Abstract
Capsule The density of Black Kites in Delhi, India, may represent the highest concentration of a raptor recorded in the world and has not declined since the 1960s.
Aims To estimate the density, phenology, breeding success and diet of Black Kites in Delhi.
Methods During 2013, Black Kite nests were surveyed in 24 plots of 1 km2 distributed throughout Delhi. A sample of 151 nests was checked regularly to record laying date, breeding success and diet.
Results The average density was 15 nests/km2. The majority of nests were on trees (91%) and the rest on artificial structures. Mean laying date was 31 January and the laying season was protracted over four months. Mean number of fledged young was 0.73, 1.09 and 1.53 per territorial, breeding and successful pair. Diet was dominated by scavenged meat and by rats, pigeons and doves abundant in the city.
Conclusions Density has been stable since 1960–1970s and probably represents the highest ever recorded for a raptor. This is probably promoted by a combination of (i) availability of rubbish, (ii) few predators and (iii) high tolerance by people. The conservation status of this raptor seems satisfactory, but removal of mature trees for rapid development may result in local declines or re-distributions, suggesting the need for continued monitoring.
The Black Kite Milvus migrans is a medium-sized raptor, currently considered as one of the most numerous and successful birds of prey of the world (Ferguson-Lees & Christie Citation2001). It is a generalist, opportunistic feeder, capable of reaching extremely high densities where food concentrations allow it (e.g. review in Sergio et al. Citation2005, Malhotra Citation2007) and may occupy habitats which range from fully natural to completely urban (Ortlieb Citation1998, Ferguson-Lees & Christie Citation2001). Such opportunism and capability to exploit human-modified habitats has afforded this species a generally favourable conservation status, with frequent reports of recently increasing populations, despite some local declines (Bijlsma Citation1997, Sergio et al. Citation2003, Thiollay & Bretagnolle Citation2004).
This capability to adapt to human landscapes reaches its extreme in populations that nest in fully urban conditions, as frequently observed in Asia and Africa (Desai & Malhotra Citation1979, Brown et al. Citation1982, Ali & Ripley Citation1983, Naoroji Citation2006). In these settings, kites are reported to use the urban ecosystem not only for nesting but also for feeding on human offal, road kills, animal carcasses and rubbish, sometimes forming spectacular concentrations of thousands of individuals at rubbish dumps of large cities (Brown et al. Citation1982, Owino et al. Citation2004, Naoroji Citation2006, Malhotra Citation2007). When these dumps are located in the proximity of airports, the concentration of kites often generates serious management problems because of the risk of collisions with planes (Satheesan Citation1996, Owino et al. Citation2004). It is remarkable that, despite their overall abundance and frequent proximity to humans, Black Kites have been very rarely studied, except for two or three intensively investigated populations, all of them located in Europe and in non-urban settings (Viñuela et al. Citation1994, Blanco Citation1997, Sergio et al. Citation2003, Citation2011).
In the Indian subcontinent, where we conducted our research, the govinda sub-species is well distributed with dense populations in all the major urban centres (Naoroji Citation2006), which has attracted many anecdotal observations, as reported in several issues of the Journal of the Bombay Natural History Society (Hanxwell Citation1892, Fischer Citation1906, Ali Citation1926, Abdulali Citation1968, Citation1972, Mahabal & Bastawade Citation1985, Malhotra Citation1991). However, quantitative data for this biogeographic region are extremely scarce and previous studies, all of them conducted in the 1970s, have focused on: (1) a coarse estimation of the size of the overall Delhi population (Galushin Citation1971) and (2) data on the breeding ecology of the high-density colony of the Delhi Zoo (Desai & Malhotra Citation1979). Here, we report comprehensive quantitative data on the density, nest spacing, phenology, breeding success and diet of a fully urban population located within Delhi, India. We then compare the current estimates with historical records and with studies on other kite sub-species.
METHODS
Study area
Black Kites were surveyed in 2013 in 24 plots (details below) within an overall area of 1500 km2 pertaining to the city of Delhi, India. Delhi is a mega-city of 16 million inhabitants in constant, rapid expansion (Census Organization of India Citation2011). The overall city comprises both urban and semi-urban areas under poor solid waste management, which affords plenty of food to Black Kites in the form of rubbish, carrion and remains from slaughterhouses. The climate is semi-arid, with 64 cm of annual precipitation, mainly concentrated in July and August. Temperature ranges from a mean maximum of 39.6°C to a minimum of less than 8.2°C in the winter (India Meteorological Department Citation2013). The vegetation of the general region falls within the ‘northern tropical thorn forest’ category (Champion & Seth Citation1968).
