Abstract
Capsule Mediterranean Great Tits showed a marked increase in levels of circulating carotenoids during moult and autumn.
Aims To study seasonal variation in plasma carotenoid content during a whole annual cycle for Great Tits Parus major inhabiting a Mediterranean woodland.
Methods We used a sample of 71 adult male Great Tits captured in NE Spain during a whole year. Data were organized into four seasons (winter, breeding, moult and autumn). We sampled blood to extract plasma. Carotenoid plasma concentrations were analysed by High-performance liquid chromatography (HPLC).
Results Lutein and zeaxanthin content varied seasonally, with a marked increase in levels during moult and autumn. Within the moulting period, levels increased gradually as the season progressed. This pattern differed significantly from that previously described in northern European populations, where high values appear mainly in the breeding season. Carotenoid concentrations (lutein: 1.2 ± 0.25, zeaxanthin: 0.07 ± 0.03 µg mL−1) were also lower than in northern Europe.
Conclusion Mediterranean Great Tits show a very different pattern of circulating carotenoids and lower levels than northern European populations. The increasing pattern of carotenoid availability found within the moulting period raises the need to control for the effect of sampling date when analysing data on carotenoid concentrations at different times of year.
Carotenoids have a prominent role in animal health, acting as powerful antioxidant and immuno-stimulating agents (Olson & Owens Citation1998, Møller et al. Citation2000). Carotenoid pigments are also utilized by many species as integumentary colorants, being responsible for most of their red, orange and yellow displays (Hill & McGraw Citation2006a, Citation2006b). Carotenoids are of additional great interest in birds because of the potential trade-off between using them for physiological processes related to health and for plumage pigmentation (Lozano Citation1994, Olson & Owens Citation1998). In line with this, extensive recent literature has demonstrated that carotenoid-based colour displays may function as honest signals of health and individual quality and are commonly used by individuals as an important criterion for mate choice (Hill Citation2006, Citation2011).
Birds cannot synthesize these molecules de novo but must acquire them through the diet, either directly by consuming plants or indirectly via herbivorous prey (Olson & Owens Citation1998). Similar to other nutritional constraints, carotenoid availability may therefore vary seasonally according to seasonal variations in food quality and quantity (Hill Citation1995, Isaksson et al. Citation2007). Physiological factors related to variation in uptake efficiency, storage or use can also vary seasonally (Negro et al. Citation2001, Hill & McGraw Citation2006b). These variations can be detected through the analysis of carotenoid levels in blood plasma (Isaksson et al. Citation2007). However, despite the importance of these data, there exist only a few studies describing temporal variation in circulating carotenoid levels in wild bird populations (Hill Citation1995, McGraw & Gregory Citation2004, Isaksson et al. Citation2007, Deviche et al. Citation2008).
The Great Tit Parus major has been the focus of several studies on food-limited expression and condition dependence of carotenoid signals (Slagsvold & Lifjeld Citation1985, Fitze et al. Citation2003, Senar et al. Citation2003, Tschirren et al. Citation2003, Biard et al. Citation2006, Isaksson et al. Citation2007). Males have a yellow, carotenoid-based ventral plumage derived from the direct deposition of dietary lutein and zeaxanthin into feathers, apparently without any conversion of the molecules (Stradi Citation1998). They feed on an omnivorous diet relatively rich in carotenoids (Slagsvold & Lifjeld Citation1985, Partali et al. Citation1987) which includes a wide range of larval and adult invertebrates, fruits and seeds (Gosler Citation1993). In the present study, we examined carotenoid levels in blood plasma in a Mediterranean population of Great Tits in NE Spain during a full annual cycle. We were focused solely on circulating lutein and zeaxanthin levels, since they have been found to be the main carotenoid pigments in the plasma and feathers of Great Tits (Partali et al. Citation1987, Stradi Citation1998). According to previous work on Great Tits from northern deciduous forests, we should expect plasma carotenoid content to be highest during the breeding period and lower at other times of the year (Partali et al. Citation1987, Isaksson et al. Citation2007). However, this pattern might be substantially different in populations inhabiting mixed Mediterranean woodlands, since these habitats differ considerably in food phenology (Blondel et al. Citation2001, Citation2010, Pagani-Nuñez et al. Citation2011) and hence, pigment occurrence (Debussche et al. Citation1987). Hence, the aim of the paper was to describe seasonal variations in plasma carotenoid content in a Mediterranean population of the Great Tit. Given that plumage coloration may be dependent on when the birds have moulted (Hill Citation1994), we also investigated whether carotenoid concentrations in plasma varied during the moulting period.
