study and those previously conducted on continental Europe.
Only 16 of 27 nests successfully fledged one or more chicks, resulting in an overall Mayfield nest survival rate of 52%. This is 15–20% lower than that found in the Swedish (Wiktander et al. Citation1994, Wiktander et al. Citation2001) and German (Rossmanith Citation2005, Rossmanith, Höntsch et al. 2007) studies and considerably lower than that previously recorded in the UK from nest record data (Glue & Boswell Citation1994). Breeding success in other woodpecker species is generally high – in the region of 70–100% (Winkler et al. Citation1995).
Chick-stage daily nest survival was positively related to the combined provisioning rate of both parents, but particularly the provisioning rate of the male. The primary cause of nest failure was apparent starvation of chicks after one or both parents no longer attended the nest. In total, 30% of the nests in the present study suffered loss of the female (n = 8), including four that went on to be successful. This was also found to be the case in Sweden, where Wiktander et al. Citation(2000) observed that 42% of nests were deserted by the female (colour-ringed individuals meant that this could be confirmed and death of the female excluded), although overall breeding success was higher there (Wiktander et al. Citation2001). Desertion of an active nest by the female, leaving the male to sole parental care is thought to be an adaptive breeding strategy in Lesser Spotted Woodpeckers. In Sweden it has been found that in most years female Lesser Spotted Woodpeckers have lower survival compared with males (Wiktander Citation1998), apparently related to their higher energy demands during the breeding season (Olsson Citation1998, Wiktander Citation1998), suggesting a higher cost of reproduction for the females compared with the males. Wiktander Citation(1998) found that the breeding season was the period of highest mortality for these birds, supporting the idea that they are under stress at this time. Therefore, their offspring-desertion behaviour may be regarded as an adaptive life-history decision (Székely et al. Citation1996). We would expect to see compensation by the male for the reduced feeding by the female, which is exactly what Wiktander et al. Citation(2000) found in Sweden; the males matched the combined rate of both sexes after the female ceased feeding. In the present study, albeit with small sample sizes, we have shown that this does not appear to be the case. Males increased their feeding rates at nests where the female was lost, but not sufficiently to match the expected rate from nests where both sexes continued to feed and the strategy was more likely to result in nest failure. As rainfall was also associated with the probability of nest failure, we suggest that poor weather in the chick stage of breeding puts additional pressure on the males. The evidence suggests that males are unable to increase their provisioning rate, particularly during bad weather. Furthermore, there was a suggestion that rainfall was higher at those nests where females were lost and that nest went on to fail. In comparison, nests where the female was lost but which went on to be successful experienced lower rainfall. This could indicate that overall food shortage in our study areas is the root cause of nest failure but that this interacts with the female reproductive strategy of leaving the male to attend the nest and results in poor breeding success overall.
Information on food availability in woodlands and its change through time is limited. Defoliating caterpillars of oaks and other species are known to follow long-term cycles of ten years or more (Harding Citation2000, Hogstad Citation2005) but there is evidence for long-term decline in common and widespread moth species in the UK (Conrad et al. Citation2006). However, work on Wood Warblers Phylloscopus sibilatrix in Wales suggests that overall abundance of caterpillars has not changed significantly in 25 years (J. Mallord, pers. comm.), although there is considerable annual and inter-site variation. Scale is likely to be a factor and it is suggested that more work should be carried out to monitor long-term changes of invertebrates in woodlands.
There is a possibility that the birds are suffering from phenological mismatch. The first-egg dates are slightly late compared with some woodland passerines (although in line with many migrant species) and the peak abundance of defoliating caterpillars from oak trees – a key resource for woodland birds – would have passed before young hatched (K. Smith, pers. obs.; caterpillar peak in these years was 10–15 May in Hertfordshire and was probably similar in our study areas). However, Lesser Spotted Woodpeckers are known to lay their eggs later than tits in the same woodlands (Wiktander et al. Citation2001), yet still successfully fledge chicks.
Olsson et al. (Citation1999) found evidence of a carryover effect from the pre-breeding into the breeding season. They found that birds in territories with good feeding conditions in the pre-breeding period were able to nest early, which resulted in more fledged young per breeding attempt than birds in poorer territories. Therefore, we suggest further investigation of causes of low breeding success should include pre-breeding food availability as well as that during the breeding period. In the pre-breeding period the birds feed mainly on invertebrates associated with dead wood (Olsson Citation1998) and much more work is needed to understand their foraging ecology at this time.
