Abstract
Capsule The pattern of moult of juvenile Common Chiffchaffs Phylloscopus collybita wintering in two distant localities of the Iberian Peninsula, Málaga (south) and Barcelona (north) differed. Individuals wintering in the northern locality moulted more contour than flight feathers, and vice versa, while sexes did not differ; individuals moulting more contour feathers arrived later and individuals moulting more flight feathers arrived earlier. Taken together, our results suggest that the pattern of moult of juvenile Common Chiffchaffs may depend on the location to which they migrate, in addition to the geographic origin and the time of breeding.
The process of moult is one of the most interesting, relevant, and ecologically meaningful stages of avian life-histories (Newton Citation2009). A common moult pattern in most European passerines is that adults have a complete post-breeding moult while juveniles only moult partially (Jenni & Winkler Citation1994). Inter-individual differences in the extent of moult among juvenile migratory passerines are generally mainly attributed to time constraints related to different geographical origins (Jenni & Winkler Citation1994). This perspective has been supported by further research focused on inter-population and inter-species comparisons of plumage quality (Serra Citation2001; de la Hera et al. Citation2010) and under captive-controlled conditions (Hall & Fransson Citation2000). In addition, it has been shown that moult extent could have a genetic basis (Battley Citation2006; de la Hera et al. Citation2013).
The extent of moult has also been related to fitness parameters such as body condition, and to environmental factors, such as food availability. Feather renewal is a costly process for birds (Senar et al. Citation1998; López et al. Citation2005; Moreno-Rueda Citation2010) that produces a direct positive effect on fitness (Pap et al. Citation2007; Vagasi et al. 2011). Furthermore, it has broadly been shown that the number of feathers moulted is constrained by an individual's body condition (Gosler Citation1991; Senar et al. Citation1998; Norman Citation1999; López et al. Citation2005; Pagani-Núñez & Hernández-Gómez Citation2013). On the other hand, habitat and food quality are in turn relevant factors for body condition, playing a major role in the process of moult (Borras et al. Citation2004; Butler et al. Citation2006; Pap et al. Citation2008; Broggi et al. Citation2011). Consequently, in addition to the geographic origin, we may assume that extrinsic factors such as food availability and intrinsic factors such as body condition are significant determinants of moult patterns displayed by birds (Murphy Citation1996).
As well as geographic origins (Jenni & Winkler Citation1994), food availability (Barta et al. Citation2008) and body condition (Gosler Citation1991), the extent of moult is also dependent on migratory behaviour (de la Hera et al. Citation2009b). In species showing partial migration, such as Blackcaps Sylvia atricapilla, it has been found that early migrants could decrease feather quality to increase feather growth rates (de la Hera et al. Citation2009a). This notion is also consistent when comparing different species in relation to the extent of moult, because warblers displaying longer migrations moult less extensively (Norman Citation1997; Svensson & Hedenström Citation1999).
This hypothesis, that birds displaying longer migrations will moult less extensively, is likely to be relevant for another partial migrant, the Common Chiffchaff Phylloscopus collybita. This small passerine displays a short-distance migration to its wintering quarters (a broad area covering the Mediterranean basin and western Africa) after moult completion (Cramp Citation1992). It shows a sex-differential pattern of migration, with females migrating further south (Catry et al. Citation2005). Otherwise, they have more time to moult than long-distance migrant warblers, showing great plasticity in the extent and timing of both moult and migration (Norman Citation1990; Cramp Citation1992; Jenni & Winkler Citation1994; Nikolaus Citation2000). In a previous study (Catry et al. Citation2007) it has been shown that females, that moulted more tail feathers, were in better condition and were dominant over males in their wintering quarters. Given this great behavioural variability and complexity, we think that the Common Chiffchaff is a highly suitable species to assess moult extent as a possible function of the locality to which they have migrated. In this regard, we investigated the variability across time and space in the extent of moult of juvenile Common Chiffchaffs wintering in the Iberian Peninsula to ascertain the relative influence of the timing of migration and of the location to which they migrated on this trait. We hypothesized that moult extent should diverge between locations because, in addition to latitudinal and time effects, different wintering conditions may promote different moult patterns.
