Abstract
Capsule Predation was the main cause of nest failure, but predation rates have remained unchanged since the 1980s. Eurasian Jays Garrullus glandarius were the most common predator.
Aims To quantify, and compare, nest predation rates for 1982–84 and 2009–11, and to identify predators of Wood Warbler Phylloscopus sibilatrix nests in Welsh oakwoods.
Methods During 2009–11, 167 Wood Warbler nests were monitored and purpose-built miniature nest cameras deployed at 73 of them. Nest predation rates were compared with 67 nests monitored during 1982–84.
Results Of 167 nests monitored from 2009 to 2011, 62 failed due to predation (32/73 camera nests, 30/94 non-camera nests), giving an overall Daily Survival Rate (DSR ± se) of 0.979 ± 0.003. This was not significantly different from the rate during 1982–84 (0.967 ± 0.006). In 2009–11, the DSR of nests declined temporally during the season at both the egg and chick stages. For chick stage nests, DSR varied annually and nonlinearly with age of nestlings. There was no evidence for an effect of cameras at either stage. Of 32 camera nests lost to predation, the predator was identified from 28, resulting in 30 predators being identified. There was one case of multiple predators at a single nest. The majority of nest predation was carried out by birds (28/30), predominantly Eurasian Jays (18/28), but also Common Buzzards Buteo buteo (5/28), Great Spotted Woodpeckers Dendrocopos major (3/28) and Eurasian Sparrowhawks Accipiter nisus (2/28). There was one predation by both a Eurasian Badger Meles meles and a Red Fox Vulpes vulpes. There were no records of Grey Squirrels Sciurus carolinensis depredating nests.
Conclusions Nest predation rates were similar in both periods, suggesting that increased rates of nest predation have not been driving the decline of the Wood Warbler population in Wales. Deployment of nest cameras did not affect nest survival rates and were successful in identifying nest predators, the majority of which were avian, especially Eurasian Jays. Knowledge of the identity of nest predators can aid the development of conservation measures.
For local populations to remain stable there needs to be a balance between annual productivity and immigration on the one hand and annual mortality and emigration on the other (Newton Citation1998). A number of factors can influence reproductive success, such as parasitism, food supply and weather (Newton Citation1998), but the single greatest cause of nest failure for most bird species is predation (Ricklefs Citation1969). Nest predation is an important natural phenomenon that has driven the evolution of a number of life-history traits, including clutch size, the length of nesting periods and nest site selection (Martin & Li Citation1992, Martin Citation1995, Bennett & Owens Citation2002). However, in recent years, increasing rates of nest predation have been implicated in the declines of a number of species (e.g. gamebirds: Tapper et al. Citation1996; waders: Grant et al. Citation1999; neotropical migrants: Thompson Citation2007). Where populations are limited by increased nest mortality, this has lead to calls for increased predator control or the development of novel conservation measures to counter the impact of predation (Fletcher et al. Citation2010, Smith et al. Citation2011a).
Elucidating the causes of nest failure is important as it can aid in the development of appropriate conservation management (Thompson Citation2007). Traditionally, the identity of nest predators has been inferred from nest remains and artificial nest studies (Rearden 1951, Green et al. Citation1987, Major Citation1991), both of which have been shown to be unreliable (Larivière Citation1999, Thompson & Burhans Citation2004). In recent years the use of miniature nest cameras has revolutionized the study of nest predation. Although not completely free of bias (Richardson et al. Citation2009), nest cameras have for the first time allowed the accurate identification of predator species. Although initially most studies were conducted in North America (reviewed by Weidinger Citation2008a), cameras have now been used on a wide range of European birds (Schaefer Citation2004, Perkins et al. Citation2005, Bolton et al. Citation2007, Weidinger Citation2009), including declining woodland species (Stevens et al. Citation2008).
Populations of woodland birds in the UK have undergone steep declines in recent decades, especially woodland specialists and long-distance migrants (Gregory et al. Citation2007, Hewson & Noble Citation2009), and increasing rates of nest predation have been suggested as a possible cause (Fuller et al. Citation2005). However, there is currently little evidence to support this hypothesis, either from broad-scale surveys of woodland bird changes (Amar et al. Citation2006, Newson et al. 2010b) or studies of individual species (Siriwardena Citation2005, Citation2006).
Wood Warblers Phylloscopus sibilatrix are long-distance migrants, wintering from Sierra Leone to western Kenya (Urban et al. Citation1997). Their breeding range is almost totally confined to Europe, with the largest population in Russia (Birdlife International Citation2004). Although some populations have remained stable, there have been declines in many countries in Northern and Western Europe, resulting in a ‘moderate decline’ in the European population between 1980 and 2005 (PECBMS Citation2011). It is currently on the UK Red List of birds of conservation concern (Eaton at al. Citation2009), the species having declined by 63% between 1995 and 2010 (Baillie et al. Citation2010), although this masks some regional variation. Repeat surveys of woodland birds in 2003/04 (Amar et al. Citation2006) showed that the largest declines since the mid-1980s were in south-east and south-west England (–76% and –67%, respectively), with lesser reductions in Scotland (–44%) and Wales (–31%). Following range contraction (Gibbons et al. Citation1993), Wood Warblers are now found predominantly in northern and western regions of the UK, and tend to be associated with mature upland oak woods that, often as a result of sheep grazing, have poorly developed understorey and ground vegetation (Hope-Jones Citation1972, Hogstad & Moksnes 1986, Bibby Citation1989). Although ground-nesting species in forest habitats may suffer lower mortality rates than those in higher shrub layers (Martin Citation1993, Wesołowski & Tomiałojć Citation2005, Yanes & Suarez Citation1995), Wood Warbler nests can suffer high rates of predation (Wesołowski Citation1985). In this study, we aim to (1) quantify nest predation rates for two periods (1982–84 and 2009–11) in one of their remaining population strongholds in the UK, the Western Atlantic Oakwoods in mid Wales, and compare them to assess whether increased predation could have driven the population decline, and (2) identify predators of Wood Warbler nests.
