Abstract
Capsule The Ring Ouzel in central Europe occurs in a mosaic of mountain forests and open areas.
Aims To determine the habitat selection of the Ring Ouzel on a landscape scale in the western Carpathians. It was hypothesized that occurrence of the Ring Ouzel is associated with upper subalpine Carpathian forests and the presence of anthropogenic open habitats.
Methods The study compared habitat characteristics of Ring Ouzel breeding areas and randomly selected control plots.
Results The Ring Ouzel occurred between 685 and 1316 m asl, preferring middle and upper subalpine Carpathian forests. Significant differences between the Ring Ouzel breeding areas and randomly selected control plots were found for five of the landscape-scale habitat variables: altitude; distance to built-up areas; distance to roads; distance to small mountain meadows; and distance to clear-cut areas. A logistic regression model indicated that the important parameters influencing the occurrence of the Ring Ouzel were altitude and distance to the nearest clear-cut and small mountain meadow.
Conclusion The Ring Ouzel preferred higher altitudes and the proximity of clear-cuts, which presumably are a physionomical substitute for the timberline and alpine meadows. However, this species avoids the presence of small mountain meadows, presumably because of succession of a dense layer of vegetation on former mountain pastures. The presence of a cultural landscape in the Carpathians may have allowed the Ring Ouzel to colonize the lower mountain areas.
Human activity has resulted in entire communities of organisms associated with specific types of habitats being threatened on a continental scale (Gaston et al. Citation2003). Birds associated with mountain environments and the boreal and arctic zones of Europe are at highest risk in the context of forecasted climate change (Beniston et al. Citation1997, Lemoine et al. Citation2007). Mountain fauna is associated with specific vegetation zones, and the presence of these is, in turn, strongly determined by climatic conditions. Current observed global warming will presumably lead to an elevation of the upper limits of vegetation layers, and consequently to the disappearance of some species associated with specific zonal habitat conditions (Beniston et al. Citation1997, Pounds et al. Citation1999, Thomas et al. Citation2004, Huntley et al. Citation2006).
The Ring Ouzel Turdus torquatus is associated with boreal and mountain plant communities. This species occurs in the western Palearctic in three geographically isolated areas, comprising distinct subspecies: the boreal T. t. torquatus occurring in the British Isles and Scandinavia; the mountain T. t. alpestris occurring in the Pyrenees, Massif Central, Alps, Carpathians, and Balkans; and T. t. amicorum occurring in Asia Minor (Cramp Citation1988). Recent studies indicate a decline in number of the Ring Ouzel in various parts of its range (Janiga & Poxton Citation1997, BirdLife International Citation2004, Burfield & Brooke Citation2005, Sim et al. Citation2010) and not very optimistic scenarios for the future (Beale et al. Citation2006, von dem Bussche et al. Citation2008). However, data on the Carpathian population of the Ring Ouzel is vague and basically limited to information on the range of occurrence and population estimates (Hudec Citation1983, Janiga & Poxton Citation1997, Danko et al. Citation2002, Tomiałojć & Stawarczyk Citation2003, Šťastný et al. Citation2006, Dyrcz & Mielczarek Citation2007). There are no long-term studies that would indicate or predict population trends in central Europe.
European policy on nature protection introduces legal regulations to combat adverse changes in the environment. The EU Birds Directive and the Natura 2000 network form a system in which the protection of endangered species is a priority. Unfortunately, limited knowledge about the Ring Ouzel, which is currently listed as a species of Least Concern at the global level (IUCN Citation2011), makes it difficult to assess its conservation status with much confidence. Against this background, information on the central European population essential for the preservation of the species on a global scale is negligible. Surprisingly, gaps in knowledge pertain to nearly every aspect of the biology and ecology of the species in its Carpathian range. In light of potential risks and scenarios of population trends, a better understanding the ecology of the Ring Ouzel is a priority.
British populations of the Ring Ouzel are almost entirely associated with open upland habitats, especially heather moorland (Buchanan et al. Citation2003, Sim et al. Citation2007), while continental populations are associated with more forested mountain environments. Alpine populations of the Ring Ouzel occur in a wide zone from the lower subalpine zone up to high rocky areas; yet, they prefer the transitional area near the upper limits of the forest (von dem Bussche et al. Citation2008). Of great importance to the distribution of the species is the structure of vegetation, and especially the presence of coniferous tree stands with an open canopy and the presence of subalpine meadows poor in nutrients (von dem Bussche et al. Citation2008). In addition, the Ring Ouzel avoids compact tree plantations, fertile meadows, and built-up areas (Buchanan et al. Citation2003, von dem Bussche et al. Citation2008), which indicates the negative effects of anthropogenic transformation of the environment in mountain conditions. One of the potential causes of the decline in number of the Ring Ouzel is climate change (Beale et al. Citation2006). Predicted climate warming may lead to habitat changes, which is of particular relevance for the mountain species associated with highly specific zonal conditions (von dem Bussche et al. Citation2008).
Ring Ouzels in the Carpathians and Sudetes inhabit the Mountain Pine Pinus mugo zone, the transition zone at the upper limits of the forest, the upper subalpine zone, and sparsely reach the lower subalpine zone (Dyrcz Citation1973, Tomiałojć & Stawarczyk Citation2003, Danko et al. Citation2002). However, the habitat selection of the Carpathian population and the factors affecting it are unknown. The aim of the present study was to determine the distribution of the Ring Ouzel and its habitat selection at the landscape scale. It has been hypothesized that the optimal occurrence of the species in central European mountains is associated with the upper subalpine spruce forest zone and the presence of anthropogenic open areas in the landscape, which may be a structural equivalent of the upper limits of the forest and meadows of the alpine zone. The present study tested this hypothesis by comparing the species' actual distribution with random sample localities. We also reviewed the literature from central Europe to determine the vertical distribution and density of the Ring Ouzel at a greater, regional scale.