We recorded the number of contour and flight feathers moulted by 269 juvenile Common Chiffchaffs wintering in two distant localities of the Iberian Mediterranean coast, Málaga and Barcelona (south and northeast Spain, around 1000 km apart), across two seasons (the winters of 2011–12 and 2012–13). These two localities cover both extremes of the wintering area of the Common Chiffchaff in the Iberian Peninsula. We should note that Common Chiffchaffs perform their moult close to their breeding grounds and before the onset of migration (Cramp Citation1992).
In Barcelona, sampling with mist nets was from 26 November to 30 January; while in Málaga sampling was from 7 October to 18 February. We temporally restricted sampling in the northern locality to avoid individuals in active migration that could have reached Málaga later in the season. We used a tape-lure in Barcelona to increase our sample size. It is known that tape-lures increase the male/female ratio in this species by around 20%, but its use does not affect any other parameter, such as biometry or age (Lecoq & Catry Citation2003; Seoane et al. Citation2003). Sexes were determined based on wing length following Catry et al. (Citation2007). This method showed 99% confidence levels when compared to molecular sexing. We only used unambiguously sexed birds to assess sex-related effects. Juvenile Chiffchaffs perform a partial post-juvenile moult that makes aging feasible: age-classes were determined based on differences in abrasion among retained and moulted feathers (Svensson Citation1992). All the members of the team have wide experience aging Chiffchaffs, and worked together for ageing and sexing to minimise inter-observer effects. If there was some doubt with a given individual, we excluded it from our analysis. We ringed all birds, and consequently excluded recaptured individuals from analyses.
We recorded the number of contour (great coverts (up to nine), carpal covert (one), and alula complex (up to three)) and flight (rectrices (up to six), tertials (up to three) and secondaries (up to six)) feathers moulted on birds' right side (). Passerines normally have ten great coverts, but in the case of the Common Chiffchaff only nine are visible. We focused on the extent of moult instead of on feather quality because we think that this is a more direct approach. We assigned five points for each feather moulted and then we summed a score for each feather tract (Gosler Citation1994; Newton Citation2009). This methodology allows comparisons with other species and with other stages of the annual cycle in which the individuals may show active moult. We combined different feather tracts in two groups because our aim was to test whether Common Chiffchaffs prioritize the moult of contour or flight feathers (Pagani-Núñez & Hernández-Gómez Citation2013), as a function of the locality to which they have migrated.
Table 1. Mean ± se of the number of feathers moulted in different feathers tracts, in addition to wing length (mm), by juvenile Common Chiffchaffs P. collybita wintering in two distant localities of the Iberian Peninsula. We show within brackets the relative proportions by locality for each group. The number of feathers only corresponds to one wing and half the tail. The alula complex includes the carpal covert. Total n = 269 (undetermined individuals: n = 59).
First, we assessed the consistency of the sample between sexes, localities and years by means of a log-linear analysis of frequency tables (test of partial associations). We used sex (male or female), locality (Málaga or Barcelona) and year (2011–12 or 2012–13) as factors. We also assessed the correlation between moult scores of contour and flight feathers at the individual level. Then, we obtained a general linear model using the moult scores of contour feathers as dependent variable. As fixed factors we included sex, locality, date, and their interactions. We did the same using the moult scores of flight feathers as dependent variable.
Log-linear analysis of frequency tables showed that our sample was consistent among years, sexes, localities and their interactions (all P > 0.05). The only significant variable was the interaction between sex and locality (P < 0.01), because sex-ratio was strongly biased towards males in the northern locality, Barcelona, compared to the southern locality, Málaga (). Moult scores of contour and flight feathers were not correlated at the individual level (r = 0.06; n = 269; P = 0.32). Common Chiffchaffs moulted (on their right side) from 0 to 8 great coverts (scores 0–40), from 0 to 4 (scores 0–20) feathers of the alula complex (including carpal covert), from 0 to 3 tertials (scores 0–15), from 0 to 1 secondary (scores 0–5), and from 0 to 2 rectrices (scores 0–10).
