Abstract
Capsule Folivorous caterpillars constituted the majority of nestlings’ food in a primeval forest. Blue Tit broods only partially matched the caterpillar peak, and the mismatch did not affect food composition or nesting success.
Aims To describe factors influencing the timing of reproduction in Blue Tits under primeval conditions (Białowieża National Park, Poland) and to check whether they schedule breeding so as to synchronize broods with a seasonal caterpillar peak.
Methods We gathered information on phenology of leaf development, seasonal availability of folivorous caterpillars (frass collection), timing of Blue Tit breeding, composition of its nestling food, and nest fate over a three-year period.
Results Caterpillars constituted c. 74% of nestling diet, but only 17–65% of broods matched the caterpillar peak in any season. Neither total nest loss, nor frequency of brood reduction depended on the level of mismatch. Caterpillar availability was probably adequate every year, regardless of the amount of mismatch, and no selective advantage of precise matching was detectable. Phenological events at all trophic levels occurred earlier in warmer springs. Egg-laying coincided with tree bud burst and appearance of caterpillars, but was not critically dependent on their timing.
Conclusion The observations are consistent with the view that Blue Tits under primeval conditions in Białowieża National Park, Poland, breed as early as possible, rather than synchronizing their breeding with the caterpillar peak later in the season.
Synchronizing reproduction in relation to varying environmental conditions constitutes a major problem for all organisms living in seasonal environments. Breeding should be timed so the chances of producing offspring are highest. Lack (Citation1950) proposed that this should be the time when food for rearing young is most plentiful. He specifically suggested that ‘the breeding season of tits is adapted to the caterpillar season’. Though several authors (Perrins Citation1965, Citation1970, Daan et al. Citation1988, Barba et al. Citation1995, van Noordwijk et al. Citation1995, Verboven & Visser Citation1998, Nilsson Citation1999, Drent Citation2006, Naef-Daenzer et al. Citation2001) indicated that the availability of food for egg-producing females and numerous other factors could be more important selective forces, Lack's hypothesis has been regularly repeated in papers on the timing of passerine breeding (Immelmann Citation1971, Zandt et al. Citation1990, Visser & Lambrechts Citation1999, Tremblay et al. Citation2005, Both & Visser Citation2005). Lack's hypothesis depends on two conditions that have to be met simultaneously: (1) folivorous caterpillars constitute a key food source for nestlings and (2) reproduction is lower before and after the peak of caterpillar supply, because lower food supply acts via undernourishment of the young resulting in fewer/lower quality fledglings by birds that breed too early or too late.
The widespread signs of climate warming observed in recent decades in many parts of Europe (Sparks & Menzel Citation2002, Visser et al. Citation1998, Citation2003, Both et al. Citation2004, Dunn Citation2004, IPCC Citation2007) have raised concerns as to the possible negative effects of warmer springs on the reproduction of birds. The (presumably) co-adapted phenology of trees, folivorous caterpillars, and their avian predators could be disrupted by mismatches at numerous links in the food chain. Any mismatch could, via changes in the nourishment of young, result in lowered reproductive success and a lower survival rate of young. Many studies make these claims (review in Visser et al. Citation2012), but most do not provide any information on the actual composition of the nestling diet (Dunn & Winkler Citation2010, Cholewa & Wesołowski Citation2011) or food availability.
Tests of the temporal match–mismatch hypothesis (Cushing Citation1990) often ignore the effect on reproduction of varying levels of food abundance across years (Zandt et al. Citation1990, Bańbura et al. Citation1994, Maziarz & Wesołowski Citation2010) or habitats (van Balen Citation1973, Tremblay et al. Citation2005). For example, birds breeding in a poor habitat or during a season with low caterpillar abundance would be unable to feed their young adequately, even if they perfectly matched the seasonal food peak with the food requirements of their nestlings (Tremblay et al. Citation2003). On the other hand, birds breeding in a rich habitat or during a caterpillar outbreak could have access to a superabundant food supply during long periods of time, mismatching the peak by a wide margin without any selective disadvantage (Tremblay et al. Citation2003, Durant et al. Citation2005). The temporal match–mismatch hypothesis also takes no notice of the potential effect of breeding density on food availability, which – other things being equal – would result in lower per capita food availability in more densely populated areas. Studies on relationships between the tits and their food supply have, as a rule, been carried out on highly productive, dense populations of birds breeding in nest-boxes. The nest-box plots are also usually situated in woods dominated by a single tree species (Wesołowski Citation2011). All of these factors could affect the outcome of predator–prey relationships to an unknown extent, and data from birds using tree holes in less disturbed habitats are still missing to determine the biological importance of phenological matching in more ‘natural’ populations.
The Białowieża Forest in eastern Poland is one of the rare undisturbed habitats left in Europe. Fragments of primeval forest are strictly protected within the Białowieża National Park (BNP; Tomiałojc´ et al. Citation1984, Tomiałojc´ & Wesołowski Citation2005, Wesołowski Citation2007a). Here, food in the breeding season is usually superabundant and interspecific competition usually plays only a minor role (Wesołowski Citation2003, Citation2007b). The invertebrate fauna is diverse and not controlled, with outbreaks of folivorous caterpillars occurring regularly at eight to ten-year intervals (Wesołowski & Rowiński Citation2006, Citation2008, Wesołowski et al. Citation2009). The importance of such resource pulses, though, is reduced by the presence of a diverse array of alternative prey types (Rowiński Citation2001, Maziarz & Wesołowski Citation2010).
Extensive data on numbers and reproduction of Blue Tits have been gathered in BNP since 1975 (Tomiałojc´ et al. Citation1984). Blue Tits breed in various types of old-growth habitats, but their numbers are greatest in deciduous forests at around 2.6–3.6 pairs/10 ha, and exceptionally as high as 6–10 pairs/10 ha (Wesołowski & Tomiałojc´ Citation1997, Wesołowski et al. Citation2010). Tits breed exclusively in tree holes (Wesołowski & Rowiński Citation2012) and the onset of breeding is highly variable across years – depending on spring temperatures it can differ by as much as 30 days (Wesołowski & Cholewa Citation2009). Blue Tits have not substantially advanced their laying dates in the long term, despite observed warming in the second half of April (Wesołowski & Cholewa Citation2009).
During three years of intensive observations in the rich deciduous forests of BNP we gathered observations of Blue Tits breeding and collected data on nestling food, spring temperatures, tree phenology (bud burst), and the abundance and development of folivorous caterpillars. First of all, we used these data to test whether Blue Tits feed mostly caterpillars to their young, and if they synchronize their reproduction with the peak of caterpillar availability as predicted by Lack's hypothesis. Furthermore, we analysed variation in temperature and the timing of phenological events at three different trophic levels to see (1) to what extent the birds can use the timing of earlier events to predict when the future caterpillar peak would occur and (2) what birds can do to improve temporal match between the increasing requirements of their broods and varying food resources.
METHODS
Study area
The Białowieża Forest complex is situated in the middle of the European plain, at the Polish-Belarusian border (co-ordinates of Białowieża village: 52°41′ N and 23°52′ E). The western part of the forest (613 km2, ca 45% of the area) belongs to Poland. The forest represents a remnant of the vast lowland forests that once covered large parts of temperate Europe. Its present unique features result from its considerable size and an exceptionally good state of preservation (Tomiałojc´ & Wesołowski Citation1990, Wesołowski Citation2005, Citation2007a). The majority of the tree stands in the Polish part are now under management, but a 47.5 km2 block of the best preserved primeval old-growth stands has been retained within the strictly protected part of BNP.
The preserved block of primeval stands are multi-storey, mixed-species, and uneven-aged. They contain many veteran trees (the tallest Norway Spruce Picea abies can reach 52 m, and several other species reach 42–45 m, Niechoda & Korbel Citation2011), and a large amount of standing dead timber and fallen trees (20–25% of total wood volume, Bobiec Citation2002). For more information and photos see Tomiałojc´ & Wesołowski (Citation1990, Citation2004), Wesołowski (Citation2007a), Wesołowski et al. (Citation2010).
Data were gathered in four large sample plots (33–54 ha), 1–2 km apart, covering a total area of about 185 ha of BNP. Three plots (C, M, and W) were situated in oak-lime-hornbeam stands composed mostly of Hornbeam Carpinus betulus, Small-leaved Lime Tilia cordata, Pedunculate Oak Quercus robur, Spruce Picea abies, and Continental Maple Acer platanoides. The fourth plot (K) was located in a swampy riverine forest made up mainly by Alder Alnus glutinosa, Ash Fraxinus excelsior, and Spruce. However, as Blue Tits breeding in plot K had access to drier Hornbeam covered ‘islands’ or valley slopes we treated all the plots as a single ‘deciduous’ habitat (for detailed descriptions see Wesołowski et al. Citation2002, Citation2006, Tomiałojc´ & Wesołowski Citation1990, Citation2005). Natural tree holes were available in excess in all plots (Walankiewicz Citation1991, Wesołowski Citation1996, Citation2001) and only these were used by breeding Blue Tits (Wesołowski & Rowiński Citation2012): neither nest-boxes nor additional food were available.