METHODS
Sampling was performed at approximately weekly intervals from January to December 2005. Great Tits (n = 71) were trapped in baited funnel traps (Senar et al. Citation1997) during winter (December–March), breeding (April–mid-June), moult (mid-June to September) and autumn (October–November). Breeding birds were also caught in nest boxes. Birds were trapped at Can Catà field station (NE Spain). The habitat in this area is a mixed forest ranging from pure evergreen oak at the bottom of the valleys to pure pine forests on hill tops. For a more detailed study of patterns within the moulting period, we increased our sample size (n = 10) with a sample of birds trapped at Sarrià (n = 13), a locality in the suburban area of Barcelona of orchards and small forests, and close to Can Catà. Birds were marked for individual recognition with numbered aluminium rings. Sex and age were determined according to Svensson (Citation1992) and Jenni & Winkler (Citation1994). An adult was defined as a bird known to have hatched at least two years before the calendar year of capture. Body mass was measured for each individual to the nearest 0.1 g. A portable colorimeter Minolta CR200 (Minolta Corporation 1994©) was used to determine hue, chroma and lightness (LCH) of the yellow plumage of breast (Figuerola et al. Citation1999). The Great Tit presents a peak of UV in the yellow breast plumage (Senar & Quesada Citation2006). Our colorimeter measures colour within the visible range (400–700 nm), but does not collect the UV range (320–400 nm). However, we have previously shown that the reflectance of the UV peak is highly correlated with the peak of the yellow–red spectrum (500–700 nm; Senar & Quesada Citation2006).
Blood samples (maximum 200 μl/bird) were collected from the brachial vein into heparinized microhematocrit tubes, kept on coolers and centrifuged at 11 000 rpm for 10 minutes within 8 hours of capture. Plasma was separated from blood cells and stored at −20° until analysed. Carotenoids were extracted from thawed plasma by adding three parts of acetone (3:1, v/v). The mixtures were introduced in a room temperature bath and sonicated for 5 minutes in order to accelerate the extraction process. We subsequently centrifuged the samples at 13 000 rpm for 10 minutes, obtaining a supernatant with the carotenoids in solution. HPLC was carried out in a Jasco PU-2089 Plus instrument equipped with a quaternary pump (Jasco Analítica Spain S.L.). Carotenoid analyses were performed using a reverse-phase C18 column (Phenomenex Synergi 4μ) and a pre-column of the same material with a particle size of 5 μm. Samples were pre-filtered using Original equipment manufacturer (OEM) Nylon filters (0.45 μm, 4 mm) and later injected with a Rheodyne 7725i Valve equipped with a 20 μl loop (Rheodyne, Rohnent Park, CA, USA). The eluent system was a gradient described in Minguez-Mosquera & Hornero-Mendez (Citation1993), except that the flow rate was 1 ml min–1. Data were acquired between 195 and 650 nm with a multiwavelength detector MD-2010 Plus (Jasco Analítica Spain S.L.). Spectra and retention time of lutein and zeaxanthin were compared with those obtained using a pure standard obtained from fresh green plants by means of thin layer chromatography (Minguez-Mosquera & Hornero-Mendez Citation1993). Quantification was performed using an external standard calibration curve at 450 nm from injection of progressive concentrations of the reference pigment.
Statistical analyses were performed with Statistica 6.0 and results were considered significant at the 0.05 level. We included only samples from adult males, in order to avoid differential effects associated with age and sex. Each bird was only used once in order to avoid pseudoreplication. Generally, we used the first capture of each individual in the analyses, but in six cases we used the second or third capture in order to obtain a balanced sample size between seasons. Data were organized into the four seasons as described above (winter, breeding, moult and autumn). Sample size per period ranged between 10 and 29 birds. A general linear model (GLM) was performed, with lutein and zeaxanthin concentrations as dependent variables and season as independent variable. Lutein and zeaxanthin values were log transformed. Since many individuals had 0 values of concentration, we added 1 to each value prior to transformation. We also included as covariates, the body mass, wing length and colour (LCH) variables of each individual. Even though the carotenoid content of the bait used in our traps (i.e. peanuts) was low (0.02 µg g−1), we decided to control for the effect of potential supplementary carotenoids in our analysis by introducing the number of times that individuals were captured during the year as a continuous variable.
