Abstract
Capsule Boldness defines the extent to which animals are willing to take risks in the presence of a predator. Late, but not early, in the breeding season, Israeli nestling Barn Owls displaying larger black feather spots were more docile, feigned death longer and had a lower breathing rate when handled than smaller-spotted nestlings. Larger-spotted breeding females were less docile if heavy but more more docile if light. The covariation between personality (boldness vs. timid) and melanin-based colouration is therefore conditional on environmental factors.
In various species of animals, individuals differ in their behaviour in a particular context such when confronting a predator or when exploring a new environment, and they often show different levels of physical activity, aggressiveness and social interactions. When individual differences in behaviour persist over time and/or across situations, these behaviours are referred to as ‘personality traits’. The evolutionary stability of variation in personality implies that there is no single behavioural optimum (Wolf et al. Citation2007). Inter-individual personality differences may reduce the level of competition and facilitate the exploitation of a larger range of ecological niches (Réale et al. Citation2007). Hence, it is not surprising if the correlation between personality traits, so-called behavioural syndromes, depends on ecological factors such as the level of predation (Sih et al. Citation2004, Bell & Sih Citation2007). It may therefore be beneficial to advertise personality to potential mates or to social competitors. For instance, the type of anti-predator behaviour (avoidance/attack) may be associated with colour patterns, either because in the context of sexual selection a particular colouration signals the ability to avoid predators, or because colouration is a naturally selected trait useful in avoiding predators.
There have been several recent studies published on the observation that differently coloured individuals of the same species behave differently. Such covariation between colouration and behaviour has not only been reported in a wide range of wild animals, including fish (Kittilsen et al. Citation2009), reptiles (Mafli et al. Citation2011), mammals (Trut Citation1999, Kontiainen et al. Citation2009) and birds (Sternalski & Bretagnolle Citation2010, Mateos-Gonzàlez & Senar Citation2012) but also in domesticated animals (Pérez-Guisado et al. Citation2006, Bennett & Hayssen Citation2010, Kim et al. Citation2010). This suggests that colouration could reveal personality (Réale et al. Citation2007). Personality encompasses several components that can all covary with colouration, such as exploration (Mateos-Gonzàlez & Senar Citation2012), aggressiveness (Senar Citation2006) and boldness (Mafli et al. Citation2011).
Behavioural traits are particularly malleable and sensitive to environmental conditions, which explains why their within-individual repeatability and heritability is usually relatively low (Réale et al. Citation2007). Since behaviours such as those associated with the avoidance of predators will evolve towards higher values in populations where predators are particularly abundant or efficient, their covariation with other phenotypes may not necessarily prevail in all populations. For instance, in the three-spined stickleback Gasterosteus aculeatus predation risk has generated a correlation between boldness and aggressiveness, as a consequence of both behavioural plasticity and natural selection (Bell & Sih Citation2007). This example demonstrates that results obtained in one population may not be applicable to other populations or species. Studies carried out in one population therefore need to be replicated in other populations in order to determine their general applicability.
We found in the Swiss Barn Owl Tyto alba alba/guttata that the degree of eumelanin-based colouration of the ventral body side, in the form of black spots of varying size located on the tip of the feathers, is associated with a type of anti-predatory behaviour (Van den Brink et al. Citation2012). Compared to nestlings displaying small black spots, those exhibiting larger spots hissed more intensely in the presence of humans (a behaviour intended to scare away predators), feigned death longer and had a lower breathing rate under stress. Furthermore, offspring raised by foster parents were more docile (i.e. less agitated when handled) if born from large-spotted rather than small-spotted fathers, and also feigned death for longer if born from large-spotted rather than small-spotted mothers. Thus, the degree of eumelanin-based colouration, a strongly heritable trait (h2 = 0.82, Roulin et al. Citation2010), appears to be genetically correlated with behaviour. The small-spotted individuals adopted a bold and proactive behaviour by displaying agitation while being handled, whereas the large-spotted individuals were more timid and docile when handled. Our aim here was to replicate that study in another population of Barn Owls Tyto alba erlangeri in Israel. This population is of particular interest because Barn Owls in the Middle East are less eumelanic than those in Switzerland (Roulin et al. Citation2009). We specifically examined the covariation between the size of the black feather spots, docility when handled, the time spent feigning death and breathing rate. Because behaviour is sensitive to many factors, such as body condition or date, we investigated whether covariations are conditional upon these factors.