Field procedures
Because many areas of the city were private properties not accessible to the public, it was impossible to design a very large continuous study area. Also, because Black Kites in our area can attain extremely high densities, small-sized plots distributed over a wide area were judged to be better suited to sample all available conditions than a single continuous plot of necessarily limited extent. Therefore, we designed a network of 24 sample plots, each one of approximately 1 km2 of homogenous accessibility and distributed throughout the city covering all types of potential nesting habitats. However, a standardized shape or a standardized surface of 1 km2 could not be attained for all plots because of constraints imposed by private properties and logistical difficulties of access. Private properties had similar landscape features to the surrounding areas of the city and we are confident that their exclusion did not bias our density estimates. However, because of the above, nests which were located at the periphery of each sample plot were not employed to generate estimates of nest spacing (nearest neighbour distance henceforth referred as NND), unless a complete nest census had been conducted also for the area bordering the quadrat.
We surveyed each quadrat repeatedly every few weeks, starting from the pre-incubation period, by walking slowly and carefully inspecting all potential nest structures (trees, buildings, towers, etc.). Structures were classified as active nests when a kite individual or pair was repeatedly observed to perch in the nest or its immediate surroundings, or to add material to the nest. Once found, nests were checked by climbing to them, observing them from nearby vantage points, or through an eight-meter telescopic rod equipped with a video-recording camera. Nests were checked approximately every eight days. However, because of time, safety, accessibility and manpower limitations, data on breeding success were collected only at a sub-sample of nests.
A nest was classified as depredated when we found remains of plucked chicks. Cases of brood reduction (death of one chick, often caused by its siblings, subsequently fed to other nestlings) were not classified as predation events. Hatching date was calculated by backdating from the feather development of nestlings first observed when <15 day old and by comparison to reference information in Desai & Malhotra (Citation1977), Cramp & Simmons (Citation1980), Hiraldo et al. (Citation1990) and personal data by one of the authors (F.S.). Laying date was estimated by subtracting 30 day, the average incubation period (Viñuela Citation1997), from hatching date. During each visit, we collected prey remains found inside and under nests and identified them to the genus or species level assuming the smallest possible number of individuals. These items were used to estimate each prey percentage contribution by number or by mass to the diet of Black Kites.
Terminology follows Steenhof (Citation1987): a territorial pair was one that built a nest and then did or did not lay a clutch; a breeding or reproductive pair was one which laid eggs; a successful pair was one which raised at least one nestling until it was 40 day old; and breeding success was the percentage of successful territorial pairs. There was no need to correct the estimates of breeding success through Mayfield estimators, because all plots were surveyed repeatedly from the pre-laying period onwards, and because nests were easy to find and were checked very frequently (approximately every eight days). Density was calculated as number of territorial pairs per unit area and expressed as number of pairs/km2. The difference in breeding success between nests located in trees and nests built on artificial structures was tested by means of a Z-test (Zar Citation1984). Throughout, means are given ±1 se, tests are two-tailed, and statistical significance was set at α ≤ 0.05.
RESULTS
Cumulatively, we censused 244 Black Kite nests in 2013. Out of these, 223 (91.4%) were located on trees and the rest on artificial structures (17 on electricity pylons and 4 on telephone metal towers). Out of 223 tree nests, 35.5% were built on Eucalyptus spp., 23% on Ficus spp., 13.8% on Neem (Azadiracta indica), 12.7% on Jamun (Syzygium cumini) and 8.3% on Keekar (Prosopis juliflora). The mean nest density was 15.1 ± 7.9 pairs/km2 and varied between 0 and 67.1 nests/km2 (n = 24 plots). Mean NND for the whole population was 133 ± 15 m and ranged between 5 and 2315 m (n = 207 pairs).