Moult scores of contour feathers were higher in the northern locality, Barcelona, and increased with date (, ). However, the interaction with date was also dependent on the locality, with individuals showing this pattern of increase especially in the northern locality (; ). We did not find any significant effect of sex and its interactions with date and locality.
Figure 1. Changes across time in Moult scores of contour feathers of 269 juvenile Common Chiffchaffs wintering in two distant localities of the Iberian Peninsula (Málaga in the south, Barcelona in the north). Empty circles correspond to individuals from Málaga, while filled squares correspond to individuals from Barcelona. Mean values and standard errors are provided. Sample sizes for each point are shown within brackets. Within the square are highlighted the earliest migrants that could have reached southern localities across the season. It is probable that these individuals came from the Iberian Peninsula.
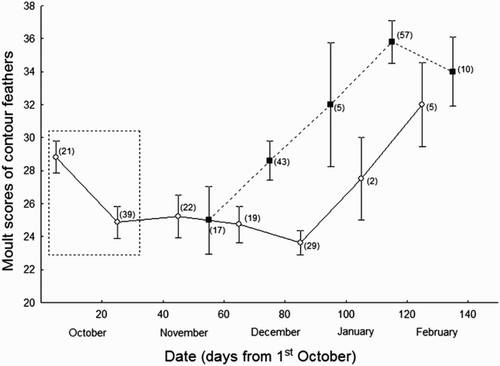
Table 2. Results of a general linear model using the number of contour feathers moulted by juvenile Common Chiffchaffs as dependent variable. We included locality (Málaga or Barcelona), sex (female or male), date, and their interactions as factors. Model fit: R2 = 0.20; F 1,209 = 10.0; P < 0.01.
In contrast, moult scores of flight feathers were higher in the southern locality and decreased with date (, ). We also found an interaction between date and locality, with individuals showing this pattern of decrease only in the southern locality (; ). Again, we did not find any significant effect of sex and its interactions with date and locality.
Figure 2. Graph showing changes across time in Moult scores of flight feathers of 269 juvenile Common Chiffchaffs wintering in two distant localities of the Iberian Peninsula (Málaga in the south, Barcelona in the north). Empty circles correspond to individuals from Málaga, while filled squares correspond to individuals from Barcelona. Mean values and standard errors are provided. Sample sizes for each point are shown within brackets. Within the square are highlighted the earliest migrants that could reach southern localities across the season. It is probable that these individuals came from the Iberian Peninsula.
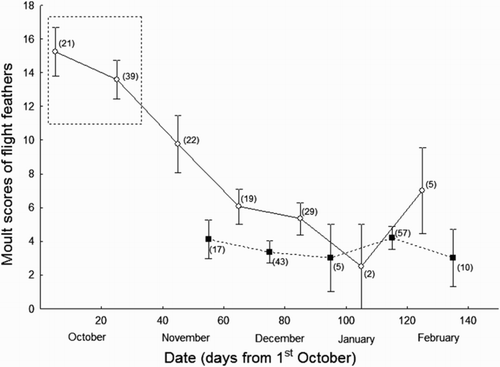
Table 3. Results of a general linear model using the number of flight feathers moulted by juvenile Common Chiffchaffs as dependent variable. We included locality (Málaga or Barcelona), sex (male or female), date, and their interactions as factors. Model fit: R2 = 0.36; F 1,209 = 21.28; P < 0.01.
We found that Common Chiffchaffs captured at the northern edge of their wintering grounds moulted more contour than flight feathers. Vice versa, individuals wintering in South Iberia moulted more flight feathers than those captured in the north. Sexes did not differ in the extent of moult after controlling for locality and date. We also found that individuals that moulted more contour feathers arrived later and individuals that moulted more flight feathers arrived earlier. This date-related pattern was especially significant for contour feathers in the northern locality and for flight feathers in the southern locality.