Field observations
Observations were carried out in 2005–07. Intensive searches for nests, aimed at finding all breeding holes, were made in two oak-lime-hornbeam areas (plot C – 48 ha and plot M – 54 ha, Wesołowski et al. Citation2006, Citation2010). Details of these areas are given in Wesołowski (Citation2002). To establish the number and distribution of Blue Tit breeding pairs in the plots, birds were followed and their movements noted on field maps before the onset of breeding. Most of the males were colour-ringed, which facilitated mapping work and nest searches. Additionally Blue Tit nest-holes were also searched for less intensively during the course of general mapping censuses (Wesołowski et al. Citation2010) in plots W (50.5 ha) and K (33 ha).
To gather data on the fate of nests the breeding holes were checked regularly, mostly from the ground. The frequency of nest visits depended on the stage of the nesting cycle (see below). After incubation had been recorded, the nest was inspected to count eggs. Lower holes were inspected from a ladder, the higher ones by climbing. We used a flashlight bulb, fixed to a flexible wire to light the cavity interiors. In some holes additional usage of a small mirror on a bendable handle was necessary. To calculate the expected fledging dates, holes with young were visited again and age of nestlings established following the criteria of Winkel (Citation1970). In the minority of inaccessible holes the expected fledging dates were calculated by adding 18 days to the recorded hatching dates, which was detected by observing holes every second day around the anticipated hatching dates. The day before the first day on which the presence of young was first recorded (female returning with food or male entering hole with food during the female's absence and leaving without it, removal of egg shells) was taken as the hatching date.
Around the expected time of fledging, holes were observed from a distance about every 24 hours, up to the day on which no parents were observed bringing food to the hole. The observation distance varied with the situation of hole and the parents' behaviour, in most cases being within c. 15–50 m. If on the previous day young were at least 18 days old (the youngest age of fledging for undisturbed broods, T. Wesołowski & P. Rowiński, unpubl. data) and no signs of attempted nest-robbing were detectable, the nest was treated as successful. If no feeding was observed at a hole containing young about to fledge (17–18 days old) parent birds (most of them colour-ringed, P. Rowiński, unpubl. data) were searched for to check whether they were collecting food and carrying it to prematurely fledged young. If they were, the nest was classified as successful. All other cases of premature cessation of parental activity (no signs of parent presence during a 90 minutes observation session) were treated as nest failures.
The fledging dates were directly recorded in the field with ±1 day precision (see above). The hatching dates were derived from direct observations of birds' behaviour around the hatch time (see above) and direct checks of nest contents in accessible holes. They produced hatching date estimates with ±1 day precision. Only in 15 broods (c 8% of all) were the hatching date values less precise, because they were back-calculated from the fledging dates (subtracting 19 days from them, ). The first-egg dates were back-calculated from observations at laying (assuming that one egg was laid per day) or from the hatching dates (assuming that incubation lasts 15 days, ). When clutch size was unknown (e.g. in holes observed only from a distance, c. 18% of nests), 25 days were subtracted from the hatching date. This comprised ten days allowed for egg-laying (where a 10-egg clutch was assumed) and 15 days for incubation.
Table 1. Timing of Blue Tit egg-laying, length of breeding stages and variation in spring weather conditions in BNP in relation to year.
To investigate changes in diet with nestling age, a brood was visited three times: when nestlings were small (c. 5 days), medium (c. 10), and large (c. 15 days). The composition of nestling diet was derived from direct observations of birds carrying food to their young. Observations were made by a team of well-trained observers using 10 × 50 binoculars or telescopes, and individuals were shifted among plots to equalize any possible observer effect. Observations were carried out between 5:00 and 19:00 (Central European Time), independent of the weather. To remove any effect of time of day consecutive observations in the same areas were taken at different hours.
The observer approached the nest close enough to be able to see clearly the bill content of a parent appearing in the vicinity. When birds saw the observer, they often perched in the vicinity of the hole with food and looked around before entering, allowing for determination of the bill contents. The observation distance varied with the situation of hole and the parents' behaviour, in most cases being within c. 15–50 m. After determining the prey, the observer moved away to permit the bird to feed its young. When the bird entered the nest too quickly for food to be seen, the record was discounted and observations continued until five beak loads of food had been determined.
A single feeding sample therefore consisted of records of food items brought by parents on five visits to the nest, without consideration of the parents' sex. Every food item seen was described to the detail of a group of organisms if recognized in the field – ‘caterpillars’ (their colour and size in relation to the bill length), ‘spiders’ (their colour and size), or something else (its shape, stiffness, presence of legs and/or wings, wings' pattern). However, no effort was taken to quantify the number of items brought on a single visit.
We gathered phenological observations at the oak-lime-hornbeam plot W, recording leaf development of the four most numerous deciduous tree species (Hornbeam, Lime, Pedunculate Oak, and Continental Maple), as well as Hazel Coryllus avellana. At ten sites, 50–200 m apart, one tree of every observed species (the same tree every spring) was used for recording the timing of bud burst and leaf development (details in Wesołowski & Rowiński Citation2006, Citation2008). It has been demonstrated that observations in this area provided representative information on tree phenology in BNP (Wesołowski & Rowiński Citation2006).
Each spring, observations commenced in the period when Hazel leaf buds were strongly swollen or had begun to break, and continued until shoots on all sample trees had developed small unfolded leaves. Every second afternoon, using a 20–60× magnification telescope, we assessed development of apical leaf buds on a three-grade scale. We classified a bud as:
0 – undeveloped: all stages from a dormant bud, to a bud with broken scales, tips of leaves visible but still forming a single bud tip
1 – broken: from small leaves with bases still hidden in bud scales but tips detached from the bud axis, to small leaves with folded blades
2 – developed: small, unfolded leaves
We assessed the development stage of ten apical leaf buds, casually selected in the southern part of a sample tree crown. On each occasion the buds were chosen anew. For each observation day we summed values for individual buds within a tree to get a leaf development score for individual trees (range 0–20). After a tree reached the maximum score we discontinued observations. Finally, to arrive at a leaf development index for a tree species, we added up tree scores for each observation day. This produced an index with values ranging from 0 to 200 (small leaves on all shoots of all trees). Though we recorded leaf development in the five most numerous deciduous woody species (Wesołowski & Rowiński Citation2006), here we use only data from the two earliest species (Hornbeam and Hazel) because other trees developed leaves too late to influence Blue Tit laying dates.
To gather data on the seasonal pattern of biomass variation of folivorous caterpillars, and to detect their seasonal peak, caterpillar frass was collected (Tinbergen Citation1960, Zandt Citation1994, Fischbacher et al. Citation1998). Frass collectors (0.25 m2 pieces of thin fabric spread among four wooden pegs) were placed under tree crowns in two oak–lime–hornbeam plots (C and M). Observations in these areas provided information representative of folivorous caterpillars variation in BNP (Wesołowski & Rowiński Citation2006, Citation2008). On each plot ten groups of trees were selected at least 100 m apart. Groups always consisted of one each of Penduculate Oak, Norway Maple, Hornbeam, and Lime. The same trees (n = 80) were used every year. To avoid losing data due to rain, frass collectors were emptied every two days (c. 48 hours) – Wesołowski & Rowiński (Citation2008). Every season frass was collected from the time of bud burst until early June when the mass of falling frass became too small to be reliably measured.
Data analysis
We use temperature data (daily means, °C) derived from the local weather station in Białowieża village, c. 1–6 km from the study areas (Wesołowski & Rowiński, Citation2008, unpubl. data). To calculate temperature sums we used mean temperatures of days with temperature > 0°C, over the periods best predicting respective events (Wesołowski & Cholewa Citation2009). These were 1 March to the date of bud burst of trees (Wesołowski & Rowiński Citation2006), and 1 March to the determinant dates of onset of laying in the (earliest) median clutches (Wesołowski & Cholewa Citation2009). The determinant date (the day on which a bird has to make its final decision about egg-laying, where temperatures after that date cannot affect timing of egg-laying; Kluyver Citation1952) was set four days before the calculated first-egg date (Wesołowski & Cholewa Citation2009). The mean daily temperature in the prelaying period was averaged over the same time periods. The mean daily temperature and temperature sums for the period of caterpillar development were calculated over the period of full bud burst of Hornbeam to the day of maximum frass fall. We took the former date as the date of caterpillar appearance, because this tree species provided c. 50% of leaves for folivorous caterpillars, and other trees (maples and early oaks) started to develop leaves at approximately the same time (Rowiński Citation2001, Wesołowski & Rowiński Citation2006).