METHODS
Study area
The study was conducted in the Żywiec Beskids which are part of the Western Beskids (Kondracki Citation2000). This area consists of several mountain ranges, of which the Wielka Racza group (highest summit, 1236 m asl) and the Pilsko group (highest summit, 1557 m asl) are distinguishable. The Western Beskids are composed of layers of sandstones and claystones of flysch formations. Among the plant communities dominating are: fertile Carpathian beech forest Dentario glandulosae–Fagetum, forming the lower subalpine zone; fir–spruce forest Abieti–Piceetum, forming the middle subalpine zone; and the upper subalpine acidophilous Carpathian spruce forest Plagiothecio–Piceetum (Matuszkiewicz Citation2001, Holeksa & Szwagrzyk Citation2004a,Citationb; Szwagrzyk & Holeksa Citation2004). Recently the Norway Spruce Picea abies has become dominant in many places as a result of artificial introduction. However, more recently deterioration of artificial spruce stands has been observed over a large area of Żywiec Beskids, along with a return to original vegetation.
The present study included the Żywiec Beskids Special Protection Area PLB240002 (Important Bird Area PL127), covering the western part of the Żywiec-Orava Beskids (Ciach Citation2010). The area largely overlaps with the Żywiec Landscape Park and the Żywiec Beskids Special Area of Conservation PLH240006.
Methods and data analyses
In 2008, a survey of breeding birds was conducted in the Żywiec Beskids Natura 2000 Special Protection Area PLB240002 site (36 962 ha). As part of the fieldwork, lasting from mid-April to late July, three surveys (mid-April to mid-May; mid-May to mid-June; and mid-June to late July) were made. Observers searched an area of 2–5 km2 daily. They moved along transects located every 200–500 m, depending on the type of terrain and its accessibility to cover the entire area. This was combined with surveys using tape playback, every several hundred meters to elicit a response from Ring Ouzels (Ciach et al. Citation2009). All observations were marked on a map with a scale of 1:25 000. Neighbouring territories were identified based on the simultaneous observation of birds displaying territorial behaviour.
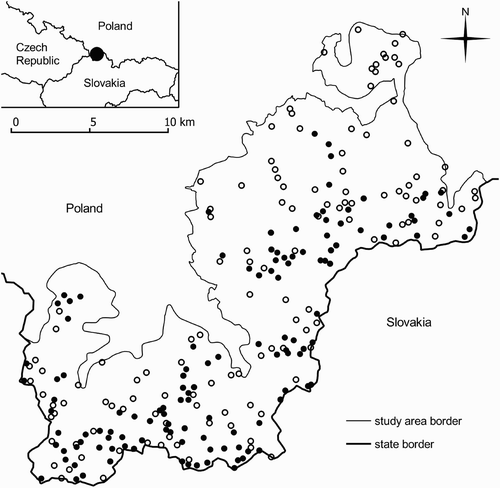
The results of the bird survey were combined with spatial data – an orthophotomap (aerial photography of the terrain), a digital elevation model (DEM), and a topographical database (GUGIK 2008, CODGIK Citation2010a,Citationb) to determine landscape-scale habitat variables for the Ring Ouzel localities and random control plots. Measurements were taken at 126 Ring Ouzel localities and 126 randomly selected control plots ().
Figure 1. The distribution of Ring Ouzel Turdus torquatus localities (•) and random points (◦) in the Żywiec Beskid Mountains Special Protection Area (southern Poland).
The centre of a breeding area occupied by a Ring Ouzel pair was specified as the centre of mass (barycentre) of a polygon, set from a minimum of three points of observation. For two observations, the centre of the distance between them was taken as the centre of a breeding area. For a single observation of a family, pair, or individual indicating occupation of a territory, the point of observation was taken as the centre of the breeding area. Data on the distribution of Ring Ouzel localities was recorded in the form of a vector layer, where the centre of a locality of the Ring Ouzel represented a point with defined geographic coordinates. In order to avoid pseudoreplication for clusters of the Ring Ouzel (where the number of breeding pairs in a locality was greater than one), the coordinates of the centre point of an area occupied by breeding pairs forming a cluster were used for analysis. Clusters were defined as a locations where centres of adjacent Ring Ouzel breeding areas were less than 125 m from each other, which corresponds to the radius of Ring Ouzel territories (Cramp Citation1988). Randomization of control areas was done using quantum gis (Quantum GIS Development Team [QGIS] Citation2010). Coordinates of the random points were generated in potential breeding habitat (forest areas), excluding habitats not suitable for species occurrence (open habitats without tree vegetation and buildings in built-up areas).
For every point of a layer with Ring Ouzel distribution and a comparative layer of random points, the following landscape-scale habitat variables were determined: (a) topographical; (b) general geographic and habitat parameters; (c) linear; and (d) surface ().
Table 1. The landscape-scale habitat variables measured in breeding areas occupied by the Ring Ouzel Turdus torquatus and random control localities.
Topographical parameters (a): altitude (ALT); slope (SLP; angle of slope); and aspect (ASP; compass direction of the slope) were determined using arcgis (ESRI Citation2010) from the digital elevation model (DEM) with a 25-m resolution, developed on the basis of aerial photographs taken in 2009 (Centralny Ośrodek Dokumentacji Geodezyjnej i Kartograficznej [CODGIK] 2010a). General geographic parameters (b): distance from watercourse networks (DWN); distance from roads (DRN); and distance from built-up areas (DBA) were taken from the Topographical Database available in the geoportal resources wms: http://sdi.geoportal.gov.pl/wms_bdt/wmservice.aspx using the geoxa viewer (Creative GIS Solutions Citation2010). The measured elements were defined as follows: the watercourse network – axes of rivers, streams, canals, and drainage ditches; the road network – axes of the roadways of paved and dirt roads, as well as foot and cycle paths; built-up areas – a complex of land covered with buildings (GUGIK Citation2008).