Based on measurements taken from natural history museums (unpubl. data of the last author), in Israel Barn Owls are on average larger than those in Switzerland (bill length: 19.54 ± 0.09 vs. 19.03 ± 0.05 mm, ancova controlling for sex: F 1,327 = 23.2, P < 0.0001; wing length: 294.7 ± 1.1 vs. 290.7 ± 0.7 mm, F 1,231 = 9.98, P = 0.002) and display smaller black spots (0.86 ± 0.06 vs. 1.18 ± 0.03 mm, F 1,352 = 24.6, P < 0.0001). In Israel brood size is also larger than in Switzerland (4.61 ± 0.12 vs. 4.25 ± 0.05 fledglings) (Chausson et al. Citation2014; unpubl. data of the second author). Personal observations indicate that in Israel Barn Owls are on average more agitated when handled than in Switzerland.
In 2012 in northern Israel (32°30´N, 35°30´E) we monitored 486 Barn Owl nestlings from 88 breeding pairs when the oldest nestling was 30 days of age, and we captured 80 of the breeding females. Immediately upon capture in their nest-box, the first author (for nestlings) and the second author (for adults) assigned a docility score between 0 and 3: minimal score (0) for individuals that did not move or hiss during manipulation; (1) hissed and/or moved very little but did not grab with bill and claws; (2) hissed, struggled, flapped wings and grabbed with their bill and claws; (3) maximal score for individuals that struggled, flapped their wings and/or hissed constantly, grabbing with their bill and claws and, when caught to be handled, lay on their back with claws raised. Females were weighed and the length of their left wing and tarsus measured to the nearest mm and 0.1 mm, respectively. As previously shown, docility is repeatable in nestlings (r = 0.27 ± 0.07, Van den Brink et al. Citation2012). The first author carried out the tonic immobility test by putting each nestling on its back and restraining it for 10 seconds with a hand on its breast. The hand was removed, and latency to the nestling moving to turn and stand again was measured. If an individual remained supine for longer than 60 seconds, the test was stopped and hence 60 seconds was the maximum duration. This duration was box-cox transformed to normalize the data set. Finally, the number of breathing breast movements during 1 minute was counted immediately after the start of disturbance and again 20 minutes later (breathing movements were not counted in 11 nestlings that were panting due to warm temperatures). Because ambient temperature can induce panting and hence influence breathing rate, we recorded ambient temperature inside the nest-boxes and statistically controlled for this factor when analysing data from breathing movements. All these methods and their rationale are explained in detail in Van den Brink et al. (Citation2012).
Because females display on average larger black spots than males (Student's t-test comparing male and female nestlings: t 348 = 11.77, P < 0.0001; 1.22 ± 0.03 vs. 0.64 ± 0.04 mm), we identified nestling sex using molecular markers (171 males and 191 females). We recorded brood size (mean ± se: 5.97 ± 0.20 nestlings; range: 1–12), female and nestling body mass, female wing length, laying date and time of the day. Forty-two breeding females were ringed as nestlings, and hence we assigned females to either the ‘yearling’ or ‘adult’ category, because in Europe it has been shown that spots increase in size between the first- and second-year of age (Dreiss & Roulin Citation2010). The second author assessed the degree of eumelanin-based plumage in breeding females and in 55-day-old nestlings by placing a 60 × 40 mm frame on the breast within which the black spots were measured to the nearest 0.1 mm. We then calculated a mean spot diameter value to be used in the statistical analyses. Two-tailed statistical analyses were carried out with the software JMP (Sall & Lehman Citation1996). We verified that associations between behaviour and spot diameter were not confounded by time of day, nestling body mass, brood size or temperature inside the nest-boxes. Dependent variables and residuals of our models were normally distributed.