We propose three alternative, and probably complementary, explanations to the fact that juvenile Common Chiffchaffs in different wintering locations had strikingly different patterns of moult. First, it may be a consequence of the different origins of individuals. Many studies have addressed this issue, stressing the relevance of latitude for moult strategies of birds and the time constraints related to different geographical origins (Mewaldt & King Citation1978; Jenni & Winkler Citation1994; but see Ryder & Rimmer Citation2003; Butler et al. Citation2006). In northern latitudes individuals has less time to moult, so that they increase feather growth rates (Hall & Fransson Citation2000; Serra Citation2001) and/or moult more feathers together (Rohwer & Rohwer Citation2013). In our case this means that southern individuals could have moulted more flight feathers because they had more time to moult. One possibility, consistent with this, is that individuals showing the most extensive moult of flight feathers, that were captured at the beginning of the season in the southern locality, came from the Iberian Peninsula. They would display a moult strategy similar to that of their close relatives, the Iberian Chiffchaff Phylloscopus brehmii (Pérez-Tris et al. 2003). Additionally, the impact of rearing conditions may influence this, by means of extrinsic factors such as food (Butler et al. Citation2006; see also Barta et al. Citation2008) and habitat quality (Heise & Rimmer Citation2000; Borras et al. Citation2004; Rohwer et al. Citation2008; Broggi et al. Citation2011), affecting feather quality and probably also moult extent. It is possible that moulting more or less contour or flight feathers may simply be a signal of the more or less favourable rearing conditions of the individuals.
Second, the conditions in the wintering locations may promote different moult patterns. It is possible to find Chiffchaffs from many breeding locations in Europe in any given wintering location (Cramp Citation1992). Our ringing group has been capturing thousands of Chiffchaffs in Málaga for more than two decades, and we have recorded individuals ringed in many of the countries of Western Europe. This divergent pattern of moult recorded between localities then suggests that individuals adjusted the type and the number of feathers moulted as a function of the locality to which they migrated to. Previous research has already showed that migrant birds may adjust their moult patterns, which has important consequences for feather quality, as a function of their migratory behaviour (de la Hera et al. Citation2009a, 2010). In our case this could mean that individuals invested more or less resources in different feather tracts as a function of their ecological demands (Gargallo Citation2013). We think that the fact that contour and flight feathers were not correlated at the individual level supports this view. Moulting more flight feathers may benefit individuals migrating earlier and towards more southerly locations if they make a higher flight effort (Swaddle et al. Citation1996; Williams & Swaddle Citation2003; Pap et al. Citation2007; Vagasi et al. 2011). Conversely, individuals migrating later and staying at the northern edge of their wintering grounds moulted more contour feathers. Further research could assess whether the investment of more resources in this feather tract resulted in higher quality feathers important for body cover. Improving insulation would benefit individuals wintering in the generally colder northern localities (Pap et al. Citation2007; de la Hera et al. Citation2009a). We also may note that our results are consistent with previous research focused on the warbler group from an inter-specific perspective (Norman Citation1997; Svensson & Hedenström Citation1999; Hall & Tullberg Citation2004).
Third and finally, different timing of reproduction may have contributed to produce the observed divergent pattern of moult. Fledging dates greatly influence the time and duration of moult of juvenile birds (Bojarinova et al. Citation1999; Rohwer Citation2013; see also Dawson Citation2004). Late fledglings generally are reared in less favourable conditions and have less time to moult. Consequently, later migrants, that moulted more contour and fewer flight feathers, may have been, in fact, juveniles from late or second broods. Similarly, it is possible that individuals captured at the end of the wintering season were coming back to their breeding grounds from southern locations.