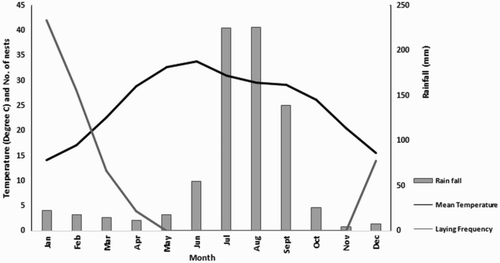
A subset of 151 nests was closely monitored for breeding success. The overall mean laying date was 31 January (n = 65, se = 3.3 days; range 19 December–13 April) and the laying season lasted almost 4 months (115 days), with a pronounced peak between the second half of January and first half of February (). When mean monthly temperature and rainfall were super-imposed on the laying frequency (), kites seemed to concentrate clutch initiation before the temperatures became excessively high and before the start of the Monsoon rains in June–July. The percentage of clutches initiated each month was negatively related to the minimum monthly temperature (linear regression: B = −1.37 ± 0.35; B for constant = 34.21 ± 7.14; n = 12; Bonferroni-corrected P = 0.006; R2 = 0.56) and quadratically related to the maximum monthly temperature (quadratic regression: B for linear term = −11.86 ± 3.66; Bonferroni-corrected P = 0.02; B for quadratic term: =0.17 ± 0.06; Bonferroni-corrected P = 0.02; B for constant = 211.37 ± 54.04; n = 12; R2 = 0.77), while egg laying stopped with the commencement of the rains and was initiated again only after the monsoon season. Finally, the number of young fledged by each pair declined with laying date (linear regression: B = −0.13 ± 0.03; B for constant = 1.66 ± 1.77; n = 65, P = 0.001; R2 = 0.17).
Figure 1. Temporal frequency of laying dates in the Black Kite population of Delhi (India) in 2013 (n = 65).
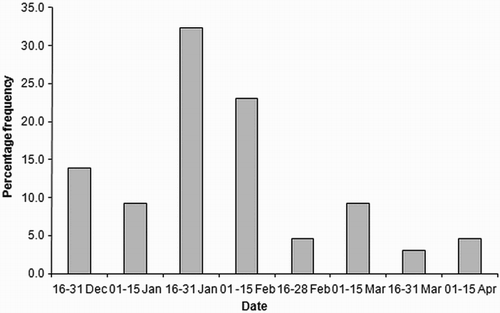
Figure 2. Mean temperature, monthly rainfall and Black Kite laying frequency in Delhi (weather data from India Meteorological Department Citation2013).
Mean clutch size was 2.09 ± 0.06 (n = 100). Mean hatching success was 64.6 ± 4.65% (n = 72 nests). Of 137 chicks first observed when less than five days old, three were depredated (all from a single nest) and six were subsequently observed dead in the nest or simply disappeared, probably because of sibling aggression (Viñuela Citation2000). The mean percentage of nestlings lost by brood reduction was 0.16 ± 0.04 per brood (n = 91 nests). The mean number of fledged young was 0.73 ± 0.07 per territorial pair (n = 151), 1.09 ± 0.06 per breeding pair (n = 100) and 1.53 ± 0.04 per successful pair (n = 72). Forty-eight per cent of territorial pairs successfully raised their nestlings to fledging age (n = 151). There was a trend for breeding success to be higher for nests on trees than for nest on the artificial structures (46% vs. 27.8%; Z = 1.8, P = 0.07, n for tree nests = 130, n for artificial substrate = 21).
Black Kite diet included all vertebrate classes but was strongly dominated, both by mass and number, by three main items: (1) remains from slaughterhouses, mainly in the form of compact chunks of meat; (2) rats and (3) medium-sized urban birds, such as doves and pigeons ().
Table 1. Diet of breeding Black Kites in Delhi, India (2012–2013), as estimated by food remains collected inside and under the nest.