Habitat parameters (c and d) were read from an orthophotomap available in the geoportal resources wms: http://sdi.geoportal.gov.pl/wms_orto/wmservice.aspx developed on the basis of source material from 2009 (CODGIK 2010b). For linear habitat parameters (c), the smallest distances from the central points of Ring Ouzel breeding areas and random buffers were measured to: canopy gaps (DCG) – areas without trees located within dense stands, with a diameter of 8–24 m; small glades (DSG) – areas without trees located within dense stands, with a diameter of 24–70 m; large glades (DLG) – areas without trees located within dense stands, with a diameter greater than 70 m; clear-cut areas (DCL) – areas without trees located within tree stands, with a minimum diameter of 24 m, where previously there was a stand. In addition, the minimum distance from any open area (MDO) was specified selecting the minimum value from the aforementioned values (regardless of their size and characteristics).
Dimensions differentiating the aforementioned open areas were determined on the basis of climatic conditions (humidity, amount of light, and temperature) of the forest edge and adjacent open habitats' shaping vegetation type and structure (Puchalski & Prusinkiewicz Citation1975). The average diameter of the locality of an individual tree in a mature stand (measured directly on the orthophotomap on 15 randomly selected trees) was 8 m. The diameter of a canopy gap surrounded by a mature tree stand (with a height of about 35 m) which is under its influence, where the intensity of light is around 25–50% of that in the open space, was 24 m. Thus the canopy gap boundaries (8–24 m in diameter) indicate an area which is under the strong influence of the surrounding tree stand, with a small amount of light reaching the ground surface and climatic conditions only slightly differing from those specific to the forest. The threshold diameter of an open area on which the surrounding tree stand has a limited influence was 70 m (twice the height of the stand). Consequently, the limits of a small glade (24–70 m in diameter) indicate open areas with a marked influence of the adjacent tree stand, with variable passage of light and a mixed nature of meadow and forest. The limits of large glades (over 70 m in diameter) indicate areas independent of the surrounding tree stand, where there is full access to light and the vegetation is typical of a meadow.
The surface habitat parameters (d) were measured in buffers with a radius of 125 m (an area of 49 087 m2; about 4.9 ha), approximately corresponding to the size of Ring Ouzel territories (Cramp Citation1988). Buffers around the Ring Ouzel localities and control points were created using quantum gis (QGIS 2010). A forest area (FAB) was defined as an area in which there is woody vegetation of various age categories and with a minimum patch diameter of 24 m. Areas without trees occurring within the forest with a minimum diameter greater than 24 m were treated as open areas and were excluded from calculation of the forest area. The length of the boundary between the forest area and open area (LFB) was measured on the external side of tree crowns in a stand. This line was drawn between adjacent trees separated from each other by less than the diameter of their crowns. The number of forest patches (NFP) was recorded; these were defined as wooded areas with a minimum area of about 0.5 ha (4646 m2), separated or surrounded by an open area.
Literature review data sources
A review of the literature from the central European mountains was carried out to determine the relationship between Ring Ouzel density and altitude on a larger regional scale. The review of the species' density distribution in reference to altitude was based on results from 57 sample plot studies conducted in habitats within the species' range of the Sudety Mountains and northern Carpathians (Dyrcz Citation1973, Kozłowski Citation1974, Cichoń & Zając Citation1991, Głowaciński Citation1991, Kieś Citation1991, S´lizowski Citation1991, Głowaciński & Profus Citation1992, Citation1996; Cichoń et al. Citation1995, Mikusek Citation1996, Kocian Citation1998, Bashta Citation1999, Citation2005; Faber Citation2000, Stój & Kawa Citation2002, Kornan Citation2004, Saniga & Saniga Citation2004, Kajtoch Citation2011, Ciach, unpubl. data; see Appendix 1).
Statistical analyses
Statistical procedures were performed using statistica 8.0 software (StatSoft Citation2008) according to Zar Citation(1999). Data distribution of the landscape-scale habitat variables differed from normality (Shapiro–Wilk W-test). In order to normalize the data distribution, square root transformation was used. The Student's t-test was used to analyze differences in the landscape-scale habitat variables. We used multiple t-tests as a preliminary examination to present general differences between variables. All tests were considered significant with P < 0.05.
A multiple logistic regression was used to test for the effects of environmental variables on the distribution of the Ring Ouzel. Habitat parameters in Ring Ouzel breeding areas and random locations were compared with stepwise logistic regression using backward selection procedure. To select variables, first the correlation structure of all predictors was analyzed. To limit multicollinearity, selection of an appropriate set of predictors was based on the Pearson correlations matrix. Whenever a correlation was equal to or exceeded 0.28 (Graham Citation2003), the variable with lower biological meaning was dropped. A set of ten variables – ALT; SLP; ASP; DWN; DCG; DSG; DLG; DCL; MDO; NFP – were used as a starting model. A backwards selection procedure was run until a model was obtained in which all of the variables analyzed were significant (P < 0.05). To confirm that other variables had no effect, each of removed variables was re-inserted singly into the final model (none of these were significant within the minimum model; P > 0.05).
A distance-weighted least-squares smoothing procedure (StatSoft 2008) was applied to illustrate the relationship between the Ring Ouzel's density and altitude at the larger regional scale of the Carpathians and Sudety Mountains. In this smoothing procedure the influence of individual points decreases with the horizontal distance from the respective points on the curve.
RESULTS
Population size, density, and distribution
As a result of a survey made in the Żywiec Beskid Mountains Special Protection Area, 126 Ring Ouzel breeding areas were identified, in which a total of 208 breeding pairs were found. The density of the Ring Ouzel population calculated in the total area of the study area was 0.06 pairs/10 ha, while based on the forest area it was 0.07 pairs/10 ha.