The frass samples were dried at c. 50°C to a constant weight, cleaned of debris, and weighed to the nearest 0.001 g. The measured values were halved (frass was collected every two days), to obtain the daily frass fall value. Thus calculated values were used to compute mean daily mass of frass (g/0.25 m2/day) per tree species. To account for differences in contributions to a total leaf area provided by various tree species (Rowiński Citation2001) we calculated mean frass fall for a plot, by weighting the species' frass fall by its contribution. Finally, as there was no difference in the timing of bud burst across deciduous stands and tree species (Wesołowski & Rowiński Citation2006) within a season, we pooled the frass data from the two plots in the analysis. There was no prolonged rain (known to disrupt frass collection; Zandt Citation1994, Wesołowski & Rowiński Citation2008) around the peak dates, so the peak values of frass fall measured required no corrections (Maziarz & Wesołowski Citation2010).
Recognition of prey to a species level was in most cases impossible in the field. Therefore, on the base of the field descriptions, we classified all prey items into three main categories (types): ‘caterpillars’, ‘spiders’, and ‘others’. The latter category included insects in which wings were visible (e.g. Diptera and Hymenoptera) or not (e.g. Coleoptera and Heteroptera), as well as all other nondescript items (such as e.g. ‘small dark ball’). It was further possible to split caterpillars into ‘green’ and ‘other’ types. In most cases the ‘green’ category referred to Winter Moth Operophtera brumata (Geometridae) caterpillars, which constituted 84% (2006) to 98% (2005) of the folivorous caterpillars on the branches of Hornbeams sampled from the ground (P. Rowiński, unpubl. data). For each observation we calculated the proportion of these food types brought during the five feedings. If multiple categories of food were brought on a single visit, this visit was equally partitioned between the food categories observed (e.g. green caterpillars – 0.5 visit, spiders – 0.5 visit). The average share of food types brought to the nest in visits at the three different nestling ages was also calculated. In the final analysis we used only the broods for which a full set (three) of feeding observations was gathered (n = 80). Due to nest loss (Wesołowski & Rowiński Citation2012) we had also gathered an incomplete (one to two feeding samples) set of feeding data for 35 additional broods. However, after checking that their inclusion would not qualitatively change the results, these were not included in the analyses presented here.
Time of day did not have any effect on the composition of food brought to Blue Tit nestlings; there was no correlation (r s = 0.07 or less, n = 238) between the hour of observation and share of any food category. Therefore, we ignored this variable in all further analyses.
To account for inter-year differences in the timing of breeding we calculated relative laying and hatching dates, i.e. differences between an absolute first-egg date/hatch date and the median dates of the first-egg/hatch in the respective seasons. We calculated as well the degree of a brood's synchrony with the caterpillar peak date, by subtracting the season's peak date from the hatch date +10 days of individual broods. This permitted us to create three ‘synchrony’ groups: ‘synchronous’ – broods in which maximum nestling food demand (= 10 days old nestlings) fell within 3 days from the caterpillar peak, ‘precocious’ – maximum food demand > 3 days before the caterpillar peak, and ‘delayed’ – maximum food demand > 3 days after the peak.
To check if the synchrony of broods with the caterpillar peak influenced their productivity, for each of the above synchrony groups we calculated the proportion of nest loss at the nestling stage, as well as the partial brood losses in the successful broods for which feeding observations were conducted. The total breeding loss was expressed as the percentage of unsuccessful nests among those in which young had hatched. As complete nest histories were available, there was no need to apply Mayfield corrections. The difference between the number of eggs hatched in successful broods and the number of young fledging from them was taken as a measure of partial brood loss.
Statistics
Individual Blue Tits were rarely detected in BNP the following spring (c. 6% of females, unpubl. data). Thus, each year we observed largely different individuals. Therefore, we treated each brood as an independent case. To investigate overall changes in diet with year, synchronization of Blue Tit broods with the caterpillar peak, and nestling age, we used repeated-measures manova. Variation in the proportion of individual food categories was then analysed with univariate statistics (anova). The data were arcsin transformed before these analyses. To analyse factors affecting total brood loss we used the generalized linear probit model, with degree of synchrony and year as independent variables. We checked effects of the above variables on the partial brood loss by performing a general linear models' analysis. In the remaining analyses we employed non-parametric tests; all probability values are two-tailed.
RESULTS
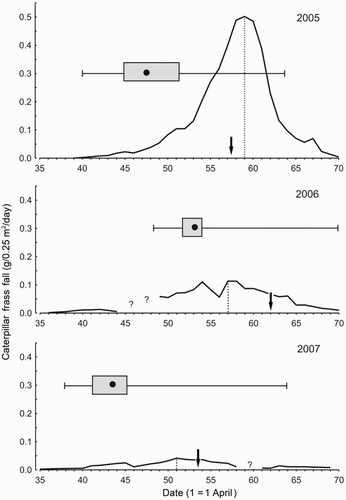
The earliest observations of preparations for breeding were made in 2007: hole cleaning – 6 April, bringing moss – 7 April. Egg-laying commenced on 13–23 April in different years (), and the latest recorded clutch was initiated on 23 May. Late clutches that were initiated several days after the main period (after 5 May, n = 6) may have been early replacements, but, as the females were unmarked, this could not be confirmed. Timing of egg-laying differed across the years (Kruskall–Wallis anova, H 2,185 = 91.9, P < 0.001), with the median laying date being ten days earlier in 2006 than in 2007 (). Laying was highly synchronized within a season, with 75% of clutches initiated within nine to ten days after the earliest ones, and half of them commencing within only four to five days (). The inter-year differences in commencement of laying followed variation in the spring temperatures: mean daily temperature (K–W anova, H 2,185 = 129.2, P < 0.001) and temperature sums (H 2,185 = 83.7, P < 0.001) were lower and similar in 2005–06 but much higher in 2007 (). Thus, in 2007, the earlier Blue Tits bred after being exposed to much higher temperatures. As some late clutches could be replacement attempts, we repeated calculations using only the first 75% broods in each season, but results were virtually the same (K–W anova, H 2,143, P < 0.001) in both comparisons.
Blue Tits laid 3–16 eggs (median = 11), and half of clutches (n = 134) contained 10–12 eggs. Clutch size did not vary across years (K–W anova, H 2,134 = 1.6, P = 0.46) and tended to decline with the relative date of clutch commencement (n = 134, r s = −0.19, P = 0.02). This relationship, though, was almost entirely due to smaller clutches being laid in the latter part of the season, and was not detectable among clutches initiated during the main period of laying (within five days from the yearly median, n = 101, r s = 0.01, P = 0.92).
The incubation period lasted 11–22 days in different broods, with median durations of 15 days in 2005 and 2007 and 12 days in 2006 (, K–W anova, H 2,71 = 25.7, P < 0.001). Within a season, Blue Tits with later relative laying dates did not incubate for fewer days (r s = 0.04, P = 0.72). Young stayed in nests for 17–22 days (median = 19 days), without substantial variation across years (K–W anova, H 2,118 = 4.1, P = 0.13).
Each spring buds of Hazel broke before those of Hornbeam (). The pattern of variation found in the onset of Hazel bud break and full bud burst was the same for both stages of Hornbeam bud burst (). In all cases these inter-year differences were significant (Friedman anova 2,10, χ2 = 18.2–20.0, P < 0.002). The dates of bud burst onset differed by three weeks, but the full bud burst dates varied less at 14–18 days (). Laying dates of Blue Tits followed the same inter-year pattern, but varied by only ten days (cf ).
Table 2. Timing of bud burst and variation in spring temperatures in BNP in relation to year.
Within a season the order of bud burst events followed increasing daily temperature and temperature sums (), but this was not always visible in the inter-year comparisons. The mean daily temperatures in the periods preceding the individual bud burst stages were similar in 2005 and 2006, but much higher in 2007 (, Friedman anova 2,30, χ2 = 45.3–45.7, P < 0.001). The temperature sums to time of the bud burst also differed across seasons (, Friedman anova 2,30, χ2 = 30.6–50.5, P < 0.001) but in this case they were higher in the earliest and latest springs and lower in the intermediate (2005) season.