METHODS
Study area
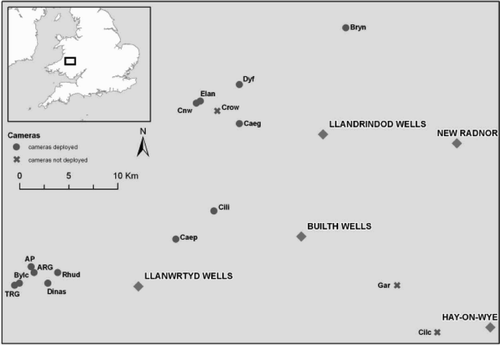
The study was carried out between 2009 and 2011 in 16 woodland sites (median area = 17.1 ha, range, 3.9–38.2), in Powys and Carmarthenshire, mid Wales, UK, within an area of about 1000 km2 (). The median distance between neighbouring study woods was 1.6 km (1.4 km for 13 sites where cameras were deployed; range 0.4–16.5). Study woods were dominated by Sessile Oak Quercus petraea, with lesser amounts of Birch Betula spp. and Rowan Sorbus aucuparia, and a poorly developed understorey consisting mainly of Hazel Corylus avellana with occasional Hawthorn Crataegus monogyna and Holly Ilex aquifolium bushes. There was a sparse ground cover of mosses, grasses, Bracken Pteridium aquilinum, Bilberry Vaccinium myrtillus and Bramble Rubus fruticosus. The surrounding landscape is a mixture of permanent pasture and, at higher altitudes, moorland and coniferous plantations. In 1982–84, nests were monitored at four of the same woods in Carmarthenshire (Stowe Citation1987).
Figure 1. Map showing the location and abbreviated names of 16 study sites, and whether cameras were deployed on each site. AP and ARG are both part of the Allt Rhyd y Groes National Nature Reserve; bylc, TRG and Dinas are all part of the Dinas-Gwynfredd RSPB reserve; elan, cnw, crow and dyf are all part RSPB Elan Valley reserves; all other sites are privately owned.
Data collection
Territories were identified by the presence of singing males on weekly visits to each wood. Surveys were conducted from the time of arrival of birds in mid–late April until the end of the breeding season (mid July) in each year. Within territories, females were located usually by their vocalisations, and watched for 30–60 minutes to confirm whether she was nesting, or until a nest was found (female Wood Warblers will readily return to the nest at all stages, even if a person is standing nearby). Nests were visited every 3–4 days to ascertain their fate and pin-point the date of failure as accurately as possible. First Egg Dates (FED) were either determined at nests visited during egg-laying, or were back-calculated after chicks had hatched, assuming an incubation period of 13 days and the laying of one egg per day with incubation starting after the last egg has been laid (Fourage 1968). When hatching date was not known, the age of nestlings was estimated by comparing the degree of feather development with that of nestlings of known age. Nests were assumed to have failed if all eggs, or chicks younger than 10 days old, disappeared from the nest, and there were no signs from nearby adults to suggest that chicks had fledged. During 1982–84, visits to nests usually stopped when chicks were 9 days old, after which they were assumed to have fledged unless evidence at the nest suggested otherwise. Fledging of nests at which cameras were deployed was confirmed by checking images stored on the memory card. At depredated nests, the state of the nest and the presence of any remains were recorded.
Camera system
A maximum of 12 (6 in 2009, 12 in 2010 & 2011) purpose-built camera systems (for details, see Bolton et al. Citation2007, Stevens et al. Citation2008) were deployed at nests in each year. Cameras were initially deployed randomly and then, when these initial nests failed or were successful, redeployed at nests of any age, even quite near to estimated fledging date as we wanted to maximize our chances of recording predation events. Nests were only precluded from use due to logistical reasons, such as proximity to a public footpath or on difficult terrain. The cameras (3 cm × 2 cm × 2 cm) were placed 0.5–1.5 m from the nest, and camouflaged with vegetation. Each camera was connected by means of a cable to the recording unit and 12 V battery, which were placed several metres from the nest in a camouflaged waterproof covering, thereby allowing batteries and cards to be changed while minimizing disturbance. Images of nest activity were recorded by means of a video motion detection facility, such that movements near the nest would trigger the camera, while minimizing the impact of swaying vegetation. Six infrared light-emitting diodes (LED) were attached in an array around the lens to provide illumination at night. The median (range) time a camera was at a nest was 11 days (2–26), and were set up 7 days (0–24) days after the nest was found. Cameras were not deployed during the building or laying phases to reduce the risk of nest desertion (Schaefer Citation2004). The majority of cameras were deployed at nests containing eggs (55%, n = 40), the average (median, range) age of the nest (days after first egg date) was 10 days (5–18). The remainder (45%, n = 33) were deployed during the nestling phase, when the age of chicks was 5 days (0–9). Battery-life was about 5 days, but to ensure that we did not miss any predation events, and to be consistent with visitation rates to non-camera nests, batteries and memory cards were changed every 3–4 days. Once cameras were set, we retreated and confirmed that the female returned to the nest, which usually happened within 5 minutes. On two occasions when the female did not quickly settle, we removed the camera equipment and returned on a subsequent day and placed the camera slightly further away. On both occasions, the female returned to the nest immediately.