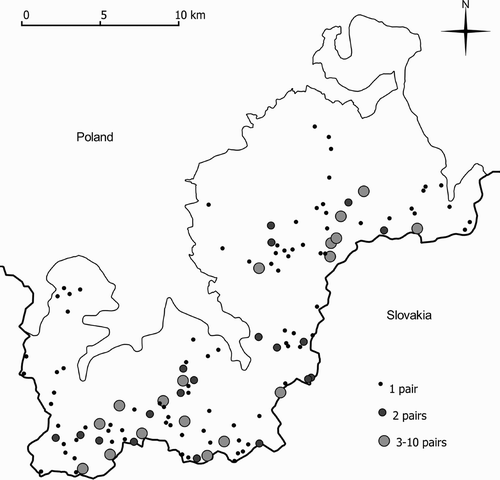
The distribution of breeding areas of the Ring Ouzel was characterized by clusters, indicating a semi-colonial occurrence of the species (). Among all of the breeding areas identified, 29% (n = 126) had more than one breeding pair (19 areas had 2 breeding pairs; 18 areas had between 3 and 10 breeding pairs). Breeding areas occurring in clusters accounted for 57% (n = 208) of all those identified, of which 39% were breeding areas occurring in clusters of 3 to 10.
Landscape-scale habitat variables influencing the occurrence of the Ring Ouzel
Significant differences between the distribution of Ring Ouzel breeding areas and random points in the Żywiec Beskids were found for five landscape-scale habitat variables: DBA; DRN; DSG; DCL; and ALT (). However, the distance from built-up areas and roads, despite being significantly greater in Ring Ouzel localities compared with random points, also significantly correlated with altitude (r = 0.54, P < 0.0001 for built-up areas; r = 0.31, P < 0.0001 for roads), illustrating that these simple univariate comparisons are likely to be confounded, and are presented for illustration of raw data only.
Table 2. The mean values and standard deviations of the landscape-scale habitat variables of Ring Ouzel Turdus torquatu s localities (n = 126) and random control points (n = 126) in the Żywiec Beskid Mountains Special Protection Area (southern Poland).
The logistic regression model indicated a significant influence of three parameters on the occurrence of the Ring Ouzel: ALT, DCL and DSG (). Of these, altitude most strongly and positively influenced the occurrence of the Ring Ouzel. The distance from small glades also had a positive influence, indicating avoidance of this kind of open area. The distance from clear-cuts had a negative influence on the presence of the species' breeding areas, indicating a preference for this kind of open area.
Table 3. Logistic regression model of Ring Ouzel Turdus torquatus occurrence in the Żywiec Beskid Mountains Special Protection Area (southern Poland).
Density of the Ring Ouzel in the Carpathians and Sudetes
Based on the published results from 57 sample plots of the Sudetes and northern Carpathians (Appendix 1), mean density of the Ring Ouzel was 0.4 pairs/10 ha and varied between 0 and 4.9 pairs/10 ha. The Ring Ouzel inhabited a wide spectrum of environments, from low-lying alluvial forests, through solid beech forests, mixed forests consisting of the European Beech Fagus sylvatica, the Silver Fir Abies alba and Norway Spruce, to the upper subalpine solid Norway Spruce forests, solid Mountain Pine patches and high mountain meadows with clusters of Mountain Pine (Appendix 1).
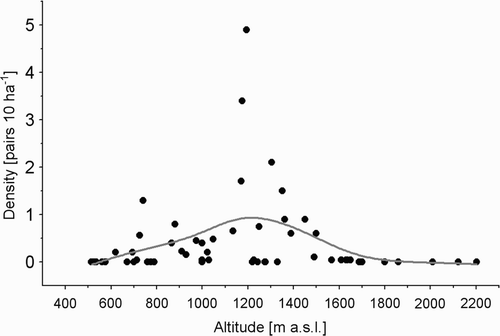
However, the analysis of density distribution of the Ring Ouzel based on the results from sample plots by using the distance-weighted least-squares smoothing procedure showed its clear dependence on altitude (). The Ring Ouzel has a wide range of occurrence, between 600 and 1700 m asl. However, an increase in density of the species occurs in the range 900–1500 m asl, in the upper subalpine and middle subalpine zones, reaching more than 0.5 pairs/10 ha. Across the study areas, the density of the Ring Ouzel peaked at 1200 m asl and was approximately 1 pair/10 ha (). The recorded densities decreased in the forests of lower subalpine zone and Mountain Pine zone.
DISCUSSION
Altitudinal distribution and the role of habitat-climate zones
The altitudinal extent of the Ring Ouzel in the Carpathians is lower than that found in the Alps and Jura Mountains (von dem Bussche et al. Citation2008). Differences in the altitudinal distribution of this species may result from altitude variation in the extent of vegetation-climatic zones in different mountain ranges (Hess Citation1965). The main range of the Ring Ouzel in the Alps (1500–2000 m asl) is approximately equivalent to the upper limits of the coniferous forest and the Mountain Pine and Rhododendron sp. zone. In the northern Carpathians this species occurs between 500 and 1700 m asl, inhabiting the Mountain Pine zone, the coniferous forests of the upper subalpine zone, sparsely reaching the deciduous and mixed forests of lower and middle subalpine zones (Bocheński Citation1960, Głowaciński & Profus Citation1992, Tomiałojć & Stawarczyk Citation2003). The results of the literature review indicated, however, that the highest density of the Ring Ouzel was in the coniferous forests of the upper subalpine zone, but also in the mixed forests of the middle subalpine zone of the Carpathians. The latter forest type (being the highest part of the lower subalpine zone) is characterized by specific climatic conditions which are favorable for the occurrence of the Silver Fir, increased occurrence of Norway Spruce, and the reduction in occurrence of European Beech (Aleksandrowicz Citation1972).
In central Europe, the Ring Ouzel finds suitable habitats in lower locations in the upper subalpine and middle subalpine vegetation-climate zones (Marisova & Vladyshevsky Citation1961, Hudec Citation1983, Janiga & Poxton Citation1997, Danko et al. Citation2002). The first is covered with acidophilous spruce forests: the Carpathian Plagiothecio–Piceetum and the Sudetic Calamagrostio villosae–Piceetum, where the main species in the communities is the Norway Spruce (Holeksa & Szwagrzyk Citation2004a). The latter community is covered with fir–spruce forest Abieti–Piceetum, with the Silver Fir and dominant Norway Spruce (Holeksa & Szwagrzyk Citation2004b). However, the species composition of forest communities alone is probably not a key factor in the Ring Ouzel's habitat selection in the Carpathians. Elements of forest structure such as age, vertical structure, density of forest stands and the occurrence of a layer of shrubs, as well as the proximity of meadows and pastures, may play a significant role.