We conclude that, in addition to the geographic origin and the timing of migration, migratory passerines may prioritize the moult of different feather tracts as a function of the locality to which they migrate. Further research may assess whether the extent of moult in this species is correlated to different geographic origins, and experimental translocation would confirm the hypothesis of moult being a function of local wintering conditions. Additionally, it would be of major interest to ascertain whether this divergent pattern of moult exists in similar species.
Acknowledgements
We want to thank José Antonio Cortés, our ringing teacher, for his help in the field and for his wise counsels and insightful comments. We also thank Jaime Resano-Mayor and two anonymous referees for their help improving an earlier version of the manuscript. Birds were handled with the permission of the Consejería de Medio Ambiente, Junta de Andalucía, and the Departament de Medi Ambient, Generalitat de Catalunya. Rings were provided by the Avian Migration Center (CMA) and the Catalan Ringing Office (ICO).
References
- Barta, Z., McNamara, J.M., Houston, A.I., Weber, T.P., Hedenström, A. & Feró, O. 2008. Optimal moult strategies in migratory birds. Philos. Trans. R. Soc. B 363: 211–229. doi: 10.1098/rstb.2007.2136
- Battley, P.F. 2006. Consistent annual schedules in a migratory shorebird. Biol. Lett. 2: 517–520. doi: 10.1098/rsbl.2006.0535
- Bojarinova, J.G., Lehikoinen, E. & Eeva, T. 1999. Dependence of postjuvenile moult on hatching date, condition and sex in the Great Tit. J. Avian Biol. 30: 437–446. doi: 10.2307/3677016
- Borras, A., Cabrera, T., Cabrera, J. & Senar, J.C. 2004. Interlocality variation in speed of moult in the Citril Finch Serinus citrinella. Ibis 146: 14–17. doi: 10.1111/j.1474-919X.2004.00199.x
- Broggi, J., Gamero, A., Hohtola, E., Orell, M. & Nilsson, J.Å. 2011. Interpopulation variation in contour feather structure is environmentally determined in Great Tits. PloS One 6: e24942. doi: 10.1371/journal.pone.0024942
- Butler, L.K., Rohwer, S. & Rogers, M. 2006. Prebasic molt and molt-related movements in Ash-throated Flycatchers. Condor 108: 647–660. doi: 10.1650/0010-5422(2006)108[647:PMAMMI]2.0.CO;2
- Catry, P., Lecoq, M., Araújo, A., Conway, G., Felgueiras, M., King, J.M.B., Rumsey, S., Salima, H. & Tenreiro, P. 2005. Differential migration of chiffchaffs Phylloscopus collybita and P. ibericus in Europe and Africa. J. Avian Biol. 36: 184–190. doi: 10.1111/j.0908-8857.2005.03445.x
- Catry, P., Bearhop, S. & Lecoq, M. 2007. Sex differences in settlement behaviour and condition of chiffchaffs Phylloscopus collybita at a wintering site in Portugal. Are females doing better? J. Ornithol. 148: 241–249. doi: 10.1007/s10336-007-0130-8
- Cramp, S. 1992. The Birds of the Western Palearctic, Vol. 6. Oxford University Press, Oxford.