The temperature sum required for the full bud burst of Hornbeam remained relatively stable among years but to accumulate that amount of warmth it took almost two weeks longer in 2005–06 than in 2007 (). Blue Tit laying dates varied in parallel () but they settled to lay eggs (determinant dates of median clutches) before the buds on Hornbeam had begun to burst in 2005–06, while in 2007 they chose to lay when buds had almost fully burst (cf Tables 1 & 2). The earliest laying Blue Tits, though, made their decisions to lay when hardly any buds had burst, even on the earliest developing Hazels.
As caterpillars appeared on developing leaves immediately after bud burst, we treated the date of Hornbeam bud burst completion (the tree species providing c. 50% of leaves for folivorous caterpillars, Rowiński Citation2001) as the date of caterpillar appearance. This was 1 May in 2005 and 2006 and 18 May in 2007. Caterpillar development was strongly dependent on temperature, and the amount of temperature accumulated to reach the peak frass fall was 353–382 degree days (). Because of colder weather, it took longer to reach the peak frass fall in 2007 (33 days) than in 2005–06 (27–28 days), .
Table 3. Development of folivorous caterpillars, weather conditions, and synchrony of Blue Tit broods with the caterpillar peak in BNP in relation to year.
The maximum food requirements of Blue Tit broods coincided with the caterpillar peak period (±3 days) to a varying degree, from about 65% of broods in 2007 to only 17% in 2006, when 83% of broods were delayed (). Precocious broods were observed only in 2005. The high synchrony of broods in 2007 was due to a prolongation of caterpillar development that year (), but if they had reached the peak – as in the other seasons – after 27–28 days then 87% of Blue Tit broods would appear delayed.
The total brood loss (from hatching to fledging) amounted to 20.7% of nests (n = 58) in 2005, 17.0% (n = 47) in 2006, and 31.9% (n = 47) in 2007. The differences across years were not significant (probit model, 2 df, W = 3.0, P = 0.23), and the brood loss rate did not depend on synchrony with the caterpillar peak (1 df, W = 1.5, P = 0.22), amounting to 31.3% in the precocious, 24.2% in the synchronous, and 18.8% in the delayed broods.
Partial losses of nestlings occurred in 38.8% (n = 68) of successful broods, with up to 7 young/brood being lost. The brood reduction frequency did not vary significantly across the synchrony groups (K–W anova, H 2,60 = 3.11, P = 0.21), yet strongly reduced broods (> 2 young lost) were concentrated (7/8) in the delayed group. This resulted in a significant relationship between the degree of brood synchrony and the number of young lost (F = 4.4, P = 0.039, no effect of year F = 0.2, P = 0.85). However, in only three cases, when only a single parent was observed to fed young, was the strong brood reduction likely to have been caused by undernourishment of young.
Abundance of folivorous caterpillars was highest in 2005 (peak frass fall – 0.50), lower in 2006 (0.11), and lowest in 2007 (0.04 g/0.25 m2/24 h), a greater than 12-fold difference (). In 2005 the mass of frass fall exceeded the 2007 peak values for 26 days and in 2006 for four to six days. Blue Tits did not respond numerically to this variation; they indeed bred at the highest density – 4.1 pairs/10 ha – in 2005, but their numbers dropped to 2.6 pairs/10 ha in the intermediate 2006 spring and increased to 3.1 pairs/10 ha in the trough season of 2007.
Figure 1. Distribution of Blue Tit hatching dates (box-whiskers) in relation to variation in caterpillar abundance (caterpillar frass production; solid line) in deciduous forest in BNP. Medians (dots), 25–75% (boxes) and minimum–maximum values (whiskers) are given; arrows indicate dates of the maximum food requirements of nestlings hatched at the median dates; and vertical dotted lines show days of peak frass production. Question marks – frass fall data unavailable due to rain.
Caterpillars constituted on average 74.2% (n = 80 broods, 240 feeding samples, each consisting of five parental visits with food) of Blue Tits nestling food, and over three quarters were green caterpillars. The remaining food types were spiders (17.2%) and ‘other insects’ (8.6%). The latter group consisted mostly of small insects (dipterans and beetles) or unrecognizable ‘small balls’. Winged insects (dipterans, transparent wings) were found only in c.12% of observations classed as ‘other’ food.
Despite huge variation in the caterpillar supply, nestling diet did not vary across years (manova: Wilks' λ = 0.06, F 6,386 = 1.3, P = 0.24). However, nestling diet changed within a season with nestling age (λ = 0.90, F 6,386 = 3.55, P = 0.002) and between synchronized and later broods (λ = 0.92, F 3,193 = 5.5, P < 0.002). There was a tendency for the relationship between nestling food composition and synchrony with the caterpillar peak to be dependent on the study year (λ = 0.94, F 6,386 = 210, P < 0.053). The remaining interactions were not significant (P = 0.08–0.53).
Overall nestling food composition did not change significantly between years ( & ), though spiders were brought more frequently in 2007 and other insects in 2005 (). The proportion of green caterpillars in nestling food significantly increased with age in 2006, from 46% in ‘small’ to 66% in ‘medium’ and ‘large’ nestlings (Friedman anova, χ2 = 6.6, P = 0.038) but not in other years. The proportion of other caterpillars tended to increase with nestling age () but the differences were significant only in 2007(Friedman anova, χ2 = 6.0, P = 0.05). The share of all caterpillars in the diet of ‘small’ young (57–68%) increased to 74–88% in the ‘medium’ and ‘large’ nestlings in 2005–06, but no difference was detectable in 2007 (). Changes in the proportion of spiders with nestling age approached significance (, P = 0.066) but the pattern of variation was inconsistent across years. The proportion of other non-caterpillar invertebrates tended to decline with nestling age () but the pattern was variable among seasons.
Table 4. The annual variation in the mean share of food types brought to the nest in relation to year in deciduous forest in BNP.
Table 5. The effect of year, nestling age, and synchrony of brood with the caterpillar peak on proportion of food types in the of diet Blue Tit nestlings. As ‘precocious’ broods were found exclusively in 2005 only two synchrony classes (‘synchronous’ and ‘delayed’) could be used in the analysis. Displayed are F values for 240 visits at 80 nests in the deciduous forest at BNP (univariate results for repeated-measures manova).
Table 6. The mean share of food types brought to young in relation to the nestling age and synchrony with the caterpillar peak in 2005–07 in the deciduous forest in BNP.
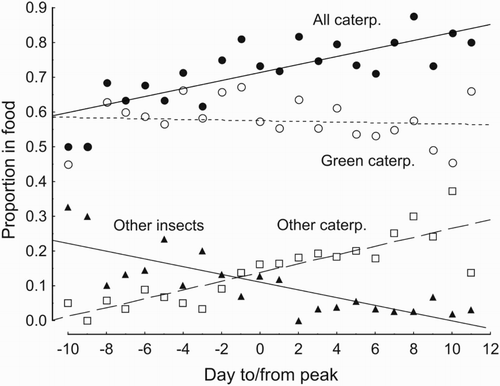
Caterpillar availability (measured by frass fall) changed very quickly within a season (). It took about three weeks for frass fall to reach the maximum seasonal values and just ten days after this date the amount of frass declined, depending on year, to c. 3–18% of the peak amount. In the low caterpillar season (2007) the amount of frass declined to a barely measurable level (). Contrary to this variation in caterpillar availability, the proportion of green caterpillars fed to the late broods was similar, and the proportion of other caterpillars higher, than in the synchronous broods (Tables 5 & 6). In fact, the proportion of caterpillars in nestling food increased throughout the season, from about 60% in the beginning to almost 80% at the end (). This increase was mostly at the cost of ‘other insects’, for which the proportion tended to decline with nestling age (, ) but otherwise remained stable throughout the nesting season (, B = −0.1, F = 0.2, P = 0.64).
Figure 2. Variation in proportion of different food types brought to Blue Tit nestlings in relation to the time from the seasonal caterpillar peak in deciduous forest in BNP. Mean values are shown for all observations done on a given day, irrespective of nestlings' age. Day ‘−10’ includes samples collected 10–14 days before the peak, day ‘11’ includes samples collected 11–17 days after the peak. Pooled data from 2005–07. Regression results: date from the peak with all caterpillars, B = 0.78, F = 31.9, P < 0.001; green caterpillars, B = −0.09, F = 0.7, P = 0.16; other caterpillars, B = 0.86, F = 59.4, P < 0.001; other insects B = −0.80, F = 35.0, P < 0.001; spiders (not shown) B = −0.11, F = 0.2, P = 0.64.