Analysis
Daily Nest Survival Rates (DSR) were calculated according to Mayfield Citation(1975), and the egg and chick stages compared as a simple Chi-squared test of the proportion depredated, and following the methodology of Johnson Citation(1979). DSR are quoted for nest losses due to predation only. The effect of deploying cameras on nest survival rates was tested within a Generalized Linear Mixed Model (GLMM) following the logistic exposure model of Shaffer Citation(2004). Each line of data represented an individual nest exposure day, allowing the modelling of variables that vary in time, e.g. age of nest and date, as well as categorical variables that may change from one day to the next, such as whether or not a camera was deployed at a nest. A binomial dependent variable indicated whether a nest was successful or not on a given day. To account for multiple lines of data referring to each nest, ‘nest identity’ was included as a random factor; including ‘site’ as a second random factor caused the model to fail to converge, due to the overlap in the variability being explained by the random terms, ‘nest identity’ being nested within ‘site’. Other variables included in models were ‘year’ (three-level factor, pairwise comparisons estimated from lsmeans procedure), ‘day’ (days from 1 April) and ‘age’ (number of days from FED or age of nestlings); ‘age’ and ‘day’ were also included in analyses as quadratic (nonlinear) terms. DSR of nests during laying/incubation and the nestling phase were modelled separately. The final models were obtained by backward deletion of non-significant variables until only significant (P < 0.05) variables remained. Analyses were conducted on two data sets: (1) 2009–11, all sites, including a two-level factor ‘camera’, and (2) 1982–84 and 2009–11, all sites, including two-level ‘period’. GLMMs were fitted using procedure glimmix of sas version 9.2, with binomial distribution and logit link term (SAS Institute Inc. Citation2002–08).
RESULTS
A total of 176 nests were found during 2009–11, of which 8 were excluded from analyses as they were found during building but were never seen to contain eggs. One other nest was excluded as it was deserted during egg-laying, and colour ringing indicated the replacement of the original male; another nest was subsequently started. This left a total of 167 nests that were monitored. Of these, 63 (38%) nests were found during nest-building, 73 (44%) during incubation, and 31 (18%) when adults were provisioning chicks. Cameras were deployed at 73 nests, of which 32 (44%) failed due to predation; of the 94 non-camera nests, 30 (32%) were lost to predators (χ2 1 = 1.57, P = 0.2 (with Yates correction for one degree of freedom). There was no evidence from camera nests that these may have been abandoned and their contents taken by scavengers). In addition to the loss of 37% (n = 62) of nests due to predation, other causes of failure were: desertion (3%, n = 5 (camera nests, n = 1)), brood starvation (2.4%, n = 4 (camera nests, n = 1)) and the predation of the female (0.6%, n = 1). In seven of the nine cases of desertion and starvation there was no re-nesting attempt, suggesting that predation of the female could have been the ultimate cause of these failures too. Of 67 nests monitored in 1982–84, 39% (n = 26) were lost to predation; other causes of nest failure were desertion (1.5%, n = 1), eggs being washed away (1.5%, n = 1) and predation of the female (3%, n = 2).
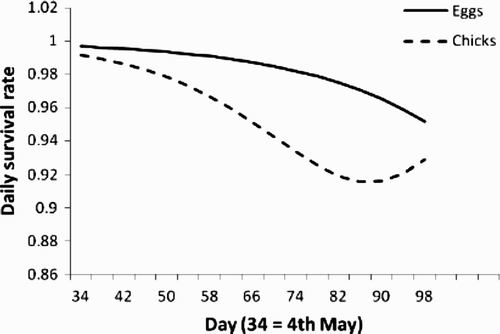
In 2009–11, the DSR (losses to predation only; 1982–84 in parentheses) was 0.979 ± 0.003 (0.966 ± 0.006). DSR at the egg stage was greater in 2009–11 than in 1982–84 (0.986 versus 0.952, respectively), and declined over the course of the season in the first period only (day × period interaction, ) but there was no difference between periods at the nestling phase. The overall probability (Mayfield estimate (annual range)) of a nest surviving was 0.57 (0.47–0.66) during 2009–11, and 0.37 (0.26–0.72) during 1982–84. In 2009–2011 DSR varied between nests at different reproductive stages: egg, 0.989 ± 0.003; chick, 0.969 ± 0.005, z = 3.8, P < 0.0001 (1982–84, egg, 0.976 ± 0.008; chick, 0.955 ± 0.011, z = 1.5, P > 0.1). Predation of nests during the nestling phase varied between years (; pairwise comparisons, 2009 versus 2010, t 174 = 1.45, P = 0.15, 2009 versus 2011, t 77 = 1.77, P = 0.08, 2010 versus 2011, t 146 = 3.26, P = 0.001); DSR declined temporally during both the laying/incubation and nestling phases (; ). There was a nonlinear relationship with the age of nestlings, declining over the first seven days then increasing. The deployment of cameras had no effect on DSR at either reproductive stage ().