- Dawson, A. 2004. The effects of delaying the start of moult on the duration of moult, primary feather growth rates and feather mass in Common Starlings Sturnus vulgaris. Ibis 146: 493–500. doi: 10.1111/j.1474-919x.2004.00290.x
- Gargallo, G. 2013. Feather selection and replacement patterns demonstrate that Goldfinches Carduelis carduelis fix postjuvenile moult extent prior to moult initiation. J. Ornithol. 154: 219–230. doi: 10.1007/s10336-012-0888-1
- Gosler, A.G. 1991. On the use of greater covert moult and pectoral muscle as measures of condition in passerines with data for the Great Tit Parus major. Bird Study 38: 1–9. doi: 10.1080/00063659109477061
- Gosler, A.G. 1994. Mass-change during moult in the Great Tit Parus major. Bird Study 41: 146–154. doi: 10.1080/00063659409477211
- Hall, K.S.S. & Fransson, T. 2000. Lesser Whitethroats under time-constraint moult more rapidly and grow shorter wing feathers. J. Avian Biol. 31: 583–587. doi: 10.1034/j.1600-048X.2000.310419.x
- Hall, K.S.S. & Tullberg, B.S. 2004. Phylogenetic analyses of the diversity of moult strategies in Sylviidae in relation to migration. Evol. Ecol. 18: 85–105. doi: 10.1023/B:EVEC.0000017848.20735.8b
- Heise, C.D. & Rimmer, C.C. 2000. Definitive prebasic molt of Gray Catbirds at two sites in New England. Condor 102: 894–904. doi: 10.1650/0010-5422(2000)102[0894:DPMOGC]2.0.CO;2
- de la Hera, I., Pérez-Tris, J. & Telleria, J.L. 2009a. Migratory behaviour affects the trade-off between feather growth rate and feather quality in a passerine bird. Biol. J. Lin. Soc. 97: 98–105. doi: 10.1111/j.1095-8312.2008.01189.x
- de la Hera, I., Díaz, J.A., Pérez-Tris, J. & Luis Tellería, J. 2009b. A comparative study of migratory behaviour and body mass as determinants of moult duration in passerines. J. Avian Biol. 40: 461–465. doi: 10.1111/j.1600-048X.2008.04689.x
- de la Hera, I., Pérez-Tris, J. & Tellería, J.L. 2010. Relationships among timing of moult, moult duration and feather mass in long-distance migratory passerines. J. Avian Biol. 41: 609–614. doi: 10.1111/j.1600-048X.2010.05075.x
- de la Hera, I., Reed, T.E., Pulido, F. & Visser, M.E. 2013. Feather mass and winter moult extent are heritable but not associated with fitness-related traits in a long-distance migratory bird. Evol. Ecol. 27: 1199–1216. doi: 10.1007/s10682-013-9639-x
- Jenni, L.W. & Winkler, R.R. 1994. Moult and Ageing of European Passerines. Academic Press, London.
- Lecoq, M. & Catry, P. 2003. Diurnal tape-luring of wintering chiffchaffs results in samples with biasedsex ratios. J. Field Ornithol. 74: 230–232.
- López, G., Figuerola, J., Varo, N. & Soriguer, R.C. 2005. White Wagtails Motacilla alba showing extensive post-juvenile moult are more stressed. Ardea 93: 237–244.
- Mewaldt, L.R. & King, J.R. 1978. Latitudinal variation of postnuptial molt in Pacific Coast White-crowned Sparrows. Auk 95: 168–174. doi: 10.2307/4085508
- Moreno-Rueda, G. 2010. Experimental test of a trade-off between moult and immune response in house sparrows Passer domesticus. J. Evol. Biol. 23: 2229–2237. doi: 10.1111/j.1420-9101.2010.02090.x
- Murphy, M.E. 1996. Energetics and nutrition of molt. In Carey, C. (ed.) Avian Energetics and Nutritional Ecology, 158–198. Chapman & Hall, New York.
- Newton, I. 2009. Moult and plumage. Ring. Migr. 24: 220–226. doi: 10.1080/03078698.2009.9674395
- Nikolaus, G. 2000. Partial winter primary moult in Chiffchaffs Phylloscopus collybita. Ring. Migr. 20: 31–33. doi: 10.1080/03078698.2000.9674225
- Norman, S.C. 1990. A comparative study of post-juvenile moult in four species of Sylvia warbler. Ring. Migr. 11: 12–22. doi: 10.1080/03078698.1990.9673956
- Norman, S.C. 1997. Juvenile wing shape, wing moult and weight in the family Sylviidae. Ibis 139: 617–630. doi: 10.1111/j.1474-919X.1997.tb04684.x
- Norman, S.C. 1999. Biometrics and post-juvenile moult in the Goldcrest Regulus regulus. Ring. Migr. 19: 175–180. doi: 10.1080/03078698.1999.9674179
- Pagani-Núñez, E. & Hernández-Gómez, S. 2013. Extent of post-juvenile moult in wintering Common Chiffchaffs Phylloscopus collybita is related to indices of individual quality. Catalan J. Ornithol. 29: 35–42.