To examine the pattern of lutein and zeaxanthin variation during moult (when carotenoids in circulation are transferred to feathers for coloration), we utilized a sample of 23 Great Tits captured from mid–June to September at both localities. Individual birds were used only once on their first capture to avoid pseudoreplication. We recorded occurrence of contour feather moult in the breast area, by recording the presence of feathers growing. This allowed us to confirm the moulting period of the species in our area. We considered sampling date as the number of days after the onset of the population-wide moult period, which was defined as 1st June. We ran another GLM, with lutein and zeaxanthin concentrations during moult as dependent variables, moult date as independent variable and locality as a random factor. Locality was controlled for in our model because it is known to influence both timing of moult and carotenoid accessibility (Ferns & Hinsley Citation2008). Number of captures from the beginning of the moult (1 June) was additionally introduced as a continuous variable in the analysis in order to control for the effect of potential supplementary carotenoids (see before).
RESULTS
Plasma lutein and zeaxanthin concentrations in adult Great Tit males were highly variable (lutein mean: 1.2 ± 0.25 se, range: 0–14.8 µg ml−1; zeaxanthin mean: 0.07 ± 0.03 se, range: 0–1.5 µg ml−1; n = 71) and varied seasonally (). Both lutein and zeaxanthin concentrations peaked during moult and remained high in autumn. Both declined sharply afterwards, reaching an annual minimum through the winter and during breeding (; post-hoc planned comparisons between lutein during moult-autumn vs. winter-breeding: F1,61 = 15.2, P = 0.0002; zeaxanthin: F1,61 = 10.0, P = 0.003). Body mass, wing length and plumage coloration (LCH) at the time of trapping had no effect on circulating carotenoid levels (). The number of visits to our baited traps and hence, the carotenoid uptake due to food supplementation, also had no effect on circulating carotenoid levels ().
Figure 1. Seasonal changes in plasma concentration of lutein and zeaxanthin (means ± se) in adult male Great Tits sampled in 2005 at Can Catà field station, near Barcelona city, Spain (n for each season is provided).
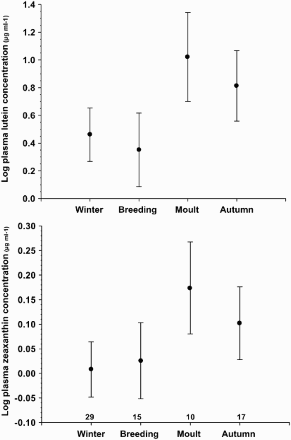
Table 1. Results from a GLM with plasma carotenoid concentrations (lutein and zeaxanthin; μg mL−1) in adult Great Tit males as dependent variables (n = 71).
Lutein and zeaxanthin concentrations during moult were significantly dependent on the date the samples were collected (), so that both levels increased as the moulting season progressed (). We found no effect of locality, or number of visits to our baited traps, on circulating carotenoid levels during moult ().
Figure 2. Changes in plasma lutein and zeaxanthin concentrations within the moulting period in adult male Great Tits sampled in 2005 (n = 23). Day zero corresponds to June 1.
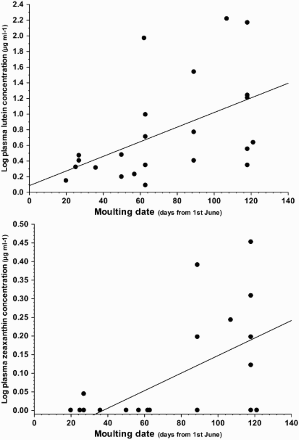
Table 2. Results from a GLM analysing variation in plasma carotenoid concentrations (lutein and zeaxanthin; μg ml−1) during the moulting period in adult Great Tit males captured at two different localities (Can Catà and Sarrià) at or near Barcelona city, Spain (n = 23).