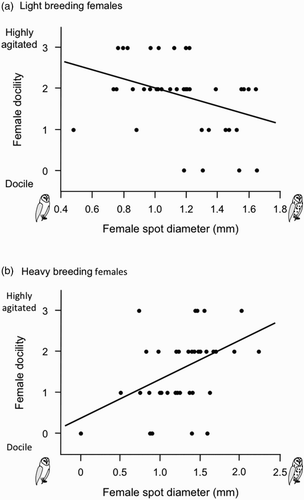
Larger-spotted females bred earlier in the season (Pearson's correlation: r = 0.29, n = 80, P = 0.009; a), a relationship that remained significant after controlling for age in 42 individuals of known age (ancova, spot size: F 1,39 = 24.9, P < 0.0001; age category: F 1,39 = 12.1, P = 0.0013). Brood size was not correlated with female spot diameter but decreased as the season progressed (multiple regression analysis: F 1,77 = 0, P = 1.0 and F 1,77 = 8.1, P = 0.006, respectively). Larger-spotted females were heavier (r = 0.26, n = 80, P = 0.018; b), a relationship that remained significant even after controlling for date or female wing length (not shown). We thus analysed the relationship between female docility and plumage spottiness in interaction with laying date and female body mass. In adult females a score of 0 for the degree of docility when handled was assigned to 10 individuals (12.5%), a score of 1 to 20 (25.0%), a score of 2 to 37 (46.3%) and a score of 3 to 13 individuals (16.2%). The degree of female docility was related to the size of their black spots in interaction with their body mass (multiple regression analysis, female body mass: F 1,76 = 3.6, P = 0.06, heavier birds tended to be more docile; female spot diameter: F 1,76 = 0.03, P = 0.86; interaction: F 1,76 = 14.0, P = 0.0003). By dividing the sample of females into heavy and light, based on median body mass (389 g), we found that in heavier individuals larger-spotted females were less docile (Pearson's correlation: r = 0.44, n = 41, P = 0.004, a), whereas in lighter individuals larger-spotted females were more docile (r = −0.37, n = 39, P = 0.022, b). These relationships remained significant even after controlling for date. The degree of female agitation was neither associated with female body mass, laying date nor with brood size alone, nor in interaction with the diameter of their black feather spots (P-values > 0.15).
Figure 1. Diameter of black feather spots in relation to laying date (a) and body mass (b) in breeding female Barn Owls.
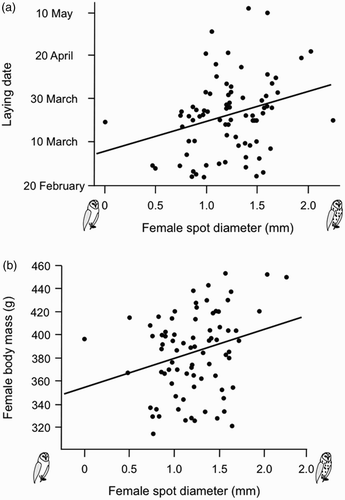
Figure 2. Relationship between docility when handled and diameter of black feather spots in light and heavy breeding females. Light and heavy females were defined by median female body mass (389 g).
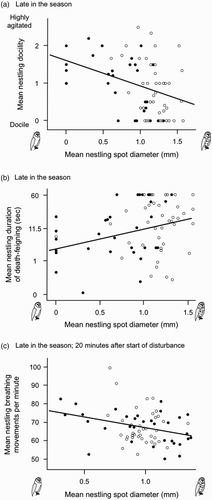
In nestlings, a score of 0 for the degree of docility when handled was assigned to 129 individuals (27.0%), a score of 1 to 177 (37.0%), a score of 2 to 138 (28.9%) and a score of 3 to 34 individuals (7.1%). Nestling docility was related to the size of their black feather spots in interaction with date (linear mixed model on mean male and mean female siblings, and with nest as random variable explaining 48.0% of the variation; nestling sex: F 1,103.9 = 0.03, P = 0.86; date: F 1,78.18 = 10.5, P = 0.002 (nestlings were more docile late than early in the season); nestling spot size: F 1,130.6 = 0.4, P = 0.52; interaction date by nestling spot size: F 1,100.5 = 6.2, P = 0.015). By pooling nestlings handled early and late in the season based on median date (21 May), we found that larger-spotted nestlings were more docile late in the season (linear mixed model: F 1,69.57 = 5.8, P = 0.019, a; nestling sex: F 1,48.87 = 0.9, P = 0.33), a relationship that did not apply early in the season (P-values > 0.25). A similar interaction was noted for the tonic immobility test (linear mixed model on mean male and mean female siblings, and with nest as random variable explaining 31.9% of the variation; nestling sex: F 1,112.1 = 0.51, P = 0.47; date: F 1,79.32 = 3.1, P = 0.08; nestling spot size: F 1,132.6 = 1.5, P = 0.22; interaction date by nestling spot size: F 1,116.7 = 3.6, P = 0.05) because larger-spotted nestlings took longer to erect only late in the season (linear mixed model: F 1,68.69 = 5.2, P = 0.026, b; nestling sex: F 1,52.19 = 1.5, P = 0.23). Immediately after having opened the nest-boxes, smaller-spotted nestlings showed more breathing breast movements than larger-spotted nestlings and females more than males (66.4 ± 1.4 vs. 62.3 ± 1.5) (linear mixed model with nest as random variable explaining 42.6% of the variation, nestling spot size: F 1,120.5 = 4.2, P = 0.04; nestling sex: F 1,96.47 = 4.0, P = 0.04; ambient temperature: F 1,67.12 = 1.9, P = 0.17), independent of date. Twenty minutes later the number of breathing breast movements were more frequent (paired t-test: t 471 = 5.1, P < 0.0001). On this occasion, we again found that breathing movements were more frequent in females than males (69.0 ± 1.4 vs. 64.1 ± 1.4; F 1,96.46 = 6.7, P = 0.01) and in smaller-spotted individuals (F 1,118.5 = 7.2, P = 0.008). However, in the same model the interaction between nestling spot diameter and date was significant (F 1,96.44 = 5.2, P = 0.02; date: F 1,72.09 = 0.01, P = 0.94; ambient temperature: F 1,65.88 = 2.8, P = 0.10) because late in the season the negative association between spot diameter and the number of breast movements was stronger (linear mixed model with nest as random variable with breast movements as dependent variable; effect of spot diameter: F 1,67.68 = 10.2, P = 0.002; c) than early in the season (F 1,46.01 = 0.3, P = 0.57).