DISCUSSION
Blue Tits in BNP fed young mostly (c. 74% of diet) with folivorous caterpillars. The proportion of caterpillars in the diet did not decline, despite a more than tenfold drop in supply across the study years, nor with the increased mismatch with the yearly caterpillar peak. In fact, the proportion of caterpillars was highest in the diet of the largest nestlings in late broods. These results are consistent with outcomes of other surveys: caterpillars comprised one of the two most frequent food types in 88% of Blue Tit nestling food analyses (review in Cholewa & Wesołowski Citation2011). Therefore, one expectation of Lack's (Citation1950) hypothesis has been fulfilled: folivorous caterpillars do constitute a key component of the Blue Tit nestling diet in BNP.
However, the second requirement of temporal matching between the seasonal caterpillar peak and the peak of nestling food demands has not been well supported by our data. Although we used a rather relaxed definition of ‘matching’ (a week around the peak) a variable fraction (17–65%) of Blue Tit broods in BNP matched the caterpillar peak in any season. The same phenomenon has been found in other areas, where Blue Tits matched the peak only in some years (review in Zandt et al. Citation1990, Bańbura et al. Citation1994, García-Navas & Sanz Citation2011a). The degree of overlap between the birds and caterpillars was largely a result of variation in the caterpillar development rates, which was weather-dependent. It took 27–33 days to reach the frass fall peak during the study years, but it could be anywhere between 21 and 35 days (Rowiński Citation2001). The high match in 2007 was due to a retardation of caterpillar development, and if the caterpillars had developed quickly (21 days) that year then all broods would have appeared to be delayed. As the laying birds cannot predict what the weather would be like during the period of caterpillar growth, and have rather limited means of adjusting the pace of their breeding after clutch initiation (a few days, see below), it seems there is no way in which the birds could guarantee a match with the peak (Perrins Citation1991).
Such a perfect match would appear irrelevant, however. Within the range of conditions studied in BNP, the mismatching of broods caused no visible fitness loss, as neither total nest loss nor brood reduction in the successful broods depended on the match. Negative effects of the mismatch were undetectable even in the low caterpillar season of 2007 (Wesołowski et al. Citation2009), when caterpillar peak supply was approximately ten times lower than in 2005 and breeding density of tits remained relatively high. We cannot rule out the possibility, however, that some weaker effects could have occurred, such as poorer condition of (presumably undernourished) fledglings from the mismatched broods (Tremblay et al. Citation2003). Unfortunately we were unable to collect nestling weight data because these birds often nested in inaccessible tree holes (frequently > 20 m above the ground, Wesołowski & Rowiński Citation2012). Nevertheless, even if some of the mismatched young fledged in a poorer condition, this would not necessarily result in their lower survival. The review of Nilsson (Citation1999) showed that the survival of young tits was far more dependent on their fledging early than on their condition at fledgling. Similarly, Naef-Daenzer et al. (Citation2001) showed that heavier young of Parus major and Periparus ater fledging from late broods had better survival, but young from the early broods survived equally well, independent of their body mass.
If matching the caterpillar peak is so important, then one would expect to find some means used by the birds to allow them to make up for the discrepancies. There are several ways in which such compensation might be possible. If the birds perceive that they are too early, they could delay the appearance of nestlings by postponing laying in nests that are already built, laying more eggs, or laying and incubating eggs with interruptions, which might slow down nestling development (Cresswell & McCleery Citation2003). On the other hand, if they sense that they are too late, then they could speed up breeding by laying fewer eggs, with no interruptions, or begin incubation before the clutch is completed (Nilsson & Svensson Citation1993, Daan et al. Citation1988, Wesołowski Citation2000, Citation2013, Naef-Daenzer et al. Citation2004, Matthysen et al. Citation2011, García-Navas & Sanz Citation2011b). Signs of such responses in BNP are rather weak. Independent of the year's phenology, Blue Tits laid the same number of eggs, their clutch size did not decline within the main laying period, and the length of the nesting cycle did not change across seasons. The only observation consistent with this idea was the shorter incubation time found in 2006, which decreased the mismatch of broods in that year. However, as this was the latest breeding season in absolute terms, birds could be also in a hurry for other reasons, independent of the necessity to match the caterpillar peak (Nilsson Citation1994 and below).
Fine tuning of breeding to the caterpillar peak is important only when nestling food supply remains inadequate except for the brief period around the peak (Dunn & Winkler Citation2010). If periods with a more than adequate amount of food last longer, or if they have broad plateaus of abundance rather than sharp peaks (Tremblay et al. Citation2003, Dunn et al. Citation2011), then there is little selective advantage for perfect matching. This seemed to be the case in BNP, where in > 30 years of observations, caterpillars were more abundant in 75% of seasons than in the low season of 2007 (Wesołowski et al. Citation2009, Maziarz & Wesołowski Citation2010), and the mass of frass fall also exceeded the 2007 peak values (c. 165 mg/day/m2) for long periods of time (Wesołowski & Rowiński Citation2008, Maziarz & Wesołowski Citation2010). Furthermore, comparisons of the peak frass mass in BNP (which usually exceed 400 mg/day/m2 and can reach c. 8000 mg/day/m2 during the outbreaks) with figures from other areas in which the caterpillar supply was adequate (Fischbacher et al. Citation1998, reviews in Rowiński Citation2001 and Tremblay et al. Citation2003, Naef-Daenzer et al. Citation2004) indicate that the caterpillars in BNP tend to be superabundant in most years.
The apparent lack of a functional reaction by Blue Tits to the highly variable caterpillar supply in BNP leads to the same conclusion: Blue Tits managed to feed a high proportion of caterpillars to their nestlings despite the strong seasonal decline in their supply, even in the 2007 season in which their availability was less than a tenth of that observed in 2005. This ability of Blue Tits to maintain a high proportion of caterpillars in the nestling diet, despite large variation in supply, has also been observed in other areas (Bańbura et al. Citation1994, Tremblay et al. Citation2005, García-Navas & Sanz Citation2011a). Even in areas in which caterpillars were sparse, the birds compensated by collecting them from larger areas (Tremblay et al. Citation2005). These offsetting capabilities have their limits, though. When the supply of caterpillars declines to a very low level, their share in diet does decline. In BNP, species breeding later in the spring had relatively fewer caterpillars in the nestling diet: Wood Warbler Phylloscopus sibilatrix nestlings received 50% caterpillars a week after the peak but their share declined to 30% a week later. Nestlings of Red-breasted Flycatcher Ficedula parva received 40% caterpillars in mid-June but only 10% two weeks later (Maziarz & Wesołowski Citation2010, Mitrus et al. Citation2010, C. Mitrus pers. comm.). Also in Blue Tits, the very low caterpillar supply due to either poor habitat or large mismatch with the caterpillar peak could force tits to replace caterpillars with other items, and these diet shifts have resulted in reduced nesting success (Blondel et al. Citation1991, Massa et al. Citation2004).
In conclusion, we suggest that in many areas and situations there may be a super-abundance of caterpillars for breeding Blue Tits. Therefore, matching the peak of caterpillar abundance may not be important for this species most of the time, and could become crucial only locally in ‘low’ caterpillar years, or in marginal habitats (Perrins Citation1991, Tremblay et al. Citation2003). Although folivorous caterpillars are very important in the diet of Blue Tits, and the rearing period of tit nestlings largely coincides with the availability of caterpillars, we did not find strong evidence that the precise temporal matching of their nestling time to the seasonal caterpillar peaks is indeed required. As a consequence, the mismatch induced by climate warming may not be as detrimental in our study population as often envisaged (Visser et al. Citation2003, Both et al. Citation2004).
If a mismatch is not important, then what other selective pressures could shape the timing of Blue Tit breeding seasons? Generally, there should be a pressure to breed as early as possible. Birds commencing breeding early would have more opportunity to lay replacement clutches after nest loss, more time to rear second broods, and could survive better. Their young could have a greater chance of avoiding post-fledging predation or of finding a place to settle (Kluijver Citation1951, Perrins Citation1965, Drent Citation1984, reviews in Svensson & Nilsson Citation1995, Nilsson Citation1999, Naef-Daenzer et al. Citation2001, Ramsay & Otter Citation2007). However, as breeding too early could be costly to adults (Nilsson Citation1994, Svensson & Nilsson Citation1995), birds should start to breed immediately after the benefits of early breeding outweigh the costs, i.e. at the first moment in which a combination of the bird's body state and environmental conditions permit it.