DISCUSSION
Adult male Great Tits in our study area showed a marked seasonality in circulating lutein and zeaxanthin levels throughout the year, with concentrations being highest in moult and autumn (mid-June to November) and relatively low at other times of the year. Seasonal differences found are probably not due to differences in carotenoid storage or release, because previous studies have shown that Great Tits store carotenoids in very small quantities and with no long-term carry over effects (Isaksson & Andersson Citation2008, C. Isaksson, pers. comm.; see, however, Peters et al. Citation2011). Differences found could be due to carotenoid use. Some studies have shown, for instance, that carotenoid plasma levels drop during stress and immune challenges or moult (Blas et al. Citation2006, Peters et al. Citation2007, Citation2011, Biard et al. Citation2009, Alonso-Alvarez & Galvan Citation2011). However, oxidative stress does not seem to influence carotenoid mobilization in Great Tits (Isaksson & Andersson Citation2008). The fact that seasonal effects on carotenoid profiles were independent of body mass (and wing length), implying no effect of body condition on carotenoid profiles, supports this view. Additionally, under this hypothesis, we should expect carotenoid levels to be lower during moult, because this is a period with high physiological carotenoid demands, but this was not the case. In fact, carotenoid levels during moult were at their maximum levels. Alternatively, changes in plasma carotenoids could reflect changes in particular foraging preferences at different seasons. We have recently demonstrated that Great Tits show a specific appetite for carotenoids and try to maximize the intake of these pigments (Senar et al. Citation2010). Hence, if tits do not ingest carotenoids in some seasons, it may be because they are less available.
An additional alternative is that Great Tits could switch to a carotenoid-rich diet because of its higher environmental abundance, so that changes in plasma carotenoid concentration could reflect this diet switch. In fact, the seasonal pattern we found reflects typical seasonal dietary changes of Mediterranean Great Tit populations through the year. During spring, tits in Mediterranean areas rely on a mixed diet which is generally poor in carotenoid content, and caterpillars are far less abundant than in northern localities (Latscha Citation1990, Blondel et al. Citation2001, Pagani-Nuñez et al. Citation2011). As a consequence, the carotenoid surplus for our population is not as large as that present in deciduous forests in temperate regions (Isaksson et al. Citation2007). However, the increase in fruit abundance and frugivory throughout moult and autumn (Herrera Citation1984, Debussche et al. Citation1987, Hampe Citation2001) may contribute to explain the elevated levels of circulating lutein and zeaxanthin detected in our samples. Ripe fleshy fruits represent an exceptional source of dietary carotenoids (Olson & Owens Citation2005) and are consumed by Great Tits during late summer and autumn (August–October; Obeso Citation1987). When we specifically analysed the lutein and zeaxanthin profiles of Great Tit males during moult, we observed a relevant increase in the circulating levels throughout this period, which coincides with the progressive appearance of ripe fruits and the increase in frugivory in the species (Sorensen Citation1981). We do not necessarily imply that plasma carotenoid profile is simply a reflection of availability, but note that peaks in blood carotenoids may reflect a high carotenoid availability in diet which may then be reflected in blood profile (see also Peters et al. Citation2011).
It is interesting to point out here the general low levels of circulating carotenoids in our Great Tits (range 0–8 µg mL−1), compared to other northern populations (4–51 µg mL−1; Hörak et al. Citation2004, Isaksson et al. Citation2007). This suggests that carotenoids may be more limiting in Mediterranean populations than in other populations. It would be therefore interesting to analyse for geographic differences in plumage coloration to test whether such differences in circulating carotenoids are reflected in plumage coloration.
Some previous studies have found a correlation between the levels of carotenoids in blood and the colour of the feathers of adult plumaged birds (Hill et al. Citation1994). However, we found that carotenoid profiles throughout the year were independent of plumage coloration. This is not surprising since the colour displayed by a bird is based on the carotenoids ingested in the previous moult, and the high yearly variation in carotenoid availability (Senar & Quesada Citation2006, del Val et al. Citation2010) can easily preclude such a relationship. A proper test of the relationship should analyse carotenoid profiles during moult in relation to subsequent acquired coloration.