Figure 3. Relationship between docility when handled, tonic immobility test and breathing breast movements and diameter of black feather spots in nestling Barn Owls measured late in the season. Early and late in the season was defined by median date (21 May). Filled circles represent males and open circles females. Mean values of same-sex siblings were calculated.
Similar to the results obtained in Switzerland (Van den Brink et al. Citation2012), we found that larger-spotted individuals were more docile (with the exception of heavy females, for which the opposite was found), feigned death for longer and had a lower breathing rate under stress. Despite some slight differences from the Swiss study (e.g. nestling docility was correlated with paternal spot diameter, not with nestling spot diameter) that are still unexplained, our study suggests that the relationship between a heritable melanin-based trait and behaviour is similar in populations separated by more than 3000 km. This implies that the higher docility of owls with larger eumelanic spots is a general property of the family Tytonidae. In domestic animals docility is a heritable trait that can be selected for by humans in order to obtain individuals that can be easily handled and show less fear (Trut Citation1999, Malmkvist & Hansen Citation2002). It would therefore be interesting to investigate whether Barn Owl populations in regions that are occupied by humans at different densities, and consequently have greater or lesser contact with humans, display spots of different sizes. It would also prove valuable to determine whether owls displaying larger black spots survive better in captivity than individuals with smaller spots, and whether differently spotted captive owls react differently to the presence of humans. Our findings in Israel suggest that, compared to small-spotted Barn Owls, those displaying large spots react less strongly to stress induced by a potential predator. Thus, the link between the size of eumelanic spots and response to stress may be a general property of the family Tytonidae and other animals (Almasi et al. Citation2010).
In Switzerland, a relationship was found between the docility of offspring raised by foster parents and the size of black spots displayed by their biological fathers. This suggests a genetic basis for the covariation between melanism and behaviour. In Israel we did not perform cross-fostering experiments and hence it is not possible to distinguish genetic from environmental effects on the relationship between offspring docility and plumage traits. However, the finding that the covariation between melanin-based coloration and behaviour is conditional upon date and body mass suggests that this covariation has a strong environmental component. This is particularly important given that the covariation between docility and coloration can even reverse, since larger-spotted females were less docile if heavy and more docile if light. To understand the potential adaptive value of these results and whether body mass and date have a causal effect on the covariation between behaviour and coloration, an experimental approach is necessary. Hatchlings should be systematically cross-fostered between nests to allocate randomly genotypes among environments, and rearing conditions should be experimentally modified (for instance by modifying brood size).
Finally, in most animals, darker eumelanic individuals are more aggressive than their lighter coloured conspecifics (Senar Citation2006, Ducrest et al. Citation2008, Mafli et al. Citation2011). Thus, there is a question of why in the Barn Owl the relationship between eumelanin-based colouration and boldness is consistently reversed compared to other animals? To understand this difference between most other animals and the Barn Owl, it should be first noted that in many animals males are usually darker eumelanic than females. Hence, unsurprisingly agitation, a behaviour that is typically expressed by males, is shown by darker eumelanic individuals in most animals. Interestingly, in the Barn Owl the pattern of colouration is reversed, with females being typically darker eumelanic than males, which could potentially explain why smaller-spotted individuals are more agitated. This suggests that the relationship between docility and colouration may be due to genes that have a typical male function (e.g. aggressive behaviour). In species in which males are darker coloured than females these genes may simultaneously encode for agitation and eumelanism, whereas in species in which females are darker coloured than males, these genes may encode for eumelanism and docility. We are currently investigating whether the size of the black feather spots covaries with testosterone levels; and whether genes of the melanocortin system regulate the level of this hormone, as shown in other animals (Ducrest et al. Citation2008).