DISCUSSION
Our study confirmed that Black Kites maintained extremely high breeding densities throughout the city of Delhi, as already observed in the 1970s (Galushin Citation1971). When compared with data from other populations (reviewed in ), the density observed in the urban environment of Delhi was higher than any previously published estimate. This is then, probably, the highest density ever recorded over a large, continuous area for any bird of prey of this size. The capability to attain such a high population-level over such a large region is likely to be promoted by a combination of at least three factors. (1) First, the rubbish management plans of such a rapidly developing mega-city are inevitably poor, which results in a network of enormous, legally authorized rubbish dumps coupled with hundreds of smaller, and often illegal sites where garbage is dumped daily. At an even finer-scale, private individuals, families and shops often leave their daily garbage directly in the streets, resulting in a network of ephemeral, small piles of food. In turn, these must promote large populations of potential prey species, such as rats and pigeons. All the above, coupled with the high abundance of meat and fish shops throughout the city, sets an ideal scenario of enormous food availability for an opportunistic predator and facultative scavenger. (2) Second, the attitudes of local people towards kites, and wildlife in general, are extremely positive and tolerant, even despite the fact that some kites can be very aggressive in defending their nest against nearby passers-by. We are not aware of any cases of persecution of kites in Delhi, which is confirmed by the relative absence of fear of humans by most kites in comparison to European conspecifics. (3) Third, the city provides an environment with a low abundance of potential predators. The only potential nest predators known to occur locally are Indian Eagle Owls Bubo bubo bengalensis, House Crows Corvus splendens and Rhesus Macaques Macaca mulatta. The latter two species can be locally abundant, but are often deterred by the very aggressive and effective nest defence behaviour of parent kites.
Table 2. Breeding density and nest spacing of Black Kite populations in Europe and India (Delhi), 1966–2013.
When compared to historical records, the high density we recorded seemed remarkably stable over several decades. Rapid city-wide surveys and data from the New Delhi Zoological Park suggested only slightly higher densities in the 1970s than currently observed (Galushin Citation1971, Desai & Malhotra Citation1979; see ). This is despite enormous changes in the city's dimensions, population size and management, and despite the recent, virtual extinction of the locally abundant populations of a potential trophic competitor, the White-rumped Vulture Gyps bengalensis, the former primary scavenger (Prakash et al. Citation2003, Naoroji Citation2006). The kite population thus seems very resilient to change in terms of overall density.
The laying season was protracted over almost four months, probably as a result of the long period of warm, favourable climate and of the stable food supply provided by the urban environment (). The temporal peak and range of laying dates seemed to be arranged so that most nestlings fledged well before the high temperatures and the marked peak in precipitation caused by Monsoon rains in July–August (). The negative effect of high temperatures and rainfall on kite foraging performance, egg viability and breeding success has been reported for various European populations (Hiraldo et al. Citation1990, Viñuela Citation2000, Sergio Citation2003). The observed, lengthy range of laying dates compares to a duration of the laying season of 28 days for kite populations of the Italian Alps and to 2.8 months for the population of Doñana National Park, in the extreme south of Europe (F. Sergio, pers. data). This suggests a North-South latitudinal gradient in the length of kites' breeding seasons. Protracted breeding seasons are increasingly reported as progressively more studies of birds of prey are conducted in tropical or more southern latitudes (Simmons Citation2000, Ogada & Kibuthu Citation2012).
When compared to other populations (review in ), our estimates of breeding success were lower than in other studies and this may be a consequence of density-dependent processes in a crowded, saturated population (Newton Citation1998). The fact that similarly low levels of reproduction were reported for another saturated population (Doñana, Sergio et al. Citation2011) lends support to this impression. However, in the absence of more information, other alternative explanations cannot be discounted: for example, it is not known whether a diet based in large portion on rubbish and meat produced for human consumption could spread pathogens or toxic substances among the offspring.
Table 3. Productivity of Black Kite populations in Europe and Asia, 1966–2013.
Finally, the observed diet composition confirmed the full dependence of the local kite population on urban resources, such as meat scraps from slaughterhouses or prey species which were extremely abundant within the city, such as rats, pigeons and doves. The current picture of the diet does not suggest that kites range frequently, if at all, out of the city to capture wild prey in surrounding rural areas. This confirms that the high density attained within the urban setting is likely promoted by attraction to a dense food source.
In summary, extensive foraging opportunities, a stable favourable climate, absence of human persecution and low density of potential predators have probably contributed to one of the densest raptor populations of the world. The current conservation status of the studied population seems satisfactory, but recent urban development is causing extreme and almost complete removal of mature trees in some sectors of the city. In turn, this could limit the kite population in the future, or trigger local declines and re-distributions, especially when considering that artificial structures do not seem to fully compensate for tree absence (authors’ pers. data). Thus, given the abundance of the species and the current urban sanitary levels, the ecological service provided by kites through removal of organic rubbish must be valuable, suggesting the need for ecologically sensitive urban planning of the remaining green areas. This calls for the importance of continued monitoring of the population and its nesting requirements in future years.