It is also probable that food is more difficult to acquire during wet weather and the male may spend more time gleaning food for himself or sheltering from inclement conditions at times of extreme rainfall. Rainfall in all years was generally higher than average across our study regions (http://www.metoffice.gov.uk/climate/uk/). In addition, there were several days of extreme rainfall in excess of 20 mm during the chick-provisioning period. Projections are for average summers in the UK to be become drier because of climate change; however, extreme events and shorter periods of heavy rainfall may become more common (http://www.metoffice.gov.uk/climatechange).
There was no strong evidence of Great Spotted Woodpeckers affecting Lesser Spotted Woodpeckers in our study, at least using the parameters we measured. Predation by Great Spotted Woodpeckers accounted for the loss of only three broods. In Sweden, predation of the nest cavity was not the main cause of nest failure, while in Germany nest predation by avian predators (two Eurasian Jays Garrulus glandarius and three Great Spotted Woodpecker) was the primary cause of failure. Interactions with Great Spotted Woodpeckers were also low during nest watches and breeding success was not related to the encounter rate or the relative abundance of Great Spotted Woodpeckers in the woods. Therefore, based on these data, we cannot add to the suggestion that Great Spotted Woodpeckers are negatively influencing Lesser Spotted Woodpeckers, despite large increases in Great Spotted Woodpecker populations over recent decades (Eaton et al. Citation2009). In the same study area, Charman et al. Citation(2010) found no influence of Great Spotted Woodpecker abundance on the occupancy of woodlands by Lesser Spotted Woodpeckers. It is clear that Great Spotted Woodpeckers are nest predators of the smaller species, as found in this and other studies (Wiktander et al. Citation2001); however, this does not necessarily lead to a population-level effect. Other studies examining the impact of Great Spotted Woodpeckers on woodland birds have found little evidence of their impact (Newson et al. Citation2010, Lewis et al. Citation2007). However, it is plausible that other, more subtle effects that are hard to measure may have gone undetected in the present study. For instance, behavioural alteration may have occurred in the presence of high numbers of Great Spotted Woodpeckers; Lesser Spotted Woodpeckers may be prevented from settling in preferred areas or may be forced to select non-optimum nest-sites or nest later because of early-season interactions with Great Spotted Woodpeckers. Such interactions and their impacts are difficult to detect and could ultimately only be tested by large-scale removal experiments.
As none of our birds were individually marked we have not been able to measure survival rates. However, Wiktander Citation(1998) showed that the breeding season was the period of greatest mortality in the Lesser Spotted Woodpecker and the subsequent survival rate of males was lower when breeding conditions were poor. Thus, it is possible that the low breeding success of our birds puts them in double jeopardy; not only are they producing few young but they are also prejudicing their own survival in order to breed. The collection of data on the survival rates of birds in England would be required to test this, although given the low density and cryptic behaviour of the birds such a study would present severe methodological challenges.
Can low breeding success help explain the observed population decline?
We have shown that both the brood size of successful nests and nest survival are lower than those reported by Glue and Boswell Citation(1994) for nest record data in the period up to the late 1980s. Without data on the adult and first-year survival of Lesser Spotted Woodpeckers in the UK, it is not possible to determine with certainty the implications of this low breeding output on the population trend. However, using the mean survival rates quoted by Rossmanith, Blaum et al. (2007) from Swedish and German studies (Sweden: adult survival = 0.62; first-year survival = 0.25; Germany: adult survival = 0.62; first year survival = 0.38) and the productivity figures from the present study and those of Glue and Boswell Citation(1994), it is clear that the low productivity we have observed would be sufficient to account for the observed population decline of around 7% per annum. Using a simple deterministic population model with birds breeding in their first year, the Swedish and German survival figures and Glue and Boswell's (1994) productivity, gives modelled annual population multiplication rates of +5% and +28%, respectively; whereas using the low productivity reported in the present study gives annual population multiplication rates of –20% and –11%, respectively.