Climatic factors have an indirect impact on the occurrence of the Ring Ouzel (Beale et al. Citation2006, von dem Bussche et al. Citation2008). Predicted climate change (Beniston et al. Citation1997, Lemoine et al. Citation2007) may lead to changes in the existing altitudinal extents of forest communities in the mountains (Huntley et al. Citation1995). This may in turn result in a decline in the number of birds inhabiting particular zonal habitats (Huntley et al. Citation2006). Withdrawal of the Ring Ouzel into higher grounds under the influence of climate change is predicted in Switzerland (von dem Bussche et al. Citation2008). Therefore, changes are also probable in the distribution and population size of this species in the Carpathians, where an altitudinal shifting of the extent of the lower subalpine zone may occur, with a gradual disappearance of the upper subalpine zone. The populations of Ring Ouzel that are in the outer limits of the species' range of occurrence and live in relatively low mountains may be particularly vulnerable to climate change.
The role of landscape structure elements
The occurrence of the Ring Ouzel is determined by the presence of open areas and the associated ecotone and/or the presence of forests of low density (Marisova & Vladyshevsky Citation1961, Dyrcz Citation1973, Hudec Citation1983, Głowaciński Citation1991, Faber Citation2000, Danko et al. Citation2002, von dem Bussche et al. Citation2008). The presence of clear-cut areas, which can be considered a physionomical substitute for plant communities of the forest's upper limits, had a significant positive impact on the occurrence of the Ring Ouzel. The proximity of clear-cuts and different phases of development of forest stands (plantations, young tree stands, and older stands) creates a mosaic landscape, presumably favorable for the occurrence of the Ring Ouzel, especially in lower mountain locations.
A factor associated with habitat structure is food abundance. The diet of the Ring Ouzel consists of insects, larvae, and other small invertebrates, especially earthworms (Cramp Citation1988). Presumably, open areas and the ecotone of the forest and open areas have a greater number of organisms that are an important component of the Ring Ouzel's diet in the spring and summer (Marisova & Vladyshevsky Citation1961). Open areas on the edge of forest stands also promote the occurrence of plants of the genera Juniperus, Rubus, Fragaria, Prunus, and Sorbus, whose fruits are food for the Ring Ouzel, especially outside the breeding season (Cramp Citation1988). Dense tree stands, avoided by the Ring Ouzel (Buchanan et al. Citation2003, von dem Bussche et al. Citation2008) probably provide a poorer food base. Avoidance of very small glades by the Ring Ouzel is presumably because of their lack of open habitat characteristics. Such glades, being under the influence of surrounding tree stands, have high herbaceous vegetation – making foraging difficult – and presumably do not possess suitable invertebrates, in contrast with large, completely open areas. Moreover, dense vegetation cover may increase predation risk.
The results of the present study indicate a semi-colonial occurrence of the Ring Ouzel in higher mountain areas. The strategy of group nesting occurs for the Fieldfare Turdus pilaris which, thanks to breeding in clusters, increases the chances of successfully detecting and deterring potential predators (Andersson & Wiklund Citation1978, Wiklund & Andersson Citation1980, Wiklund Citation1982). Occurrence of Ring Ouzels in clusters may, however, be the result of the presence of optimal habitat conditions, including the availability of food, rather than for anti-predation reasons. Also, habitat fragmentation is unlikely to be responsible for the semi-colonial distribution. The number of forest patches in a buffer, which is a measure of habitat fragmentation did not differ between Ring Ouzel breeding areas and random localities.
The occurrence of the Ring Ouzel is probably not determined only by the presence of open areas, but the nature of the vegetation communities that occur in them. Among non-forest mountain communities are fens, mires, bogs, bilberry Vaccinium myrtillus shrub patches, and different types of swards. The harsh climate of high mountain areas does not allow herbaceous plants to reach large sizes and stops succession, which promotes the retention of Ring Ouzel feeding areas (Cramp Citation1988). Avoidance of low-lying open areas may be connected with the structure of vegetation, which, being too dense and compact makes it impossible to acquire food. Therefore, birds prefer nutrient-poor high mountain meadows or pastures (Buchanan et al. Citation2003, von dem Bussche et al. Citation2008), which offer a vegetation structure suitable for foraging. Functional differences between glades and clear-cuts make the latter closer to early forest succession stages. Thus, the character of vegetation cover of grasses and herbs in glades and scrubs and seedlings in clear-cuts may explain the opposite preferences toward both types of open areas. The formation of open areas for agricultural and pastoral use may allow the Ring Ouzel to colonize lower mountain areas. Anthropogenic open habitats, being a physionomical equivalent of natural meadows of subalpine and alpine zones are, in terms of landscape, a substitute for natural high mountain habitats. However, vegetation cover in such terrain may play a significant role for the Ring Ouzel distribution.
The impact of humans
The landscape of central European mountains is the result of human activity. Farming, grazing, and forest management led to the formation of a landscape mosaic of habitats in the mountains. However, the results of this work indicate that the occurrence of the Ring Ouzel might be negatively affected by the presence of human settlements, as in Switzerland (von dem Bussche et al. Citation2008). However, in high mountain environments the presence of human developments and infrastructure depend on land accessibility. With the multivariate approach used in the present study it is difficult to evaluate the effect of human settlement, since this variable is highly dependent on altitude. The negative impact of built-up areas must, therefore, be considered in terms of the specifics of our study area, where, with increasing altitude, the concentration of buildings and density of road networks decreases. The Ring Ouzel occurs in high areas, and these are often remote from human settlements and roads. However, progressive building development of mountain areas, tourist pressure, and the development of the accompanying infrastructure pose a potential threat to the population the Ring Ouzel. An equally negative phenomenon in mountains is the afforestation of open areas (Buchanan et al. Citation2003, von dem Bussche et al. Citation2008).