ACKNOWLEDGEMENTS
We thank W. Cresswell and three anonymous reviewers for improving the manuscript with their comments. We thank the Forest Departments of the Government of NCT, Delhi and Uttar Pradesh; Shri. Amitabh Agnihotri (Director of the National Zoological Park, New Delhi); the Administrative Officer of Miranada House: University of Delhi (DU); various other landowners, managers and government officials for permissions and logistic support. We heartedly thank for help in the field our field assistant Vishnu Narayan, Dr A. Tanferna and the following volunteers of the ‘Black Kite Project Group’ from Sri Venkateswara College (DU) and the University of Delhi: M. Singh, N. Goyal, S. Gupta, E. Bhartia, A. Yadav, C. Prajapati, D. Pal, V. Sindhi, K. Wadhwa, D. Ramanan, S. Prajapati, N. Verma, J. Sungra, B. Roy, A. Bedi, A. Mandal, V. Gupta, A. Dutta, P. Kumar, Raju, D. Thakur, G. Singh, Saubhagya, G. Vashishtha and M. Garg.
FUNDING
The study was funded by the Wildlife Institute of India and Raptor Research and Conservation Foundation (RRCF, Mumbai). Funding for travelling and fieldwork by F. Sergio was afforded by Project RNM-7307 of the Junta de Adalucia and CGL2011-28103 of the Spanish Ministry of Science and Innovation.
REFERENCES
- Abdulali, H. 1968. Extension of the range of the large Indian Kite Milvus migrans lineatus (Gray). J. Bombay Nat. History Soc. 65: 774.
- Abdulali, H. 1972. Some bird notes by W.F. Sinclair. J. Bombay Nat. History Soc. 69: 422–424.
- Ali, S. 1926. Mating habits of the Common Kite Milvus migrans govinda. J. Bombay Nat. History Soc. 31: 524–526.
- Ali, S. & Ripley, S. D. 1983. Handbook of the Birds of India and Pakistan: together with those of Bangladesh, Nepal, Sikkim and Sri Lanka (Vol. 1: Divers and Hawks). Compact ed. Delhi (IN): Oxford University Press. p. 226–229.
- Bijlsma, R.G. 1997. Black Kite. In Hagemeijer, W.J.M. & Blair, M.J. (eds.) The EBCC Atlas of European Breeding Birds, their Distribution and Abundance, 132–133. T & AD Poyser, London.
- Blanco, G. 1997. Role of refuse as food for migrant, floater and breeding Black Kites (Milvus migrans). J. Raptor Res. 31: 71–76.
- Brown, L.H., Urban, E.K. & Newman, K. 1982. The Birds of Africa, Vol. 1. Academic Press, London.
- Bustamante, J. & Hiraldo, F. 1990. Adoptions of fledglings by Black and Red Kites. Anim. Behav. 39: 804–806. doi: 10.1016/S0003-3472(05)80395-1
- Census Organization of India. 2011. Census of India. Available from: http://censusindia.gov.in/2011census [accessed 27 September 2013].
- Champion, H.G. & Seth, S.K. 1968. A Revised Survey of the Forest Types of India. Manager of Publications, Government of India, New Delhi.
- Cramp, S. & Simmons, K.E.L. 1980. Handbook of the Birds of Europe, the Middle East and North Africa, Vol. 2. Hawks and Bustards. Oxford University Press, Oxford.
- Danko, Š. 1989. Five young in the nest of a Black Kite (Milvus migrans). Buteo 4: 87–92.
- De Giacomo, U., Martucci, O. & Tinelli, A. 1993. L'alimentazione del Nibbio bruno (Milvus migrans) nella Tenuta di Castelporziano (Roma). Avocetta. 17: 73–78.
- Desai, J.H. & Malhotra, A.K. 1977. Growth and development of the Pariah Kite Milvus migrans govinda. Miscellaneous Rep. Yamashina Inst. Ornithol. 9: 217–226.
- Desai, J.H. & Malhotra, A.K. 1979. Breeding biology of the Pariah Kite Milvus migrans at Delhi Zoological Park. Ibis 121: 320–325. doi: 10.1111/j.1474-919X.1979.tb06849.x
- Ferguson-Lees, J. & Christie, D.A. 2001. Raptors of the World. Houghton Miffin Company, New York.