- Pap, P.L., Barta, Z., Tökölyi, J. & Vágási, I.C. 2007. Increase of feather quality during moult: a possible implication of feather deformities in the evolution of partial moult in the Great Tit Parus major. J. Avian Biol. 38: 471–478. doi: 10.1111/j.0908-8857.2007.03958.x
- Pap, P.L., Vagasi, C.I., Czirjak, G.A. & Barta, Z. 2008. Diet quality affects postnuptial molting and feather quality of the house sparrow (Passer domesticus): interaction with humoral immune function? Can. J. Zool. 86: 834–842. doi: 10.1139/Z08-060
- Perez-Tris, J., Ramírez, Á. & Tellería, J.L. 2003. Are Iberian Chiffchaffs Phylloscopus (collybita) brehmii long-distance migrants? An analysis of flight-related morphology. Bird Study 50: 146–152. doi: 10.1080/00063650309461306
- Rohwer, S. 2013. Molt intensity and conservation of a molt migrant (Passerina ciris) in Northwest Mexico. Condor 115: 421–433. doi: 10.1525/cond.2013.120090
- Rohwer, V.G. & Rohwer, S. 2013. How do birds adjust the time required to replace their flight feathers? Auk 130: 699–707. doi: 10.1525/auk.2013.13042
- Rohwer, V.G., Rohwer, S. & Barry, J.H. 2008. Molt scheduling of western Neotropical migrants and up-slope movement of Cassin's Vireo. Condor 110: 365–370. doi: 10.1525/cond.2008.8321
- Ryder, T.B. & Rimmer, C.C. 2003. Latitudinal variation in the definitive prebasic molt of Yellow Warblers. Wilson Bull. 115: 325–332. doi: 10.1676/02-131
- Senar, J.C., Copete, J.L. & Martin, A.J. 1998. Behavioural and morphological correlates of variation in the extent of postjuvenile moult in the Siskin Carduelis spinus. Ibis 140: 661–669. doi: 10.1111/j.1474-919X.1998.tb04712.x
- Seoane, J., Varo, N. & Vázquez, M. 2003. Una nota sobre el uso de reclamos sonoros para capturar Mosquiteros Comunes (Phylloscopus collybita) en otoño. Rev. Anillamiento 12: 9–13.
- Serra, L. 2001. Duration of primary moult affects primary quality in Grey Plovers Pluvialis squatarola. J. Avian Biol. 32: 377–380. doi: 10.1111/j.0908-8857.2001.320415.x
- Svensson, L. 1992. Identification Guide to European Passerines. Lars Svensson, Stockholm.
- Svensson, E. & Hedenström, A. 1999. A phylogenetic analysis of the evolution of moult strategies in Western Palearctic warblers (Aves: Sylviidae). Biol. J. Lin. Soc. 67: 263–276. doi: 10.1111/j.1095-8312.1999.tb01864.x
- Swaddle, J.P., Witter, M.S., Cuthill, I.C., Budden, A. & McCowen, P. 1996. Plumage condition affects flight performance in common starlings: implications for developmental homeostasis, abrasion and moult. J. Avian Biol. 27: 103–111. doi: 10.2307/3677139
- Vágási, C.I., Pap, P.L., Tökölyi, J., Székely, E. & Barta, Z. 2011. Correlates of variation in flight feather quality in the Great Tit Parus major. Ardea 99: 53–60. doi: 10.5253/078.099.0107
- Williams, E.V. & Swaddle, J.P. 2003. Moult, flight performance and wingbeat kinematics during take-off in European starlings Sturnus vulgaris. J. Avian Biol. 34: 371–378. doi: 10.1111/j.0908-8857.2003.02964.x