Table 1. Final models of daily survival rates (losses to predation only) of Wood Warbler nests during the egg and chick stages: (a) a comparison between 1982–84 and 2009–11, and (b) incorporating the effect of the deployment of nest cameras for nests in 2009–11. Only significant terms are included, after backwards deletion of non-significant variables, plus camera and period effects. ‘Day’ refers to days from 1 April, ‘Age’ and its quadratic term ‘Age2’ the number of days from FED, or age of nestlings (‘Day2’ was also included in analyses but was retained in neither model).
Figure 2. Estimated daily nest survival rates in relation to days from the beginning of May for nests at both the egg and chick stages; lines fitted after accounting for the parameter estimates of other significant variables, assuming the median value of each, and ‘Year’ set to 2011 (estimate = 0).
During 2009–11, birds were responsible for the majority of recorded predation events (n = 28/30, 93%), with Eurasian Jays Garrulus glandarius accounting for 64% (n = 18) of avian predation and 60% overall (; ). Other bird predators included Common Buzzard Buteo buteo, Great Spotted Woodpecker Dendrocopos major and Eurasian Sparrowhawk Accipiter nisus. Only two instances of predation by mammals were recorded, once each by Red Fox Vulpes vulpes and Eurasian Badger Meles meles. The majority of predation events by nearly all species resulted in no signs of predation being found at the nest (). On four occasions the predation event was not recorded, even though the camera continued to work afterwards. Pieces of eggshell were left at one of the nests, and in three of the four cases evidence from the cameras suggested that the predation happened at night.
Table 2. The number of predation events captured on camera for each predator species, and the condition of the nest after predation. ‘Disturbed’ nests include those where small amounts of nest material were dislodged to those where the whole nest had been ripped out. (‘Remains’ in parentheses.)
Figure 3. Nest camera images of (a) Jay, (b) Great Spotted Woodpecker, (c) Buzzard, (d) Sparrowhawk, (e) Badger and (f) Fox.

The complete depredation of a nest (no instances of partial predation were recorded) was usually carried out over more than one visit (median, 3 visits, range, 1–7) for all species apart from Fox. All visits to a nest occurred on a single day on 64% (18/28) of occasions (all species), 29% (8/28) over two consecutive days (Jay, Great Spotted Woodpecker), and 4% (1/28) each were predated three (Buzzard) and six (Sparrowhawk) days after the initial visit. There was one instance of a nest being depredated by multiple predators: Buzzard (two visits, including one on which no nestlings were removed), Great Spotted Woodpecker and Jay.
DISCUSSION
Predation was the greatest cause of mortality of Wood Warbler nests in this Welsh population. However, nest predation rates have not increased since the 1980s, suggesting predation has not been the cause of the Wood Warbler decline in Wales. In common with an increasing number of recent studies, we have demonstrated the value of deploying cameras in studying nest predation, accurately identifying the main predators responsible. Nesting Wood Warblers became quickly habituated to the presence of the cameras, there was no evidence that any nest was deserted in response to their deployment, and their presence did not affect daily nest survival rates.
Increasing rates of nest predation have been identified as a potential cause of the recent decline in woodland bird populations (Fuller et al. Citation2005), although evidence for this is equivocal (Siriwardena Citation2005, Citation2006, Amar et al. Citation2006, Newson et al. Citation2010a, Citationb). Over a similar period to these declines, the populations of a number of potential nest predators have increased (Harris et al. Citation1995, Baillie et al. Citation2010). Populations of both Jays (+48%) and Great Spotted Woodpeckers (+178%) have shown significant population increases in Wales since 1995 (Risely et al. Citation2011). The National Gamebag Census revealed a 62% increase (64% for upland regions of England and Wales) in Fox numbers between 1984 and 2009 (Aebischer et al. Citation2011), while comparison of two national surveys (1985–88 and 1994–97) suggested a 77% increase in the UK Badger population (Battersby et al. Citation2005). While there was no difference in nest survival of nests containing chicks between the two periods (1982–84 and 2009–11), survival at the egg stage was higher in the later period, mirroring the long-term trend found in analyses of nationwide data (Baillie et al. Citation2010). Whether this trend is due to changes in the predator community is unknown; however, the two instances of egg predation caught on camera were both by Jays. The proportion of nests that were lost to predation in Wales in 2009–11 (37%) was comparable to that found in the same study area in the 1980s (39%) and in studies from elsewhere in Europe, e.g. Sweden, 37% (Temrin & Jakobsson Citation1988) and Poland, 43–87% (Wesołowski & Maziarz Citation2009). However, the Wood Warbler population in Wales has remained relatively stable, so there is still a need to investigate whether nest predation has played a role in the steeper declines observed in other UK regions (Amar et al. Citation2006).