The correlation between progression of the moult period and plasma carotenoid levels within that period suggests, following the same prior reasoning, that for the whole year period, carotenoid availability could also vary during the moulting period. If this was the case, we might predict that birds which best adjusted feather replacement to the period of higher pigment abundance would incorporate more carotenoids into their plumage and develop brighter coloration. Moulting too late, however, could also be constrained by energetic costs related to a reduction of temperature and hours of daylight. Energetic constraints have in fact been found to affect plumage coloration in tits, since birds moulting at slow rates produce a more colourful plumage (Ferns & Hinsley Citation2008). More data on timing of moult and plumage coloration, and more specific information on diet and food availability in the environment during moult, are however, needed to test this prediction.
To conclude, Mediterranean Great Tits show a very different pattern of circulating carotenoids and lower concentrations than northern European populations. The reasons for this difference are not yet clear. However, whatever the reason, our results highlight the need to control for the effect of sampling date and locality when analysing data on carotenoid concentrations during the year.
ACKNOWLEDGEMENTS
We thank Nuria Fernández, Isabel García and Lluïsa Arroyo for help in the laboratory, and José Carrillo-Ortiz and Miquel Vall-Llosera Camps for field assistance. We are also very grateful to Leopoldo Gil (Can Catà) and Hermanitas de la Asunción (Sarriá) for allowing us to work in their properties. Birds were handled under permission of the Catalan Ringing Office (ICO) and the Department of Medi Ambient, Generalitat de Catalunya.
FUNDING
This work is a contribution to the CGL2009-10652 and CGL2012-38262 projects to J.C.S. and J.J.N. (Ministerio de Ciencia y Tecnología). E.D.V. was supported by the FPI grant [BES2004-5631] (Ministerio de Ciencia y Tecnología and Ministerio de Economía y Competitividad, respectively).
REFERENCES
- Alonso-Alvarez, C. & Galvan, I. 2011. Free radical exposure creates paler carotenoid-based ornaments: A possible interaction in the expression of black and red traits. Plos ONE 6: e19403. doi: 10.1371/journal.pone.0019403
- Biard, C., Surai, P.F. & Moller, A.P. 2006. Carotenoid availability in diet and phenotype of blue and Great Tit nestlings. J. Exp. Biol. 209: 1004–1015. doi: 10.1242/jeb.02089
- Biard, C., Hardy, C., Motreuil, S. & Moreau, J. 2009. Dynamics of PHA-induced immune response and plasma carotenoids in birds: Should we have a closer look? J. Exp. Biol. 212: 1336–1343. doi: 10.1242/jeb.028449
- Blas, J., Perez-Rodriguez, L., Bortolotti, G.R., Vinuela, J. & Marchant, T.A. 2006. Testosterone increases bioavailability of carotenoids: Insights into the honesty of sexual signaling. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 103: 18633–18637.
- Blondel, J., Dervieux, A., Maistre, M. & Perret, P. 2001. Feeding ecology and life history variation of the Blue Tit in Mediterranean deciduous and sclerophyllous habitats. Oecologia 88: 9–14. doi: 10.1007/BF00328397
- Blondel, J., Aronson, J., Bodiou, J.Y. & Boeuf, G. 2010. The Mediterranean Region: Biological Diversity in Space and Time. Oxford University Press, Oxford.
- Debussche, M., Cortez, J. & Rimbault, I. 1987. Variation in fleshy fruit composition in the Mediterranean region: The importance of ripening season, life-form, fruit type and geographical distribution. Oikos 49: 244–252. doi: 10.2307/3565758
- Deviche, P., McGraw, K.J. & Underwood, J. 2008. Season-, sex-, and age-specific accumulation of plasma carotenoid pigments in free-ranging white-winged crossbills Loxia leucoptera. J. Avian. Biol. 39: 283–292. doi: 10.1111/j.0908-8857.2008.04164.x
- Ferns, P.N. & Hinsley, S.A. 2008. Carotenoid plumage hue and chroma signal different aspects of individual and habitat quality in tits. Ibis 150: 152–159. doi: 10.1111/j.1474-919X.2007.00759.x
- Figuerola, J., Pascual, J. & Senar, J.C. 1999. The use of a colorimeter in field studies of Blue Tit Parus caeruleus coloration. Ardea 87: 269–275.