Locally, the Ring Ouzel is associated with mountain meadows in which there is grazing (Marisova & Vladyshevsky Citation1961, Głowaciński Citation1991, Burfield Citation2002). One of the main components of the Ring Ouzel's food in the spring is larvae of dung beetles and flies (Geotrupinae and Diptera) (Marisova & Vladyshevsky Citation1961). Thus, an important element in the protection of this species may prove to be the maintenance of extensive forms of land-use which generate an adequate food base in mountain areas. In addition, grazing causes inhibition of natural succession in open areas, which are an important element of Ring Ouzel habitats and provide short grass-dominated vegetation, where they can obtain their key invertebrate food (Burfield Citation2002). Historical data indicate that the number of livestock in the Carpathians was much higher than presently. However, owing to a lack of historical data on Ring Ouzel distribution or abundance, the impact of former land-use on this species is impossible to assess. The maintenance of semi-natural open areas in the mountains is, however, beneficial for a whole variety of plant and animal species, indicating the Ring Ouzel's umbrella role in habitats of extensively used high mountain meadows and pastures.
The density and dynamics of the Ring Ouzel population
Information regarding earlier numbers of the Ring Ouzel in the present study area (Żywiec Beskids) is vague (Ferens Citation1950) and it is not possible to compare it with current population estimates (Ciach et al. Citation2009). The population density recorded in present study was relatively low. However, this is the result of calculating densities at the landscape scale. Similar densities have been indicated on a landscape scale for the Wyspowy Beskids (Kajtoch Citation2011). Ring Ouzel density evaluated on non-random sample plots is much higher and can reach up to 5 pairs/10 ha (Głowaciński Citation1991, Głowaciński & Profus Citation1992, Faber Citation2000, Saniga & Saniga Citation2004). Nonetheless, these values are overestimates which may be the result of calculating densities from small sample plots, as well as methodological error owing to locating sample plots in a non-random way in places of numerous (sometimes semi-colonial) occurrence of the species. The results of the literature review indicate that the average density of the Ring Ouzel under optimum altitude conditions is more realistically about 1 pair/10 ha.
Lack of data on population dynamics in Poland (Tomiałojć & Stawarczyk Citation2003, Chylarecki & Jawińska Citation2007), and in the entire Carpathians, makes it difficult to assess correctly the species' conservation status, but it might be more threatened than its current classification as a species of Least Concern suggests. The present study establishes a baseline against which future changes should be monitored to determine trends of the Ring Ouzel in space and time. This species is generally overlooked by European Union (EU) member states because is not listed on Annex I of the Birds Directive. However, as a migratory species it should be subject to the same conservation measures as Annex I species, under Article 4.2 of the Birds Directive. The EU holds around 60% of the global population of the Ring Ouzel, and the Carpathians are the highest-density region for this species in the world. The EU member states in the region should be encouraged to take responsibility for this species' population.
ACKNOWLEDGEMENTS
We are grateful to the anonymous referees for their constructive comments on the manuscript.
REFERENCES
- Aleksandrowicz , B. W. 1972 . Typologiczna analiza lasu , Warsaw : PWN .
- Andersson , M. and Wiklund , C. G. 1978 . Clumping versus spacing out: experiments on nest predation in fieldfares (Turdus pilaris) . Anim. Behav. , 26 : 1207 – 1212 . (doi:10.1016/0003-3472(78)90110-0)
- Bashta , A. T. 1999 . Breeding bird community of monocultural spruce plantation in the Skolivski Beskids (the Ukrainian Carpathians) . Berkut , 8 : 9 – 14 .
- Bashta , A. T. 2005 . Biotope distribution and habitat preference of breeding bird communities in alpine and subalpine belts in the Tatra and Babia Gora Mts. (Southern Poland) . Berkut , 14 : 145 – 162 .
- Beale , C. M. , Burfield , I. J. , Sim , I. M.W. , Rebecca , G. W. , Pearce-Higgins , J. W. and Grant , M. C. 2006 . Climate change may account for the decline in British ring ouzel Turdus torquatus . J. Anim. Ecol. , 75 : 826 – 835 . (doi:10.1111/j.1365-2656.2006.01102.x)
- Beniston , M. , Diaz , H. F. and Bradley , R. S. 1997 . Climatic change at high elevation sites: an overview . Climatic Change , 36 : 233 – 251 . (doi:10.1023/A:1005380714349)
- BirdLife International . 2004 . Birds in Europe: Population Estimates, Trends and Conservation Status , Cambridge : BirdLife International .
- Bocheński , Z. 1960 . Ptaki pienin . Acta Zool. Cracov. , 5 : 349 – 445 .
- Buchanan , G. M. , Pearce-Higgins , J. W. , Wotton , S. R. , Grant , M. C. and Whitfield , D. P. 2003 . Correlates of the change in Ring Ouzel Turdus torquatus abundance in Scotland from 1988–91 to 1999 . Bird Study , 50 : 97 – 105 . (doi:10.1080/00063650309461300)
- Burfield , I. J. 2002 . “ The breeding ecology and conservation of the Ring Ouzel Turdus torquatus in Britain ” . University of Cambridge : PhD Thesis .
- Burfield , I. J. and de Brooke , M. L. 2005 . The decline of the Ring Ouzel Turdus torquatus in Britain: evidence from bird observatory data . Ring. Migr. , 22 : 199 – 204 . (doi:10.1080/03078698.2005.9674333)
- Ciach, M. 2010. Beskid Żywiecki Mountains. In Wilk, T., Jujka, M., Krogulec, J. & Chylarecki, P. (eds) Important Bird Areas of International Importance in Poland: 412–414. OTOP, Marki.
- Ciach , M. , Kwarciany , B. , Mrowiec , W. , Figarski , T. , Bujoczek , M. , Dyduch , M. and Fluda , M. 2009 . Beskid Z˙ywiecki PLB240002 (IBA PL127) In Chmielewski, S. & Stelmach, R. (eds) Ostoje ptaków w Polsce – wyniki inwentaryzacji, część I: 51–58. Bogucki Wydawnictwo Naukowe, Poznań
- Cichoń , M. and Zając , T. 1991 . Avifauna of Bieszczady National Park (SE Poland) in 1987 and 1988 – quantitative and qualitative data . Acta Zool. Cracov. , 34 : 497 – 517 .