- Fischer, C.E.C. 1906. Flocking of Kites. J. Bombay Nat. History Soc. 17: 525–526.
- Fiuczynski, V. 1981. Berliner milan-chronik (Milvus migrans and Milvus milvus). Beitr. Vogelkd 27: 161–196.
- Galushin, V.M. 1971. A huge urban population of birds of prey in Delhi India. Ibis 113: 522. doi: 10.1111/j.1474-919X.1971.tb05189.x
- Gedeon, K. 1994. Monitoring Greifvögel und Eulen. Grundlagen und Möglichkeiten einer Langfristigen Überwachung von Bestandsgröben und Reproduktionsdaten Jahresber. Monitoring Greifvögel Eulen Europas 1: 1–118.
- Hanxwell, T.A. 1892. Nest and eggs of the crested Black Kite. J. Bombay Nat. History Soc. 07: 403–404.
- Heckenroth, V. 1970. Der Greifvögelbestand des Bodanrücks (Bodensee) 1968 and 1969. Anz. Ornithol. Ges Bayern 9: 47–51.
- Henrioux, P. & Henrioux, J. 1995. Seize ans d'etude sur les rapaces diurnes et nocturnes dans l'Ouest lemanique (1975–1990). Nos Oiseaux 43: 1–26.
- Hiraldo, F., Veiga, J.P. & Manez, M. 1990. Growth of nestling Black Kites Milvus migrans: effects of hatching order, weather and season. J. Zool. Lond. 222: 197–214. doi: 10.1111/j.1469-7998.1990.tb05672.x
- India Meteorological Department. 2013. http://amssdelhi.gov.in/climatology/sfd1.htm [accessed 27 September 2013].
- Koga, K., Siraishi, S. & Uchida, T.A. 1989. Breeding ecology of the Black-eared Kite Milvus migrans lineatus in the Nagasaki Peninsula, Kyushu. Jpn. J. Ornithol. 57: 57–66. doi: 10.3838/jjo.38.57
- Mahabal, A. & Bastawade, D.B. 1985. Population ecology and communal roosting behaviour of Pariah Kite Milvus migrans govinda in Pune (Maharashtra). J. Bombay Nat. History Soc. 82: 337–346.
- Malhotra, A.K. 1991. Site fidelity and power of recognition in Pariah Kite Milvus migrans govinda. J. Bombay Nat. History Soc. 87: 458.
- Malhotra, A.K. 2007. Tiger of sky-Pariah Kite. PhD Thesis, Shilalekh Publishers, Delhi.
- Mammen, U. & Stubbe, V. 1995. Jahresbericht 1994 zum monitoring Greifvögeln und Eulen Europas Jahresber. Monitoring Greifvögel Eulen Europas 7: 1–78.
- Mammen, U. & Stubbe, V. 1996. Jahresbericht 1995 zum monitoring Greifvögeln und Eulen Europas Jahresber. Monitoring Greifvögel Eulen Europas 8: 1–92.
- Naoroji, R. 2006. Birds of Prey of the Indian Subcontinent. Christopher Helm/A&C Black Publishers Ltd., London.
- Newton, I. 1998. Population Limitation in Birds. Academic Press, London.
- Nore, T. 1979. Rapaces diurnes communs en Limousin pendant la periode de nidification (Buse, Bondre, Milan noir, Busards saint-martin et Cendre). Alauda 47: 183–194.
- Ogada, D.L. & Kibuthu, P.M. 2012. Breeding ecology of Mackinder's Eagle-Owls (Bubo capensis mackinderi) in farmlands of central Kenya. J. Raptor Res. 46: 327–335. doi: 10.3356/JRR-11-90.1
- Ortlieb, R. 1998. Der Schwarzmilan. Die Neue Brehm-Bücherei, Hohenwarsleben, Germany.
- Owino, A., Biwott, N. & Amutete, G. 2004. Bird strike incidents involving Kenya Airways flights at three Kenyan airports, 1991–2001. African J. Ecol. 42: 122–128. doi: 10.1111/j.1365-2028.2004.00507.x
- Petretti, F. 1992. Nibbio bruno. In Brichetti, P., De Franceschi, P. & Baccetti, N. (eds.) Fauna d'Italia, 459–465. Edizioni Calderini, Bologna, Italy.