The dominant predators of Wood Warbler nests in our Welsh study woods were Jays, accounting for 60% of all predation events recorded. Of the predators captured on camera, Jays have been found to be responsible for the majority of nest predation in other studies (Schaeffer 2004, Stevens et al. Citation2008). The declining daily nest survival rates at both the egg and chick stages can be related to the breeding season of this predator: increasing rates of predation, especially in June and July, coincided with the time when Jays were either feeding nestlings (Holyoak Citation1967; Joys & Crick Citation2004), or the post-fledging period when juveniles are still fed by their parents (Goodwin Citation1986), and the abundance of Jays is at its highest. Adult Jays predominantly provision their nestlings with Lepidopteran larvae (Owen Citation1956), which are past their peak in abundance by mid-June in our study woods (Smith et al. Citation2011b). This may further increase the risk to nesting birds as Jays switch to alternative prey. It is commonly found that nest mortality is higher in the nestling phase than during incubation due to increased parental activity at the nest (Weidinger Citation2002), although this can be offset by more concealed nests sites (Martin et al. Citation2000). Wood Warbler adults are very vocal at all stages of the nesting cycle, but especially during the nestling phase as females spend less time on the nest. Combined with increasing rates of nest visitation to feed chicks, this is more likely to attract a visually orientated predator such as the Jay (Goodwin Citation1986).
Although we recorded relatively few predation events, the identity of the dominant predator (Jays) is probably accurate, although the proportion of predation events attributable to this species may change with a greater sampling effort (Weidinger Citation2008a). In total, we recorded only six species of nest predator. Although partly due to the relatively small number of predation events (Weidinger Citation2008a), it may also reflect a comparatively depauperate predator community in Welsh oakwoods. For example, although our study woods were patchily distributed, interspersed with pasture and moorland, large numbers of Corvid predators, which are usually associated with habitat fragmentation (Andren Citation1992), were not present. In addition to Jays, the other species that we recorded (Buzzard, Sparrowhawk, Great Spotted Woodpecker, Badger and Red Fox) are all known nest predators (Schaefer Citation2004, Bolton et al. Citation2007, Stevens et al. Citation2008, Weidinger Citation2009). The relative unimportance of mammal predation is in contrast to many camera studies of nesting birds, including Wood Warbler in Switzerland (Grendelmeier et al. Citation2011) where 83% (20/24) of recorded predation events was attributable to Foxes, Martens Martes spp. and Badgers. A number of other mammal species were probably present in our woods, e.g. Stoats Mustela erminea, Weasels M. nivalis, Polecats M. putorius and European Hedgehogs Erinaceus europaeus (Harris et al. Citation1995), but were not recorded depredating nests. It is possible we underestimated the extent of mammalian predation as in three of the four nests where the camera failed to record the predation event, there was evidence that they were predated at night (i.e. there was bird activity recorded the evening before but not the morning after the failure of the nests, even though the camera continued to record images) when both cases of mammal predation occurred. Also, the presence of eggshell remains at one of the nests may be indicative of small mammal predation (Brown et al. Citation1998, Marini & Melo Citation1998, Walankiewicz Citation2002).
The only other potential predator regularly seen was the introduced Grey Squirrel Sciurus carolinensis, which was common in all of our woods. Controversy surrounds nest predation by squirrels and its potential link to widespread songbird declines (Hewson et al. Citation2004), yet there is little evidence to support this claim (Newson et al. Citation2010b). Although it is regularly recorded depredating nests in its native North America, other species are normally identified as the dominant predator in these studies (Picman Citation1988, Hanski et al. Citation1996, Fenske-Crawford & Niemi Citation1997, Farnsworth & Simons Citation2000). In Europe, the Red Squirrel Sciurus vulgaris, which would have once occurred in our study area (UK Red Squirrel Group Citation2011), also plays a relatively minor role in overall nest mortality (Walankiewicz Citation2002, Wesołowski Citation2002, Eggers et al. 2004, Weidinger Citation2009), except perhaps where there is competition for nest boxes (Shuttleworth 2001). Grey Squirrels were frequently recorded by our nest cameras but never appeared to notice the nest; Stevens et al. Citation(2008) also recorded squirrels at Spotted Flycatcher Muscicapa striata nests, but with no evidence of predation.
In common with numerous recent studies (Larivière & Messier Citation1997, Marini & Melo Citation1998, Brown et al. Citation1998, Larivière Citation1999, Farnsworth & Simons Citation2000, Williams & Wood Citation2002, Stevens et al. Citation2008), inference of predator identity from nest remains was inconclusive in the present study. Although the dominant predator (Jay) tended to leave the nest untouched (16/18, 89%), most other species did the same. There was also some intraspecific variation in both Jay and Buzzard predation, adding further uncertainty (Larivière Citation1999). The potential effect of observer activity on nest success is an important issue, not only in terms of scientific bias, but also from a conservation standpoint (Weidinger Citation2008b, Ibáñez-Álamo et al. Citation2012). Our study has provided further evidence that the deployment of cameras need not have an effect on nest success (Richardson et al. Citation2009, Schaeffer 2004, Stevens et al. Citation2008). Given the importance of identifying nest predators (and eliminating other potential suspects) in the formulation of conservation strategy, this study highlights the benefits of such techniques in studies of nest mortality especially as, with careful deployment, they have no effect on nest mortality rates.
ACKNOWLEDGEMENTS
We are very grateful to all the landowners who allowed access to their woods; to Anna Riach and Derek Gruar who helped with fieldwork in 2009; to Paul Britten for creating our study map. The study was part-funded by the Countryside Council for Wales (CCW) in 2009 and 2010. We are grateful for comments from Tomasz Wesołowski and an anonymous reviewer.