- Cichoń , M. , Przybyło , R. and Zając , T. 1995 . Awifauna Otrytu, doliny Sanu i terenów przyległych . Ochr. Przyr. , 52 : 195 – 206 .
- Creative GIS Solutions. 2010. Geoxa Viewer 2.0. Lublin, Polska.
- Chylarecki , P. and Jawińska , D. 2007 . Monitoring Pospolitych Ptaków Lęgowych – Raport z lat 2005–2006 , Warsaw : OTOP .
- CODGIK 2010a. Numeryczne Dane Wysokościowe. Available at: http://www.codgik.gov.pl/zasob/372-numeryczne-dane-wysokosciowe.html (accessed 27 December 2010).
- CODGIK. 2010b. Ortofotomapa. Available at: http://www.codgik.gov.pl/ortofotomapa.html (accessed 27 December 2010).
- Cramp , S. 1988 . The Birds of the Western Palearctic , Edited by: Cramp , S. Vol. 5 , Oxford : Oxford University Press .
- Danko , Š. , Darolová , A. and Krištín , A. 2002 . Rozsirene vtakov na Slovensku , Bratislava : Slovenska Akademia Vied. Veda .
- Dyrcz , A. 1973 . Ptaki polskiej części Karkonoszy . Ochr. Przyr. , 38 : 213 – 284 .
- Dyrcz , A. and Mielczarek , P. 2007 . “ Drozd obrożny Turdus torquatus ” . In Atlas rozmieszczenia ptaków lęgowych Polski 1985–2004 , Edited by: Sikora , A. , Rohde , Z. , Gromadzki , M. , Neubauer , G. and Chylarecki , P. 370 – 371 . Poznań : Bogucki Wydawnictwo Naukowe .
- ESRI . 2010 . ArcGIS, Version 10.0 , Redlands , CA : ESRI Inc .
- Faber , M. 2000 . Lęgowe zgrupowanie ptaków kopuły szczytowej Pilska . Not. Ornitol. , 41 : 241 – 245 .
- Ferens , B. 1950 . Ptaki żywiecczyzny . Materiały do fizjografii kraju PAU , 25 : 1 – 96 .
- Gaston , K. J. , Blackburn , T. M. and Goldewijk , K. K. 2003 . Habitat conversion and global avian biodiversity loss . Proc. Royal Soc. London, B , 270 : 1293 – 1300 . (doi:10.1098/rspb.2002.2303)
- Głowaciński , Z. 1991 . Ekologiczny zarys awifauny zlewni Kamienicy w Gorcach i Beskidzie Wyspowym (Karpaty Zachodnie) . Ochr. Przyr. , 49 : 175 – 196 .
- Głowaciński , Z. and Profus , P. 1992 . Structure and vertical distribution of the breeding bird communities in the Polish Tatra National Park . Ochr. Przyr. , 50 : 65 – 94 .
- Głowaciński , Z. and Profus , P. 1996 . Lęgowe zespoły ptaków buczyny i olszynki nadpotokowej w Bieszczadzkim Parku Narodowym . Rocz. Bieszcz. , 5 : 109 – 116 .
- Graham , M. H. 2003 . Confronting multicollinearity in ecological multiple regression . Ecology , 84 : 2809 – 2815 . (doi:10.1890/02-3114)
- GUGIK . 2008 . Baza Danych Topograficznych. Wytyczne techniczne , Warsaw : GUGIK .
- Hess , M. 1965 . Piętra klimatyczne w Polskich Karpatach Zachodnich. Zesz. Naukowe UJ . Pr. Geograf. , 11 : 1 – 258 .
- Holeksa , J. and Szwagrzyk , J. 2004a . “ Acydofilne świerczyny górnoreglowe ” . In Lasy i Bory. Poradniki ochrony siedlisk i gatunków Natura 2000 – podręcznik metodyczny , Edited by: Herbich , J. Vol. 5 , 299 – 304 . Warsaw : Ministerstwo Środowiska .
- Holeksa , J. and Szwagrzyk , J. 2004b . “ Dolnoreglowy bór jodłowo-świerkowy ” . In Lasy i Bory. Poradniki ochrony siedlisk i gatunków Natura 2000 – podręcznik metodyczny, Vol. 5 , Edited by: Herbich , J. 308 – 311 . Warsaw : Ministerstwo Środowiska .
- Hudec , K. 1983 . Fauna CSSR. Ptaci – Aves III/1 , Praha : Academia .
- Huntley , B. , Berry , P. M. , Cramer , W. and McDonald , A. P. 1995 . Modelling present and potential future ranges of some European higher plants using climate response surfaces . J. Biogeogr. , 22 : 967 – 1001 . (doi:10.2307/2845830)
- Huntley , B. , Collingham , Y. C. , Grenn , R. E. , Hilton , G. M. , Rahbek , C. and Willis , S. G. 2006 . Potential impacts of climatic change upon geographical distributions of birds . Ibis , 148 : 8 – 28 . (doi:10.1111/j.1474-919X.2006.00523.x)
- IUCN . 2011 . Turdus torquatus. In IUCN Red List of Threatened Species, Version 2010.4 Available at: http://www.iucnredlist.org/ (accessed 18 January 2011)
- Janiga , M. and Poxton , I. R. 1997 . “ Ring Ouzel Turdus torquatus ” . In The EBCC Atlas of European Breeding Birds. Their Distribution and Abundance , Edited by: Hagemeiler , W. J.M. and Blair , M. J. 542 – 543 . London : T&AD Poyser .
- Kajtoch , Ł. 2011 . Rozmieszczenie, liczebność i siedliska drozda obrożnego Turdus torquatus w Beskidzie Wyspowym . Ornis Pol. , 52 : 62 – 71 .