In conclusion, given the relationship between low breeding success and provisioning, and the implication that food may be lacking or unavailable to Lesser Spotted Woodpeckers, action to provide or increase the food source is likely to be crucial. Amar et al. Citation(2010) described changes in woodland structure in Great Britain between the 1980s and early 2000s, many of which are linked to cessation of active management and an increase in deer browsing. It is not known whether structural changes in woodland could have reduced food supplies. However, more information on the feeding ecology and diet of Lesser Spotted Woodpeckers in the pre-breeding and breeding periods and the potential for different tree species and management regimes to deliver this will be vital if practical measures are to be developed. Charman et al. Citation(2010) have described habitat requirements at a landscape scale, and work is ongoing to establish foraging behaviour and habitat needs within woods, as well as investigating how and why woodlands could have changed in terms of their value to the species.
ACKNOWLEDGEMENTS
This study was funded by RSPB and Natural England under the ‘Action for Birds in England’ partnership. We thank all the land-owners in Wiltshire, Hampshire, Worcestershire, Shropshire and South Yorkshire who allowed access to their woodlands. Assistance in the field in 2007 was given by Hugh Venables and Anne Heath. Jacqueline Weir and Chris Dunn provided assistance with the pilot project in 2005 and 2006. Tom Charman assisted with analyses and gave extensive comments on the manuscript. Ulf Wiktander gave data for , which was originally published in Wiktander et al. Citation(2000) and is reproduced here with permission of the author and John Wiley & Sons Ltd. Nigel Butcher built the camera used for nest inspections. Arjun Amar and anonymous reviewers gave comments that improved the manuscript.
REFERENCES
- Aebischer , N. J. 1999 . Multi-way comparisons and generalized linear models of nest success: extensions of the Mayfield method . Bird Study , 46 : 22 – 31 .
- Amar , A. , Redpath , S. and Thirgood , S. 2003 . Evidence for food limitation in the declining hen harrier population on the Orkney Islands, Scotland . Biol. Conserv. , 111 : 377 – 384 .
- Amar , A. , Hewson , C. M. , Thewlis , R. M. , Smith , K. W. , Fuller , R. J. , Lindsell , J. A. , Conway , G. , Butler , S. and MacDonald , M. 2006 . What's Happening to our Woodland Birds? Long-term Changes in the Populations of Woodland Birds RSPB Research Report No. 19 and BTO Research Report No. 169
- Amar , A. , Smith , K. , Butler , S. , Lindsell , J. , Hewson , C. , Fuller , R. and Charman , E. C. 2010 . Recent patterns of change in vegetation structure and tree composition of British broadleaved woodland: evidence from large-scale surveys . Forestry , 83 : 345 – 356 .
- Charman , E. C. , Smith , K , Gruar , D. J. , Dodd , S. and Grice , P. 2010 . Factors associated with wood occupancy by Lesser Spotted Woodpeckers Dendrocopos minor in England . Ibis , 152 : 543 – 555 .
- Conrad , K. F , Warren , M. S. , Fox , R. , Parsons , M. S. and Woiwod , I. P. 2006 . Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis . Biol. Conserv. , 132 : 279 – 291 .
- Eaton , M. A. , Brown , A. F. , Noble , D. G. , Musgrove , A. J. , Hearn , R. , Aebischer , N. J. , Gibbons , D. W. , Evans , A. and Gregory , R. D. 2009 . Birds of Conservation Concern 3: the population status of birds in the United Kingdom, Channel Islands and the Isle of Man . Br. Birds. , 102 : 296 – 341 .
- Fuller , R. J. , Noble , D. G. , Smith , K. W. and Vanhinsbergh , D. 2005 . Recent declines in populations of woodland birds in Britain: a review of possible causes . Br. Birds. , 98 : 116 – 143 .
- Gibbons , D. W. , Reid , J. B. and Chapman , R. A. 1993 . The New Atlas of Breeding Birds in Britain and Ireland: 1988–1991 , London : T & AD Poyser .
- Glue , E. and Boswell , T. 1994 . Comparative nesting ecology of the three British breeding woodpeckers . Br. Birds. , 87 : 253 – 269 .
- Harding , D. 2000 . “ Two decades of data on oak defoliation in a Worcestershire woodland NNR ” . In Long-term Studies in British Woodland. English Nature Science Edited by: Kirby , K. J. and Morecroft , M. D. Vol. 34 , 87 – 97 . English Nature, Peterborough, UK
- Hewson , C. M. , Amar , A. , Lindsell , J. A. , Thewlis , R. M. , Butler , S. , Smith , K. and Fuller , R. J. 2007 . Recent changes in bird populations in British broadleaved woodlands . Ibis , 149 ( Suppl. 2 ) : 14 – 28 .