- Fitze, P.S., Tschirren, B. & Richner, H. 2003. Carotenoid-based colour expression is determined early in nestling life. Oecologia 137: 148–152. doi: 10.1007/s00442-003-1323-3
- Gosler, A.G. 1993. The Great Tit. Hamlyn, London.
- Hampe, A. 2001. The role of fruit diet within a temperate breeding bird community in southern Spain. Bird Study 48: 116–123. doi: 10.1080/00063650109461209
- Herrera, C. 1984. A study of avian frugivores, bird-dispersed plants, and their interaction in Mediterranean scrublands. Ecol. Monogr. 54: 1–23. doi: 10.2307/1942454
- Hill, G.E. 1994. House finches are what they eat: A reply to Hudon. Auk 111: 221–225. doi: 10.2307/4088530
- Hill, G.E. 1995. Seasonal variation in circulating carotenoid pigments in the House Finch. Auk 112: 1057–1061. doi: 10.2307/4089042
- Hill, G.E. 2006. Female mate choice for ornamental coloration in birds. In Hill, G.E. & McGraw, K.J. (eds) Bird Coloration, Volume 2, Function and Evolution. Harvard University Press, Cambridge, MA, pp. 137–200.
- Hill, G.E. 2011. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol. Lett. 14: 625–634. doi: 10.1111/j.1461-0248.2011.01622.x
- Hill, G.E. & McGraw, K.J. 2006a. Bird Coloration. Volume 2. Function and Evolution. Harvard University Press, Cambridge.
- Hill, G.E. & McGraw, K.J. 2006b. Bird Coloration: Volume 1. Mechanisms and Measurements. Harvard University Press, Cambridge.
- Hill, G.E., Montgomerie, R.D., Inouye, C.Y. & Dale, J. 1994. Influence of dietary carotenoids on plasma and plumage colour in the house finch: Intra- and intersexual variation. Funct. Ecol. 8: 343–350. doi: 10.2307/2389827
- Hörak, P., Surai, P.F., Ots, I. & Moller, A.P. 2004. Fat soluble antioxidants in brood-rearing Great Tits Parus major: Relations to health and appearance. J. Avian. Biol. 35: 63–70. doi: 10.1111/j.0908-8857.2004.03167.x
- Isaksson, C. & Andersson, S. 2008. Oxidative stress does not influence carotenoid mobilization and plumage pigmentation. Proc. R. Soc. Lond. Ser. B Biol. Sci. 275: 309–314. doi: 10.1098/rspb.2007.1474
- Isaksson, C., Von Post, M. & Andersson, S. 2007. Sexual, seasonal, and environmental variation in plasma carotenoids in Great Tits, Parus major. Biol. J. Linn. Soc. 92: 521–527. doi: 10.1111/j.1095-8312.2007.00852.x
- Jenni, L. & Winkler, R. 1994. Moult and Ageing of European Passerines. Academic Press, London.
- Latscha, T. 1990. Carotenoids -their Nature and Significance in Animal Feeds. F. Hoffman-LaRoche, Basel.
- Lozano, G.A. 1994. Carotenoids, parasites, and sexual selection. Oikos 70: 309–311. doi: 10.2307/3545643
- McGraw, K.J. & Gregory, A.J. 2004. Carotenoid pigments in male American goldfinches: What is the optimal biochemical strategy for becoming colourful? Biol. J. Linn. Soc. 83: 273–280. doi: 10.1111/j.1095-8312.2004.00388.x
- Minguez-Mosquera, M.I. & Hornero-Mendez, D. 1993. Separation and quantification of the carotenoid pigments in red peppers (Capsicum annuum L.), paprika, and oleoresin by reversed-phase HPLC. J. Agric. Food Chem. 41: 1616–1620. doi: 10.1021/jf00034a018
- Møller, A.P., Biard, C., Blount, J.D., Houston, D.C., Ninni, P., Saino, N. & Surai, P.F. 2000. Carotenoid-dependent signals: Indicators of foraging efficiency, immunocompetence or detoxification ability? Avian. Poult. Biol. Rev. 11: 137–159.