Behaviour of Blue Tits in BNP was consistent with this hypothesis. The earliest Blue Tits began nest building on 6 April and egg-laying (determinant dates) about 10 April in 2005–07, but in 1990 (Wesołowski & Cholewa Citation2009) they commenced laying as early as the first days of April. Thus Blue Tits in BNP were already responsive to environmental cues and could begin preparations for breeding in the last days of March, i.e. days were then long enough (photoperiod can restrict the onset of breeding in Blue Tits – Lambrechts et al. Citation1997, Lambrechts & Perret Citation2000). Therefore, other factors would have to be responsible for later onset of breeding in most years, as well as for large inter-year variation of breeding dates (over a month in BNP, Wesołowski & Cholewa Citation2009).
The interannual shifts were strongly related to spring temperatures, and the birds bred earlier in warmer springs. This was found not only in BNP (this study, Wesołowski & Cholewa Citation2009) but also in all other areas except Corsica (Leclerq Citation1977, Schmidt Citation1984, Blondel Citation1985, Nilsson & Källander Citation2006). The higher temperatures could act directly on the birds' physiology, as well as indirectly, e.g. by speeding up the development of invertebrate food or they could provide a cue helping the birds to predict future conditions (Nilsson Citation1994, Svensson & Nilsson Citation1995). How Blue Tits in BNP use temperature cues remains unclear but their reaction to the increasing temperatures is variable. To induce breeding, higher daily temperatures were necessary early in the spring rather than later in the season. This suggests that temperature needs to be weighted (calibrated) against day length to trigger the start of the breeding season (Wesołowski Citation1998, Lambrechts & Perret Citation2000, Gienapp et al. Citation2005). The longer days later in the season allow more time for foraging, which could offset the lower temperatures to some extent (Perrins & McCleery Citation1989).
Blue Tits may also respond proximately to some correlate of increasing temperatures, such as the sight of developing leaves (Lack Citation1966, Slagsvold Citation1976) or increasing amount of food (Perrins Citation1970). Their laying dates in BNP varied roughly in parallel with the bud burst dates of Hazel and Hornbeam, which suggests that they may be causally linked. However, these temporal relationships were fluid: in some years the birds decided to lay eggs before the buds on Hornbeam had begun to burst, in others the Hornbeam buds had almost fully burst. The earliest laying Blue Tits, though, made their decisions to lay when hardly any buds had burst, even on the earliest developing Hazels. Such variation suggests that the Blue Tit laying decisions were not tightly linked to any peculiar stage of leaf development. Also observations in other areas suggest (review in Slagsvold Citation1976, Blondel et al. Citation1993, Visser et al. Citation2002, Nilsson & Källander Citation2006, Naef-Daenzer et al. Citation2012) that in most cases commencement of laying by Blue Tits overlapped with the bud burst of (at least some) local trees, yet without precise coupling with any specific phenological stage. However, the birds could still broadly follow a ‘green wave’ of developing leaves. In some cases Blue Tits commenced laying well in advance of the bud burst of local trees (Schmidt Citation1984, Blondel et al. Citation1993, Visser et al. Citation2002), which suggests that the sight of developing leaves by birds may not constitute a necessary requirement to trigger reproduction. Alternatively, an admixture of earlier leafing trees (e.g. Hazel) could have been used as a cue by tits in these areas.
Developing leaves provide foraging substrate for newly emerging caterpillars and other invertebrates. Therefore, even if Blue Tits do not use fresh leaves as a cue, they benefit from their appearance by gaining access to a novel and fast increasing food source. If they were unable to cross the nutritional threshold for egg formation before leaves have begun to grow, then using these extra resources would enable them to commence breeding. The idea that food for laying females is limiting (Perrins Citation1970) has been tested in several food addition experiments with Blue Tits. Supplementary feeding has usually advanced the start of laying by only few days and was effective only in some conditions (review in Nilsson & Källander Citation2006). Thus, food availability, though important, is apparently not the sole factor taken into account by Blue Tits in deciding when to lay eggs. Experiments testing the role of other purported proximate factors were even less successful: neither heating of Blue Tit nest-boxes (Yom-Tov & Wright Citation1993) nor presentation of branches with green leaves to captive Blue Tits (Visser et al. Citation2002) induced them to lay earlier.
Thus we face a paradox, observations in BNP and other areas show that the birds in a local population can shift timing of their breeding by several weeks between years, but none of the experimental field manipulations has been able to create shifts of this size. Perhaps the experiments do not capture important aspects of how birds perceive their environment. From the birds' perspective warmer night temperatures in the nest-box, or a tray full of mealworms, need not necessarily mean a ‘warm spring’ or ‘abundant food’. It may be that birds reach decisions using a combination of cues, and weigh them against one another (e.g. day length vs. temperature, see above), so it is not the level of a single factor, but the right combination of several causes that is important.
ACKNOWLEDGEMENTS
We heartily thank W. Błędowski, M. Czuchra, P. Da¸browski, M. Maziarz, G. Hebda, and B. Sklepowicz for their participation in the field work. We thank C. Mitrus for sharing unpublished information and M. Cholewa, J. Bańbura, B. Naef-Daenzer, and six anonymous referees for comments on the first draft. Linguistic help and comments of P. O. Dunn and R. Broughton are appreciated as well.
Funding
This field work was supported by the Polish Ministry of Science and Higher Education [grant number 2PO4C 075 27]. The final analysis was supported by an internal grant from the Faculty of Biological Sciences, Wrocław University (to TW).
REFERENCES
- van Balen, J.H. 1973. A comparative study of the breeding ecology of the Great Tit Parus major in different habitats. Ardea 61: 1–93.
- Bańbura, J., Blondel, J., de Wilde-Lambrechts, H., Galan, M.-J. & Maistre, M. 1994. Nestling diet variation in an insular Mediterranean population of Blue Tits Parus caeruleus: effects of years, territories and individuals. Oecologia 100: 413–420. doi: 10.1007/BF00317863
- Barba, E., Gil-Delgado, J. & Monrós, J.S. 1995. The cost of being late: consequences of delaying Great Tit Parus major first clutches. J. Anim. Ecol. 64: 642–651. doi: 10.2307/5806
- Blondel, J. 1985. Breeding strategies of the Blue Tit and Coal Tit (Parus) in mainland and island Mediterranean habitats: a comparison. J. Anim. Ecol. 54: 531–556. doi: 10.2307/4497
- Blondel, J., Dervieux, A., Maistre, M. & Perret, P. 1991. Feeding ecology and life history variation of the Blue Tit in Mediterranean deciduous and sclerophyllous habitats. Oecologia 88: 9–14. doi: 10.1007/BF00328397
- Blondel, J., Dias, P.C., Maistre, M. & Perret, P. 1993. Habitat heterogeneity and life-history variation of Mediterranean Blue Tits (Parus caeruleus). Auk 110: 511–520. doi: 10.2307/4088415
- Bobiec, A. 2002. Living stands and dead wood in the Białowieża Forest: suggestions for restoration management. Forest Ecol. Manage. 165: 121–136. doi: 10.1016/S0378-1127(01)00655-7
- Both, C. & Visser, M. 2005. The effect of climate change on the correlation between avian life-history traits. Glob. Change Biol. 11: 1606–1613. doi: 10.1111/j.1365-2486.2005.01038.x
- Both, C., Artemyev, A.V., Blaauw, B., Cowie, R.J., Dekhuijzen, A.J., Eeva, T., Enemar, A., Gustafsson, L., Ivankina, E.V., Järvinen, A., Metcalfe, N.B., Nyholm, N.E.I., Potti, J., Ravussin, P.-A., Sanz, J.J., Silverin, B., Slater, F.M., Sokolov, L.V., Török, J., Winkel, W., Wright, J., Zang, H. & Visser, M.E. 2004. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. Lond. B 271: 1657–1662. doi: 10.1098/rspb.2004.2770
- Cholewa, M. & Wesołowski, T. 2011. Nestling food of European hole-nesting passerines: do we know enough to test the adaptive hypotheses on breeding seasons? Acta Orn. 46: 105–116. doi:10.3161/000164511X625874 doi: 10.3161/000164511X625874
- Cresswell, W. & McCleery, R. 2003. How Great Tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J. Anim. Ecol. 72: 356–366. doi: 10.1046/j.1365-2656.2003.00701.x
- Cushing, D.H. 1990. Plankton production and year-class-strength in fish populations – an update of the match mismatch hypothesis. Adv. Marine Biol. 26: 249–293. doi: 10.1016/S0065-2881(08)60202-3
- Daan, S., Dijkstra, C., Drent, R. & Meijer, T. 1988. Food supply and the annual timing of avian reproduction. Acta XIX Congr. Inter. Ornithol. 1: 392–407.
- Drent, P.J. 1984. Mortality and dispersal in summer and its consequences for the density of Great Tits Parus major at the onset of autumn. Ardea 72: 127–162.