REFERENCES
- Aebischer , N. J. , Davey , P. D. and Kingdon , N. G. 2011 . National Gamebag Census: Mammal trends to 2009 Game & Wildlife Conservation Trust, Fordingbridge (http://www.gwct.org.uk/ngc)
- Amar , A. , Hewson , C. M. , Thewlis , R. M. , Smith , K. W. , Fuller , R. J. , Lindsell , J. A. , Conway , G. , Butler , S. and MacDonald , M. A. 2006 . What's Happening to our Woodland Birds? Long-term Changes in the Populations of Woodland Birds , RSPB Research Report No. 19, Sandy, Bedfordshire
- Andren , H. 1992 . Corvid density and nest predation in relation to forest fragmentation – a landscape perspective . Ecology , 73 : 794 – 804 .
- Baillie , S. R. , Marchant , J. H. , Leech , D. I. , Renwick , A. R. , Joys , A. C. , Noble , D. G. , Barimore , C. , Conway , G. J. , Downie , I. S. , Risely , K. and Robinson , R. A. 2010 . Breeding Birds in the Wider Countrside: Their Conservation Status 2010 BTO Research Report No. 565. BTO, Thetford (http://www.bto.org/birdtrends)
- Battersby, J. (Ed.) & Tracking Mammals Partnership . 2005 . UK Mammals: Species Status and Population Trends. First Report by the Tracking Mammals Partnership JNCC/Tracking Mammals Partnership, Peterborough
- Bennett , P. M. and Owens , I. P.F. 2002 . Evolutionary Ecology of Birds: Life Histories, Mating Systems and Extinction , Oxford : Oxford University Press .
- Bibby, C.J. 1989. A survey of breeding wood warblers Phylloscopus sibilatrix in Britain, 1984–1985. Bird Study 36, 56–72
- Birdlife International 2004. Birds in Europe: population estimates, trends and conservation status. Birdlife International, Cambridge, UK.
- Bolton , M. , Butcher , N. , Sharpe , F. , Stevens , D. and Fisher , G. 2007 . Remote monitoring of nests using digital camera technology . J. Field Ornithol. , 78 : 213 – 220 .
- Brown , K. P. , Moller , H. , Innes , J. and Jansen , P. 1998 . Identifying predators at nests of small birds in a New Zealand forest . Ibis , 140 : 274 – 279 .
- Eaton , M. A. , Brown , A. F. , Noble , D. G. , Musgrove , A. J. , Hearn , R. , Aebischer , N. J. , Gibbons , D. W. , Evans , A. and Gregory , R. D. 2009 . Birds of conservation concern 3: the population status of birds in the United Kingdom and the Isle of Man . Br. Birds. , 102 : 296 – 341 .
- Eggers , S. , Griesser , M. , Andersson , T. and Ekman , J. 2005 . Nest predation and habitat change interact to influence Siberian Jay numbers . Oikos , 111 : 150 – 158 .
- Farnsworth , G. L. and Simons , T. R. 2000 . Observations of Wood Thrush nest predation in a large contiguous forest . Wilson Bull , 112 : 82 – 87 .
- Fenske-Crawford , T. J. and Niemi , G. J. 1997 . Predation of artificial ground nests at two types of edges in a forest-dominated landscape . Condor , 99 : 14 – 24 .
- Fletcher , K. , Aebischer , N. J. , Baines , D. , Foster , R. and Hoodless , A. N. 2010 . Changes in breeding success and abundance of ground-nesting moorland birds in relation to the experimental deployment of legal predator control . J. Appl. Ecol. , 47 : 263 – 272 .
- Fourarge , J. G. 1968 . Le Pouillot Siffleur Phylloscopus sibilatrix Bechstein . Gerfaut , 58 : 1 – 368 .
- Fuller , R. J. , Noble , D. G. , Smith , K. W. and Vanhinsbergh , D. 2005 . Recent declines in populations of woodland birds in Britain: a review of possible causes . Br. Birds. , 98 : 116 – 143 .
- Gibbons , D. , Reid , J. B. and Chapman , R. A. 1993 . The New Atlas of Breeding Birds in Britain and Ireland: 1988–1991 British Trust for Ornithology, Scottish Ornithologists' Club, Irish Wildbird Conservancy
- Goodwin , D. 1986 . Crows of the World , 2 , London : British Museum .
- Grant , M. C. , Orsman , C. , Easton , J. , Lodge , C. , Smith , M. , Thompson , G. , Rodwell , S. and Moore , N. 1999 . Breeding success and causes of breeding failure of curlew Numenius arquata in Northern Ireland . J. Appl. Ecol. , 36 : 59 – 74 .
- Green , R. E. , Hawell , J. and Johnson , T. H. 1987 . Identification of predators of wader eggs from egg remains . Bird Study , 34 : 87 – 91 .
- Gregory , R. D. , Vorisek , P. , van Strien , A. , Gmelig Meyling , A. W. , Jiguet , F. , Fornasari , L. , Reif , J. , Chylarecki , P. and Burfield , I. 2007 . Population trends of widespread woodland birds in Europe . Ibis , 149 ( Suppl. 2 ) : 78 – 97 .
- Grendelmeier , A. , Gerber , M. , Pasinelli , G. and Arlettaz , R. 2011 . Breeding success and nest predation of wood warbler (Phylloscopus sibilatrix) in northern Switzerland (Abstract). 8th Conference of the European Ornithologists' Union (L. Fusani, T. Coppack & M. Strazds, eds.), 27–30 August 2011, Riga, Latvia
- Hanski , I. K. , Fenske , T. J. and Niemi , G. J. 1996 . Lack of edge effect in nesting success of breeding birds in managed forest landscapes . Auk , 113 : 578 – 585 .