- Petretti, A. & Petretti, F. 1981. A population of diurnal raptors in central Italy. Gerfaut 71: 143–156.
- Prakash, V., Pain, D.J., Cunningham, A.A., Donald, P.F., Prakash, N., Verma, A., Gargia, R., Sivakumar, S. & Rahmani, A.R. 2003. Catastrophic collapse of Indian White Backed (Gyps bengalensis) and Long Billed (Gyps indicus) vulture population. Biol. Conserv. 109: 381–390. doi: 10.1016/S0006-3207(02)00164-7
- Satheesan, S.M. 1996. Raptors associated with airports and aircraft. In D. M. Bird, D. E. Varland, and J. J. Negro (eds.) Raptors in human landscapes: adaptations to built and cultivated environments: Adaptations to built and cultivated environments, 315–323. Academic Press, London.
- Seelig, K., Benecke, H., Braumann, F. & Nicolm, B. 1996. Die Vogel im Naturpark Drömling. Abh. Ber. Mus. Heineanum 3, Sonderh, Germany.
- Sergio, F. 2003. From individual behaviour to population pattern: weather-dependent foraging and breeding performance in Black Kites. Animal Behav. 66: 1109–1117. doi: 10.1006/anbe.2003.2303
- Sergio, F. & Boto, A. 1999. Nest dispersion, diet and breeding success of Black Kites (Milvus migrans) in Italian Pre-Alps. J. Raptor Res. 33: 207–217.
- Sergio, F., Pedrini, P. & Marchesi, L. 2003. Reconciling the dichotomy between single species and ecosystem conservation: Black Kites and eutrophication in pre-Alpine lakes. Biol. Conserv. 110: 101–111. doi: 10.1016/S0006-3207(02)00181-7
- Sergio, F., Blas, J., Forero, M., Fernández, N., Donázar, J.A. & Hiraldo, F. 2005. Preservation of wide-ranging top predators by site-protection: Black and Red kites in Doñana national park. Biol. Conserv. 125: 11–21. doi: 10.1016/j.biocon.2005.03.002
- Sergio, F., Blas, J., López, L., Tanferna, A., Díaz-Delgado, R., Donázar, J.A. & Hiraldo, F. 2011. Coping with uncertainty: breeding adjustments to an unpredictable environment in an opportunistic raptor. Oecologia 166: 79–90. doi: 10.1007/s00442-010-1795-x
- Sermet, E. 1980. Milan noir. In Schifferli, A., Göroudet, P. & Winkler, R. (eds.) Arias des oiseaux nicheurs de Suisse, 88–89. Station Ornithologique de Sem-pach, Sempach, Switzerland.
- Simmons, R.E. 2000. Harriers of the World: Their Behaviour and Ecology. Oxford University Press, London, United Kingdom.
- Steenhof, K. 1987. Assessing raptor reproductive success and productivity. In B.A. Giron, P., Millsap B. A., Kline, K.W. & Bird D.M. (eds.) Raptor Management Techniques Manual, 157–170. Natl. Wildl. Fed., Washington, DC.
- Stubbe, V. 1961. Die Besiedelungsdichte eines Abgeschlossenen Waldgebietes (Hakel) mit Greifvögeln im Jahre 1957. Beitr. Vogelkd 7: 155–224.
- Thiollay, J.-M. 1967. Ecologie d'une population de rapaces diurnes en Lorraine. La Terre et la Vie. 114: 116–183.
- Thiollay, J.-M. & Bretagnolle, V. 2004. Rapaches nicheurs de France: distribution, effectifs et conservation. Delachaux et Niestlé, Paris, France.
- Viñuela, J. 1997. Laying order affects incubation duration in the Black Kite (Milvus migrans): counteracting hatching asynchrony? Auk 114: 192–199. doi: 10.2307/4089160
- Viñuela, J. 2000. Opposing selective pressures on hatching asynchrony: egg viability, brood reduction, and nestling growth. Behav. Ecol. Sociobiol. 45: 333–343.
- Viñuela, J., Villafuerte, R. & De Le Court, C. 1994. Nesting dispersion of a Black Kite population in relation to location of rabbit warrens. Can. J. Zool. 72: 1680–1683. doi: 10.1139/z94-224
- Zar, J.H. 1984. Bio-Statistical Analysis, 283. Prentice-Hall, Englewood Cliffs, NJ.