- Drent, R.H. 2006. The timing of birds’ breeding seasons: the Perrins hypothesis revisited especially for migrants. Ardea 94: 305–322.
- Dunn, P.O. 2004. Breeding dates and reproductive performance. Adv. Ecol. Res. 35: 67–85.
- Dunn, P.O. & Winkler, D.W. 2010. Effects of climate change on timing of breeding and reproductive success in birds. In Møller, A.P., Fiedler, W. & Berthold, P. (eds) Effects of Climate Change on Birds, 113–128. Oxford University Press, Oxford.
- Dunn, P.O., Winkler, D.W., Whittingham, L.A., Hannon, S.J. & Robertson, R.J. 2011. A test of the mismatch hypothesis: how is timing of reproduction related to food abundance in an aerial insectivore? Ecology 92: 450–461. doi: 10.1890/10-0478.1
- Durant, J.M., Hjermann, D.Ø., Anker-Nilssen, T., Beaugrand, G., Mysterud, A., Pettorelli, N. & Stenseth, N.C. 2005. Timing and abundance as key mechanisms affecting trophic interactions in variable environments. Ecol. Letters 8: 952–958. doi: 10.1111/j.1461-0248.2005.00798.x
- Fischbacher, M., Naef-Daenzer, B. & Naef-Daenzer, L. 1998. Estimating caterpillar density on trees by collection of frass droppings. Ardea 86: 121–129.
- García-Navas, V. & Sanz, J.J. 2011a. The importance of a main dish: nestling diet and foraging behaviour in Mediterranean Blue Tits in relation to prey phenology. Oecologia 165: 639–649. doi: 10.1007/s00442-010-1858-z
- García-Navas, V. & Sanz, J.J. 2011b. Short-term alterations in songbird breeding schedule lead to better synchronization with food availability. Auk 128: 146–155. doi: 10.1525/auk.2010.10094
- Gienapp, P., Hemerik, L. & Visser, M.E. 2005. A new statistical tool to predict phenology under climate change scenarios. Global Change Biol. 11: 600–606. doi: 10.1111/j.1365-2486.2005.00925.x
- Immelmann, K. 1971. Ecological aspects of periodic reproduction. In Farner, D.S., King, J.R. & Parkes, K.C. (eds) Avian Biology, Vol. 1: 342–389. Academic Press, New York.
- IPCC. 2007. Climate change 2007: synthesis report. Intergovernmental Panel on Climate Change, Geneva.
- Kluijver, H.N. 1951. The population ecology of the Great Tit, Parus m. major L. Ardea 39: 1–135.
- Kluyver, H.N. 1952. Notes on body weight and time of breeding in the Great Tit, Parus m. major L. Ardea 40: 123–141.
- Lack, D. 1950. The breeding seasons of European birds. Ibis 92: 288–316. doi: 10.1111/j.1474-919X.1950.tb01753.x
- Lack, D. 1966. Population studies of birds. Clarendon Press, Oxford.
- Lambrechts, M.M. & Perret, P. 2000. A long photoperiod overrides non-photoperiodic factors in Blue Tits' timing of reproduction. Proc. R. Soc. Lond. B 267: 585–588. doi: 10.1098/rspb.2000.1041
- Lambrechts, M.M., Blondel, J., Maistre, M. & Perret, P. 1997. A single response mechanism is responsible for evolutionary adaptive variation in a bird's laying date. Proc. Natl. Acad. Sci. USA 94: 5153–5155. doi: 10.1073/pnas.94.10.5153
- Leclerq, B. 1977. Etude phénologique des paramètres liés à reproduction des mésanges en futaie de Chênes. Rev. Ecol. (Terre et Vie) 31: 599–616.
- Massa, B., Lo Valvo, F., Margagliotta, B. & Lo Valvo, M. 2004. Adaptive plasticity of Blue Tits (Parus caeruleus) and Great Tits (Parus major) breeding in natural and semi-natural insular habitats. Italian J. Zool. 71: 209–217. doi: 10.1080/11250000409356574
- Matthysen, E., Adriaensen, F. & Dhondt, A.A. 2011. Multiple responses to increasing spring temperatures in the breeding cycle of Blue and Great Tits (Cyanistes caeruleus, Parus major). Global Change Biol. 17: 1–16. doi: 10.1111/j.1365-2486.2010.02213.x
- Maziarz, M. & Wesołowski, T. 2010. Timing of breeding and nestling diet of Wood Warbler Phylloscopus sibilatrix in relation to changing food supply. Bird Study 57: 540–552. doi: 10.1080/00063657.2010.512954
- Mitrus, C., Mitrus, J. & Sikora, M. 2010. Changes in nestling diet composition of the Red-breasted Flycatcher Ficedula parva in relation to chick age and parental sex. Animal Biol. 60: 1–10. doi: 10.1163/157075610X516529
- Naef-Daenzer, B., Widmer, F. & Nuber, M. 2001. Differential post-fledging survival of Great and Coal Tits in relation to their condition and fledging date. J. Anim. Ecol. 70: 730–738. doi: 10.1046/j.0021-8790.2001.00533.x
- Naef-Daenzer, L., Nager, R.G., Keller, L.F., Naef-Daenzer, B. 2004. Are hatching delays a cost or a benefit for Great Tit Parus major parents? Ardea 92: 229–238.
- Naef-Daenzer, B., Luterbacher, J., Nuber, M., Rutishauser, T. & Winkel, W. 2012. Cascading climate effects and related ecological consequences during past centuries. Climate of the Past 8: 1527–1540. doi: 10.5194/cp-8-1527-2012
- Niechoda, T. & Korbel, J. 2011. Puszczańskie olbrzymy. Towarzystwo Ochrony Krajobrazu, Białowieża.
- Nilsson, J.-A. 1994. Energetic bottle-necks during breeding and the reproductive cost of being too early. J. Anim. Ecol. 63: 200–208. doi: 10.2307/5595
- Nilsson, J.-A. 1999. Fitness consequences of timing of reproduction. In Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban, 234–247. Bird Life South Africa, Johannesburg.
- Nilsson, J.-A. & Källander, H. 2006. Leafing phenology and timing of egg laying in Great Tits Parus major and Blue Tits P. caeruleus. J. Avian Biol. 37: 357–363. doi: 10.1111/j.2006.0908-8857.03604.x
- Nilsson, J.-A. & Svensson, E. 1993. The frequency and timing of laying gaps. Ornis Scand. 24: 122–126. doi: 10.2307/3676361
- van Noordwijk, A.J., McCleery, R.H. & Perrins, C.M. 1995. Selection for the timing of Great Tit breeding in relation to caterpillar growth and temperature. J. Anim. Ecol. 64: 451–458. doi: 10.2307/5648
- Perrins, C.M. 1965. Population fluctuations and clutch-size in the Great Tit, Parus major L. J. Anim. Ecol. 34: 601–647. doi: 10.2307/2453
- Perrins, C.M. 1970. The timing of birds’ breeding seasons. Ibis 112: 242–255. doi: 10.1111/j.1474-919X.1970.tb00096.x
- Perrins, C.M. 1991. Tits and their caterpillar food supply. Ibis 133: 49–54. doi: 10.1111/j.1474-919X.1991.tb07668.x
- Perrins, C.M. & McCleery, R.H. 1989. Laying dates and clutch size in the Great Tit. Wilson Bull. 101: 236–253.
- Ramsay, S.M. & Otter, K.A. 2007. Fine-scale variation in the timing of reproduction in titmice and chickadees. In Otter, K.A. (ed) The Ecology and Behavior of Chickadees and Titmice. An Integrated Approach, 55–69. Oxford University Press, Oxford.
- Rowiński, P. 2001. Timing of breeding of Nuthatch Sitta europaea in relation to food resources in a natural forest. Unpublished Biol. D. thesis, Agricultural University, Warsaw [in Polish].
- Schmidt, K.H. 1984. Spring temperature and time of laying in tits. J. Orn. 125: 321–331. doi: 10.1007/BF01640480
- Slagsvold, T. 1976. Annual and geographical variation in the time of breeding of the Great Tit Parus major and the Pied Flycatcher Ficedula hypoleuca in relation to environmental phenology and spring temperature. Ornis Scand. 2: 127–145. doi: 10.2307/3676183
- Sparks, T.H. & Menzel, A. 2002. Observed changes in seasons: an overview. Int. J. Climatol. 22: 1715–1725. doi: 10.1002/joc.821
- Svensson, E. & Nilsson, J.-A. 1995. Food supply, territory quality, and reproductive timing in the Blue Tit (Parus caeruleus). Ecology 76: 1804–1812. doi: 10.2307/1940712
- Tinbergen, L. 1960. The natural control of insects in pinewoods. I. Factors influencing the intensity of predation by songbirds. Arch. Néerl. Zool. 13: 259–343.