- Hogstad , O. 2005 . Numerical and functional responses of breeding passerine species to mass occurrence of geometrid caterpillars in a subalpine birch forest: a 30-year study . Ibis , 147 : 77 – 91 .
- Johnson , D. J. 1979 . Estimating nest success: the Mayfield method and an alternative . Auk , 96 : 651 – 661 .
- Lewis , A. J.G. , Amar , A. , Cordi-Piec , D. and Thewlis , R. M.T. 2007 . Factors influencing Willow Tit Poecile montanus site occupancy: a comparison of abandoned and occupied woods . Ibis , 149 : 205 – 213 .
- Mayfield , H. F. 1975 . Suggestions for calculating nest success . Wilson Bull , 87 : 456 – 466 .
- Newson , S. E. , Rexstad , E. A. , Baillie , S. R. , Buckland , S. T. and Aebischer , N. J. 2010 . Population changes of avian predators and grey squirrels in England: is there evidence for an impact on avian prey populations? . J. Appl. Ecol. , 47 : 244 – 252 .
- Newton , I. 1998 . Population Limitation in Birds , London : Academic Press Ltd .
- Olsson , O. 1998 . Through the eyes of a woodpecker: understanding habitat selection, territory quality and reproductive decisions from individual behaviour. PhD Thesis, Lund University, Sweden
- Olsson , O. , Wiktander , U. , Holmgren , N. M.A. and Nilsson , S. G. 1999 . Gaining ecological information about Bayesian foragers through their behaviour. II. A field test with woodpeckers . Oikos , 87 : 264 – 276 .
- Risely , K. , Renwick , A. R. , Dadam , D. , Eaton , M. A. , Johnston , A. , Bailie , S. R. , Musgrove , A. J. and Noble , D. G. 2011 . The Breeding Bird Survey 2010 BTO Research Report No. 597
- Rossmanith , E. 2005 . Breeding biology, mating system and population dynamics of the lesser spotted woodpecker (Picoides minor): combining empirical and model investigation. PhD Thesis, University of Potsdam, Germany
- Rossmanith , E. , Höntsch , K. , Blaum , N. and Jeltsch , F. 2007a . Reproductive success and nestling diet in the Lesser Spotted Woodpecker (Picoides minor): the early bird gets the caterpillar . J. Ornithol. , 148 : 323 – 332 .
- Rossmanith , E. , Blaum , N. , Grimm , V. and Jeltsch , F. 2007b . Pattern-orientated modelling for estimating unknown pre-breeding survival rates: the case of the lesser spotted woodpecker (Picoides minor) . Biol. Conserv. , 135 : 555 – 564 .
- Rossmanith , E. , Blaum , N. , Höntsch , K. and Jeltsch , F. 2009 . Sex related parental care strategies in the Lesser Spotted Woodpecker Picoides minor: of flexible mothers and dependable fathers . J. Avian Biol. , 40 : 28 – 33 .
- Smith , K. W. , Butcher , N. and Eddowes , M. 2006 . Using video cameras to inspect nest content . Nest Rec. News , 22 : 14 – 15 .
- Székely , T , Webb , J. N. , Houston , A. I. and McNamara , J. M. 1996 . An evolutionary approach to offspring desertion in birds . Curr. Ornithol. , 13 : 271 – 330 .
- Wiktander , U. 1998 . Reproduction and survival in the lesser spotted woodpecker. Effects of life history, mating system and age. PhD Thesis, Lund University, Sweden
- Wiktander , U. , Nilsson , S. G. , Olsson , O. and Stagen , A. 1994 . Breeding success of a Lesser Spotted Woodpecker Dendrocopos minor population . Ibis , 136 : 318 – 322 .
- Wiktander , U. , Olsson , O. and Nilsson , S. G. 2000 . Parental care and social mating system in the Lesser Spotted Woodpecker Dendrocopos minor . J. Avian Biol. , 31 : 447 – 456 .
- Wiktander , U. , Olsson , O. and Nilsson , S. G. 2001 . Seasonal variation in home-range size, and habitat area requirement of the lesser spotted woodpecker (Dendrocopos minor) in southern Sweden . Biol. Conserv. , 100 : 387 – 395 .
- Winkler , H. , Christie , D. A. and Nurney , D. 1995 . Woodpeckers – An Identification Guide to Woodpeckers of the World , Boston , MA : Houghton Mifflin .