- Harris , S. , Morris , P. , Wray , S. and Yalden , D. 1995 . A Review of British mammals; Population Estimates and Conservation Status of British Mammals other than Cetaceans , Peterborough : Joint Nature Conservation Committee .
- Hewson , C. M. and Noble , D. G. 2009 . Population trends of breeding birds in British woodlands over a 32-year period: relationships with food, habitat use and migratory behaviour . Ibis , 151 : 464 – 486 .
- Hewson , C. M. , Fuller , R. A. , Mayle , B. and Smith , K. W. 2004 . Possible impacts of Grey Squirrels on birds and other wildlife . Br. Wildl. , 15 : 183 – 191 .
- Hogstad, O. & Moksnes, A. 1986. Expansion and present status of the wood warbler Phylloscopus sibilatrix in central Norway. Fauna Norveg. Series C, 9, 49–54.
- Holyoak , D. 1967 . Breeding biology of the Corvidae . Bird Study , 14 : 153 – 168 .
- Hope Jones, P. 1972. Succession in breeding bird populations of sample Welsh oakwoods. Brit. Birds, 65, 291–299
- Ibáñez-Álamo , J. D. , Sanllorente , O. and Soler , M. 2012 . The impact of researcher disturbance on nest predation rates: a meta-analysis . Ibis , 154 : 5 – 14 .
- Johnson , D. H. 1979 . Estimating nest success: the Mayfield method and an alternative . Auk , 96 : 651 – 661 .
- Joys , A. C. and Crick , H. Q.P. 2004 . Breeding Periods for Selected Bird Species in England BTO Research Report No. 352. BTO, Thetford
- Larivière , S. 1999 . Reasons why predators cannot be inferred from nest remains . Condor , 101 : 718 – 721 .
- Larivière , S. and Messier , F. 1997 . Characteristics of waterfowl nest depredation by the Striped Skunk (Mephitis mephitis): can predators be identified from nest remains? . Am. Mid. Nat. , 137 : 393 – 396 .
- Major , R. E. 1991 . Identification of nest predators by photography, dummy eggs, and adhesive tape . Auk , 108 : 190 – 195 .
- Marini , M. Â. and Melo , C. 1998 . Predators of quail eggs, and the evidence of the remains: implications for nest predation studies . Condor , 100 : 395 – 399 .
- Martin , T. E. 1993 . Nest predation among vegetation layers and habitat types: revising the dogmas . Am. Nat. , 141 : 897 – 913 .
- Martin , T. E. 1995 . Avian life history evolution in relation to nest sites, nest predation and food . Ecol. Monog. , 65 : 101 – 127 .
- Martin , T. E. and Li , P. 1992 . Life history traits of open- vs. cavity-nesting birds . Ecology , 73 : 579 – 592 .
- Martin , T. E. , Scott , J. and Menge , C. 2000 . Nest predation increases with parental activity: separating nest site and parental activity effects . Proc. R. Soc. Lond. B. , 267 : 2287 – 2293 .
- Mayfield , H. F. 1975 . Suggestions for calculating nest success . Wilson Bull , 87 : 456 – 466 .
- Newson , S. E. , Rexstad , E. A. , Baillie , S. R. , Buckland , S. T. and Aebischer , N. J. 2010a . Population change of avian predators and grey squirrels in England: is there evidence for an impact on avian prey populations? . J. Appl. Ecol. , 47 : 244 – 252 .
- Newson , S. E. , Leech , D. I. , Hewson , C. M. , Crick , H. Q.P. and Grice , P. V. 2010b . Potential impact of Grey Squirrels Siurus carolinensis on woodland bird populations in England . J. Ornithol. , 151 : 211 – 218 .
- Newton , I. 1998 . Population Limitation in Birds , London : Academic Press .
- Owen , D. F. 1956 . The food of nestling Jays and Magpies . Bird Study , 3 : 257 – 265 .
- PECBMS 2011. Trends of common birds in Europe, 2010 update. European Bird Census Council, Prague, www.ebcc.info.
- Perkins , A. J. , Hancock , M. H. , Butcher , N. and Summers , R. W. 2005 . Use of time-lapse video cameras to determine causes of nest failure of Slavonian Grebes Podiceps auritus . Bird Study , 52 : 159 – 165 .
- Picman , J. 1988 . Experimental study of predation on eggs of ground-nesting birds: effects of habitat and nest distribution . Condor , 90 : 124 – 131 .
- Reardon , J. D. 1951 . Identification of waterfowl nest predators . J. Wildl. Manage. , 15 : 386 – 395 .
- Richardson , T. W. , Gardalli , T. and Jenkins , S. H. 2009 . Review and meta-analysis of camera effects on avian nest success . J. Wildl. Manage. , 73 : 287 – 293 .
- Ricklefs , R. E. 1969 . An analysis of nesting mortality in birds . Smithson. Contrib. Zool. , 9 : 1 – 48 .
- Risely, K., Renwick, A.R., Dadam, D., Eaton, M.A., Johnston, A., Baillie, S.R., Musgrove, A.J. & Noble, D.G. 2011. The Breeding Bird Survey 2010. BTO Research Report 597, British Trust for Ornithology, Thetford, UK.
- SAS Institute Inc . 2002–08 . SAS/STAT Software. Release 9.2 , Cary , NC : SAS Institute Inc .