- Tomiałojć, L. & Wesołowski, T. 1990. Bird communities of the primaeval temperate forest of Białowieża, Poland. In Keast, A. (ed) Biogeography and Ecology of Forest Bird Communities, 141–165. SPB Academic Publishers, The Hague.
- Tomiałojć, L. & Wesołowski, T. 2004. Diversity of the Białowieża Forest avifauna in space and time. J. Orn. 145: 81–92. doi: 10.1007/s10336-003-0017-2
- Tomiałojć, L. & Wesołowski, T. 2005. The avifauna of Białowieża Forest: a window into the past. Br. Birds 98: 174–193.
- Tomiałojć, L., Wesołowski, T. & Walankiewicz, W. 1984. Breeding bird community of a primaeval temperate forest (Białowieża National Park, Poland). Acta Orn. 20: 241–310.
- Tremblay, I., Thomas, D.W., Lambrechts, M.M., Blondel, J. & Perret, P. 2003. Variation in Blue Tit breeding performance across gradients in habitat richness. Ecology 84: 3033–3043. doi: 10.1890/02-0663
- Tremblay, I., Thomas, D., Blondel, J., Perret, P. & Lambrechts, M.M. 2005. The effect of habitat quality on foraging patterns, provisioning rate and nestling growth in Corsican Blue Tits Parus caeruleus. Ibis 147: 17–24. doi: 10.1111/j.1474-919x.2004.00312.x
- Verboven, N. & Visser, M.E. 1998. Seasonal variation in local recruitment of Great Tits: the importance of being early. Oikos 81: 511–524. doi: 10.2307/3546771
- Visser, M.E. & Lambrechts, M.M. 1999. Information constraints in the timing of reproduction in temperate zone birds: Great and Blue Tits. In Adams, N.J. & Slotow, R.H. (eds) Proc. 22 Int. Ornithol. Congr., Durban, 249–264. Bird Life South Africa, Johannesburg.
- Visser, M.E., van Noordwijk, A.J., Tinbergen, J.M. & Lessells, C.M. 1998. Warmer springs lead to mistimed reproduction in Great Tits (Parus major). Proc. R. Soc. Lond. B 265: 1867–1870. doi: 10.1098/rspb.1998.0514
- Visser, M.E., Silverin, B., Lambrechts, M.M. & Tinbergen, J.M. 2002. No evidence for tree phenology as a cue for the timing of reproduction in tits Parus spp. Avian Sci. 2: 77–86.
- Visser, M.E., Adriaensen, F., van Balen, J.H., Blondel, J., Dhondt, A.A., van Dongen, S., du Feu, Ch., Ivankina, E.V., Kerimov, A.B., de Laet, J., Matthysen, E., McCleery, R., Orell, M. & Thomson, D.L. 2003. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. Lond. B 270: 367–372. doi: 10.1098/rspb.2002.2244
- Visser, M.E. te Marvelde, L. & Lof, M.E. 2012. Adaptive phenological mismatches of birds and their food in warming world. J. Orn. 153: 75–84. doi: 10.1007/s10336-011-0770-6
- Walankiewicz, W. 1991. Do secondary-cavity nesting birds suffer more from competition for cavities or from predation in a primaeval deciduous forest? Natural Areas J. 11: 203–212.
- Wesołowski, T. 1996. Natural nest sites of Marsh Tit (Parus palustris) in a primaeval forest (Białowieża National Park, Poland). Vogelwarte 38: 235–249.
- Wesołowski, T. 1998. Timing and synchronisation of breeding in a Marsh Tit Parus palustris population from a primaeval forest. Ardea 86: 89–100.
- Wesołowski, T. 2000. Time saving mechanisms in the reproduction of Marsh Tits Parus palustris. J. Orn. 141: 309–318. doi: 10.1007/BF02462240
- Wesołowski, T. 2001. Ground checks – an efficient and reliable method to monitor holes’ fate. Ornis Fenn. 78: 193–197.
- Wesołowski, T. 2002. Antipredator adaptations in nesting Marsh Tits Parus palustris: the role of nest site security. Ibis 144: 593–601. doi: 10.1046/j.1474-919X.2002.00087.x
- Wesołowski, T. 2003. Bird community dynamics in a primaeval forest – is interspecific competition important? Ornis Hung. 12–13: 51–62.
- Wesołowski, T. 2005. Virtual conservation: how the European Union is turning a blind eye on its vanishing primeval forests. Conserv. Biol. 19: 1349–1358. doi: 10.1111/j.1523-1739.2005.00265.x
- Wesołowski, T. 2007a. Primeval conditions – what can we learn from them? Ibis 149 (suppl. 2): 64–77. doi:10.1111/j.1474-919x.2007.00721.x doi: 10.1111/j.1474-919X.2007.00721.x
- Wesołowski, T. 2007b. Lessons from long-term hole-nester studies in a primeval temperate forest. J. Orn. 148 (suppl. 2): 395–405. doi: 10.1007/s10336-007-0198-1
- Wesołowski, T. 2011. Reports from nestbox studies: a review of inadequacies. Acta Orn. 46: 13–17. doi: 10.3161/000164511X589866
- Wesołowski, T. 2013. Timing and stages of nest building by Marsh Tits Poecile palustris in a primaeval forest. Avian Biol. Res. 6: 31–38. doi: 10.3184/175815512X13535900176861
- Wesołowski, T. & Cholewa, M. 2009. Climate variation and bird breeding seasons in a primeval temperate forest. Climate Res. 38: 199–208. doi: 10.3354/cr00789
- Wesołowski, T. & Rowiński, P. 2006. Timing of bud burst and tree-leaf development in a multispecies temperate forest. Forest Ecol. Manage. 237: 387–393. doi: 10.1016/j.foreco.2006.09.061
- Wesołowski, T. & Rowiński, P. 2008. Late leaf development in pedunculate oaks Quercus robur L.: an antiherbivore defence? Scand. J. Forest Res. 23: 386–394. doi: 10.1080/02827580802419026
- Wesołowski, T. & Rowiński, P. 2012. The breeding performance of Blue Tits Cyanistes caeruleus in relation to the attributes of natural holes in a primeval forest. Bird Study 59: 437–448. doi: 10.1080/00063657.2012.722189
- Wesołowski, T. & Tomiałojć, L. 1997. Breeding bird dynamics in a primaeval temperate forest: long-term trends in Białowieża National Park (Poland). Ecography 20: 432–453. doi: 10.1111/j.1600-0587.1997.tb00411.x
- Wesołowski, T., Tomiałojć, L., Mitrus, C., Rowiński, P. & Czeszczewik, D. 2002. The breeding bird community of a primaeval temperate forest (Białowieża National Park, Poland) at the end of the 20th century. Acta Orn. 37: 27–45. doi: 10.3161/068.037.0105
- Wesołowski, T. Rowiński, P., Mitrus, C. & Czeszczewik, D. 2006. Breeding bird community of a primeval temperate forest (Białowieża National Park, Poland) at the beginning of the 21th century. Acta Orn. 41: 55–70. doi: 10.3161/068.041.0112
- Wesołowski, T., Rowiński, P. & Maziarz, M. 2009. Wood Warbler Phylloscopus sibilatrix: a nomadic insectivore in search of safe breeding grounds? Bird Study 56: 26–33. doi: 10.1080/00063650802681540
- Wesołowski, T., Mitrus, C., Czeszczewik, D. & Rowiński, P. 2010. Breeding bird dynamics in a primeval temperate forest over 35 years: variation and stability in a changing world. Acta Orn. 45: 209–232. doi: 10.3161/000164510X551354
- Winkel, W. 1970. Hinweise zur Art- und Altersbestimmung von Nestlingen höhlenbrütender Vogelarten anhand ihrer Körperentwicklung. Vogelwelt 91: 52–59.
- Yom-Tov, Y. & Wright, J. 1993. Effect of heating nest boxes on egg laying in the Blue Tit (Parus caeruleus). Auk 110: 95–99.
- Zandt, H.S. 1994. A comparison of three sampling techniques to estimate the population size of caterpillars in trees. Oecologia 97: 399–406.
- Zandt, H.S., Strijkstra, A.M., Blondel, J. & van Balen, J.H. 1990. Two Mediterranean Blue Tit populations: are differences in the timing of breeding associated with caterpillar availability? In Blondel, J. (ed.) Population Biology of Passerine Birds, 145–155. Springer-Verlag, Berlin.