- Negro, J.J., Tella, J.L., Hiraldo, F., Bortolotti, G.R. & Prieto, P. 2001. Sex-and age-related variation in plasma carotenoids despite a constant diet in the Red-legged Partridge Alectoris rufa. Ardea 89: 275–280.
- Obeso, J.R. 1987. Uso del espacio y alimentacion de los Parus spp. en bosques mixtos de la sierra de Cazorla. Ardeola 37: 61–77.
- Olson, V.A. & Owens, I.P.F. 1998. Costly sexual signals: Are carotenoids rare, risky or required? Trend. Ecol. Evol. 13: 510–514. doi: 10.1016/S0169-5347(98)01484-0
- Olson, V.A. & Owens, I.P.F. 2005. Interspecific variation in the use of carotenoid-based coloration in birds: diet, life history and phylogeny. J. Evol. Biol. 18: 1534–1546. doi: 10.1111/j.1420-9101.2005.00940.x
- Pagani-Nuñez, E., Ruiz, I., Quesada, J., Negro, J.J. & Senar, J.C. 2011. The diet of Great Tit Parus major nestlings in a Mediterranean Iberian forest: The important role of spiders. Anim. Biodivers. Conserv. 34: 355–361.
- Partali, V., Liaaen-Jensen, S., Slagsvold, T. & Lifjeld, J.T. 1987. Carotenoids in food chain studies -II. The food chain of Parus spp. monitored by carotenoid analysis. Comp. Biochem. Physiol. 87B: 885–888.
- Peters, A., Delhey, K., Johnsen, A. & Kempenaers, B. 2007. The condition-dependent development of carotenoid-based and structural plumage in nestling Blue Tits: Males and females differ. Am. Nat. 169: S122–S136. doi: 10.1086/510139
- Peters, A., Magdeburg, S. & Delhey, K. 2011. The carotenoid conundrum: Improved nutrition boosts plasma carotenoid levels but not immune benefits of carotenoid supplementation. Oecologia 166: 35–43. doi: 10.1007/s00442-011-1921-4
- Senar, J.C. & Quesada, J. 2006. Absolute and relative signals: A comparison between melanin- and carotenoid-based patches. Behaviour 143: 589–595. doi: 10.1163/156853906776759484
- Senar, J.C., Domènech, J., Carrascal, L.M. & Moreno, E. 1997. A funnel trap for the capture of tits. Butll. GCA 14: 17–24.
- Senar, J.C., Figuerola, J. & Domènech, J. 2003. Plumage coloration and nutritional condition in the Great Tit Parus major: The roles of carotenoids and melanins differ. Naturwissenschaften 90: 234–237.
- Senar, J.C., Moller, A.P., Ruiz, I., Negro, J.J., Broggi, J. & Hohtola, E. 2010. Specific appetite for carotenoids in a colorful bird. Plos ONE 5: e10716. doi: 10.1371/journal.pone.0010716
- Slagsvold, T. & Lifjeld, J.T. 1985. Variation in plumage colour of the Great Tit Parus major in relation to habitat, season and food. J. Zool. Lond. 206: 321–328. doi: 10.1111/j.1469-7998.1985.tb05661.x
- Sorensen, A.E. 1981. Interactions between birds and fruit in a temperate woodland. Oecologia 50: 242–249. doi: 10.1007/BF00348046
- Stradi, R. 1998. The Colour of Flight: Carotenoids in Bird Plumage. Solei Press, Milano.
- Svensson, L. 1992. Identification guide to European Passerines. L. Svensson, Stockholm.
- Tschirren, B., Fitze, P.S. & Richner, H. 2003. Proximate mechanisms of variation in the carotenoid-based plumage coloration of nesting Great Tits (Parus major L.). J. Evol. Biol. 16: 91–100. doi: 10.1046/j.1420-9101.2003.00483.x
- del Val, E., Quesada, J. & Senar, J.C. 2010. Age-related differences in a carotenoid-based coloration trait are due to within-individual changes in Great Tits (Parus major). Ardea 98: 179–184. doi: 10.5253/078.098.0207