- Kieś , B. 1991 . Bird community in natural beech wood of the lower mountains forest zone of Mt Babia Góra. Acta . Zool. Cracov. , 34 : 519 – 533 .
- Kocian , L. 1998 . Bird communities of the Western Tatras – Rohace Mountains between 1870–1996 . Acta Zool. Universitatis Comenianae , 42 : 17 – 58 .
- Kondracki , J. 2000 . Geografia regionalna Polski , Warsaw : Wydawnictwo Naukowe PWN .
- Kornan , M. 2004 . Structure of the breeding bird assemblage of a primaeval beech–fir forest in the Sramkova National Nature Reserve, the Mala Fatra Mts . Biologia , 59 : 219 – 231 .
- Kozłowski , J. 1974 . Liczebność i rozmieszczenie ptaków w rezerwacie ‘Turbacz’ w Gorcach . Ochr. Przyr. , 39 : 245 – 277 .
- Lemoine , N. , Bauer , H. G. , Peintinger , M. and Böhning-Gaese , K. 2007 . Effects of climate and land-use change on species abundance in a central European bird community . Conserv. Biol. , 21 : 495 – 503 . (doi:10.1111/j.1523-1739.2006.00633.x)
- Marisova , I. V. and Vladyshevsky , D. V. 1961 . [On the biology of Turdus torquatus L. in the Ukraine.] Zoologicheskii Zhurnal , 40 : 1240 – 1245 . (in Russian)
- Matuszkiewicz , J. M. 2001 . Zespoły leśne Polski , Warsaw : Wydawnictwo Naukowe PWN .
- Mikusek , R. 1996 . Ptaki lęgowe Gór Bystrzyckich . Ptaki S´la˛ska , 11 : 81 – 114 .
- Pounds , J. A. , Fogden , M. P.L. and Campbell , J. H. 1999 . Biological response to climate change on a tropical mountain . Nature , 398 : 611 – 615 . (doi:10.1038/19297)
- Puchalski , T. and Prusinkiewicz , Z. 1975 . Ekologiczne podstawy siedliskoznawstwa leśnego , Warsaw : PWRiL .
- Quantum GIS Development Team . 2010 . Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project . Available at: http://qgis.osgeo.org/
- Saniga , M. and Saniga , M. 2004 . Influence of forest stand structure on the occurrence of bird community in Skalna Alpa National Nature Reserve in the Vel'ka Fatra Mts. (West Carpathians) . J. For. Sci. , 50 : 219 – 234 .
- Sim , I. M.W. , Burfield , I. J. , Grant , M. C. , Pearce-Higgins , J. W. and de Brooke , M. L. 2007 . The role of habitat composition in determining breeding site occupancy in a declining Ring Ouzel Turdus torquatus population . Ibis , 149 : 374 – 385 . (doi:10.1111/j.1474-919X.2007.00655.x)
- Sim , I. , Rollie , C. , Arthur , D. , Benn , S. , Booker , H. , Fairbrother , V. , Green , M. , Hutchinson , K. , Ludwig , S. , Nicoll , M. , Poxton , I. , Rebecca , G. , Smith , L. , Stanbury , A. and Wilson , P. 2010 . The decline of the Ring Ouzel in Britain . Br. Birds , 103 : 229 – 239 .
- Slizowski , J. 1991 . Bird community of spruce forest in the upper mountains forest zone on Polica (Polish Western Carpathians) . Acta Zool. Cracov. , 34 : 535 – 551 .
- Šťastný , K. , Bejček , V. and Hudec , K. 2006 . Atlas hnízdního rozšíření ptáků v České republice 2001–2003 , Praha : Aventinum .
- StatSoft, Inc . 2008 . Statistica (data analysis software system), Version 8.0 . Available at: http://www.statsoft.com/
- Stój , M. and Kawa , P. 2002 . Ptaki Ciśniańsko-Wetlińskiego Parku Krajobrazowego w Bieszczadach Zachodnich . Rocz. Bieszcz , 10 : 353 – 371 .
- Szwagrzyk , J. and Holeksa , J. 2004 . “ Żyzne buczyny górskie ” . In Lasy i Bory. Poradniki ochrony siedlisk i gatunków Natura 2000 – podręcznik metodyczny, Vol. 5 , Edited by: Herbich , J. 62 – 70 . Warsaw : Ministerstwo Środowiska .
- Thomas , C. D. , Cameron , A. , Green , R. E. , Bakkenes , M. , Beaumont , L. J. , Collingham , Y. C. , Erasmus , B. F.N. , de Siqueira , M. F. , Grainger , A. , Hannah , L. , Hughes , L. , Huntley , B. , van Jaarsveld , A. S. , Midgley , G. F. , Miles , L. , Ortega-Huerta , M. A. , Townsend Peterson , A. , Phillips , O. L. and Williams , S. E. 2004 . Extinction risk from climate change . Nature , 427 : 145 – 148 . (doi:10.1038/nature02121)
- Tomiałojć , L. and Stawarczyk , T. 2003 . Awifauna Polski. Rozmieszczenie, liczebność i zmiany , Wrocław : PTPP ‘pro Natura’ .
- von dem Bussche , J. , Spaar , R. , Shmid , H. and Schroder , B. 2008 . Modelling the recent and potential future distribution of the Ring Ouzel (Turdus torquatus) and Blackbird (T. merula) in Switzerland . J. Ornithol. , 149 : 529 – 544 . (doi:10.1007/s10336-008-0295-9)
- Wiklund , C. G. 1982 . Fieldfare (Turdus pilaris) breeding success in relation to colony size, nest position and association with merlins (Falco columbarius) . Behav. Ecol. Sociobiol. , 11 : 165 – 172 . (doi:10.1007/BF00300059)
- Wiklund , C. G. and Andersson , M. 1980 . Nest predation selects for colonial breeding among Fieldfares Turdus pilaris . Ibis , 122 : 363 – 366 . (doi:10.1111/j.1474-919X.1980.tb00891.x)
- Zar , J. H. 1999 . Biostatistical analysis , 4 , Upper Saddle River , NJ : Prentice Hall .