- Schaefer , T. 2004 . Video monitoring of shrub-nests reveals nest predators . Bird Study , 51 : 170 – 177 .
- Shaffer , T. L. 2004 . A unified approach to analyzing nest success . Auk , 121 : 526 – 540 .
- Shuttleworth , C. M. 2011 . Interactions between the red squirrel (Sciurus vulgaris), great tit (Parus major) and jackdaw (Corvus monedula) whilst using nest boxes . J. Zool. , 255 : 269 – 272 .
- Siriwardena , G. M. 2005 . Possible roles of habitat, competition and avian nest predation in the decline of the Willow Tit Parus montanus in Britain . Bird Study , 51 : 193 – 202 .
- Siriwardena , G. M. 2006 . Avian nest predation, competition and the decline of British Marsh Tits Parus palustris . Ibis , 148 : 255 – 265 .
- Smith , R. K. , Pullin , A. S. , Stewart , G. B and Sutherland , W. J. 2011a . Is nest predator exclusion an effective strategy for enhancing bird populations? . Biol. Cons. , 144 : 1 – 10 .
- Smith , K. W , Smith , L. , Charman , E. , Briggs , K. , Burgess , M. , Dennis , C. , Harding , M. , Isherwood , C. , Isherwood , I. and Mallord , J. 2011b . Large-scale variation in the temporal patterns of the frass fall of defoliating caterpillars in oak woodlands in Britain: implications for nesting woodland birds . Bird Study , 78 : 506 – 511 .
- Stevens , D. K. , Anderson , G. Q.A. , Grice , P. V. , Norris , K. and Butcher , N. 2008 . Predators of Spotted Flycatcher Muscicapa striata nests in southern England as determined by digital nest-cameras . Bird Study , 55 : 179 – 187 .
- Stowe , T. J. 1987 . The habitat requirements of some insectivorous birds and the management of sessile oakwoods. Unpublished PhD thesis, The Botany School, Cambridge University, Cambridge
- Tapper , S. C. , Potts , G. R. and Brockless , M. H. 1996 . The effect of an experimental reduction in predation pressure on the breeding success and population density of grey partridges Perdix perdix . J. Appl. Ecol. , 33 : 965 – 978 .
- Temrin , H. and Jakobsson , S. 1988 . Female reproductive success and nest predation in polyterritorial wood warblers (Phylloscopus sibilatrix). Behav . Ecol. Sociobiol. , 23 : 225 – 231 .
- Thompson , F. R. 2007 . Factors affecting nest predation on forest songbirds in North America . Ibis , 149 ( Suppl. 2 ) : 98 – 109 .
- Thompson , F. R. and Burhans , D. E. 2004 . Differences in predators of artificial and real songbird nests: evidence of bias in artificial nest studies . Cons. Biol. , 18 : 373 – 380 .
- UK Red Squirrel Group . 2011 . Red Squirrels: Population Information , Scottish Natural Heritage, http://www.snh.org.uk/ukredsquirrelgroup/popInfo.asp (accessed 28 September 2011)
- Urban , E. K. , Hilary Fry , C. H. and Keith , S. 1997 . The Birds of Africa, Volume V: Thrushes to Puffback Flycatchers , Princeton , NJ : Princeton University Press .
- Walankiewicz , W. 2002 . Breeding losses in the Collared Flycatcher Ficedula albicollis caused by nest predators in the Białowieza National Park (Poland) . Acta Ornithol , 37 : 21 – 26 .
- Weidinger , K. 2002 . Interactive effects of concealment, parental behaviour and predators on the survival of open passerine nests . J. Anim. Ecol. , 71 : 424 – 437 .
- Weidinger , K. 2008a . Identification of nest predators: a sampling perspective . J. Avian Biol. , 39 : 640 – 646 .
- Weidinger , K. 2008b . Nest monitoring does not increase nest predation in open-nesting songbirds: inference from continuous nest-survival data . Auk , 125 : 859 – 868 .
- Weidinger , K. 2009 . Nest predators of woodland open-nesting songbirds in central Europe . Ibis , 151 : 352 – 360 .
- Wesołowski , T. 1985 . The breeding ecology of the Wood Warbler Phylloscopus sibilatrix in primaeval forest . Ornis Scand , 16 : 49 – 60 .
- Wesołowski , T. 2002 . Anti-predator adaptations in nesting Marsh Tits Parus palustris: the role of nest-site security . Ibis , 144 : 593 – 601 .
- Wesołowski , T. and Maziarz , M. 2009 . Changes in breeding phenology and performance of Wood Warblers Phylloscopus sibilatrix in a primeval forest: a thirty-year perspective . Acta Ornithol , 44 : 69 – 80 .
- Wesołowski , T. and Tomiałojć , L. 2005 . Nest sites, nest depredation, and productivity of avian broods in a primeval temperate forest: do the generalisations hold? . J. Avian Biol. , 36 : 361 – 367 .
- Williams , G. E. and Wood , P. B. 2002 . Are traditional methods of determining nest predators and nest fates reliable? An experiment with Wood Thrushes (Hylocichla mustelina) using miniature video cameras . Auk , 119 : 1126 – 1132 .
- Yanes , M. and Suarez , F. 1995 . Nest predation patterns in ground-nesting passerines on the Iberian peninsula . Ecography , 18 : 423 – 428 .