ACKNOWLEDGEMENTS
We thank Kobi Meyrom for fieldwork and Valentijn van den Brink for useful comments, Naomi Paz for editorial assistance, and the Israel Hoopoe Foundation, Israel Ministry of Agriculture and Israeli Ministry of Environmental Protection for funding. We are grateful to Will Cresswell and two anonymous reviewers for useful comments.
Funding
A. Roulin was supported by a grant from the Swiss National Science Foundation [grant number 3100AO_120517] and by the foundation Addax-Oryx.
REFERENCES
- Almasi, B., Jenni, L., Jenni-Eiermann, S. & Roulin, A. 2010. Regulation of stress-response is heritable and functionally linked to melanin-based colouration. J. Evol. Biol. 23: 987–996. doi: 10.1111/j.1420-9101.2010.01969.x
- Bell, A.M. & Sih, A. 2007. Exposure to predation generates personality in three-spined sticklebacks (Gasterosteus aculeatus). Ecol. Lett. 10: 828–834. doi: 10.1111/j.1461-0248.2007.01081.x
- Bennett, A. & Hayssen, V. 2010. Measuring cortisol in hair and saliva from dogs: coat colour and pigment differences. Domest. Anim. Endocrin. 39: 171–180. doi: 10.1016/j.domaniend.2010.04.003
- Chausson, A., Henry, I., Almasi, B. & Roulin, A. 2014. Barn owl (Tyto alba) breeding biology in relation to breeding season climate. J. Ornithol. 155: 273–281. doi: 10.1007/s10336-013-1012-x
- Dreiss, A.N. & Roulin, A. 2010. Age-related change in melanin-based coloration: females that become more female-like and males more male-like with age perform better in barn owls (Tyto alba). Biol. J. Linn. Soc. 101: 689–704. doi: 10.1111/j.1095-8312.2010.01503.x
- Ducrest, A.-L., Keller, L. & Roulin, A. 2008. Pleiotropy in the melanocortin system, colouration and behavioural syndromes. Trends Ecol. Evol. 23: 502–510. doi: 10.1016/j.tree.2008.06.001
- Kim, Y.K., Lee, S.S., Oh, S.I., Kim, J.S., Suh, E.H., Houpt, K.A., Lee, H.C., Lee, H.J. & Yeon, S.C. 2010. Behavioural reactivity of the Korean native Jindo dog varies with coat colour. Behav. Proc. 84: 568–572. doi: 10.1016/j.beproc.2010.02.012
- Kittilsen, S., Schjolden, J., Beitnes-Johansen, I., Shaw, J.C., Pottinger, T.G., Sorensen, C., Braastad, B.O., Bakken, M. & Overli, O. 2009. Melanin-based skin spots reflect stress responsiveness in salmonid fish. Horm. Behav. 56: 292–298. doi: 10.1016/j.yhbeh.2009.06.006
- Kontiainen, P., Pietiainen, H., Huttunen, K., Karell, P., Kolunen, H. & Brommer, J.E. 2009. Aggressive Ural owl mothers recruit more offspring. Behav. Ecol. 20: 789–796. doi: 10.1093/beheco/arp062
- Mafli, A., Wakamatsu, K. & Roulin, A. 2011. Melanin-based colouration predicts aggressiveness and boldness in captive eastern Hermann's tortoises. Anim. Behav. 81: 859–863. doi: 10.1016/j.anbehav.2011.01.025
- Malmkvist, J. & Hansen, S.W. 2002. Generalization of fear in farm mink, Mustela vison, genetically selected for behaviour towards humans. Anim. Behav. 64: 487–501. doi: 10.1006/anbe.2002.3058
- Mateos-Gonzàlez, F. & Senar, J.C. 2012. Melanin-based trait predicts individual exploratory behaviour in siskins, Carduelis spinus. Anim. Behav. 83: 229–232. doi: 10.1016/j.anbehav.2011.10.030
- Pérez-Guisado, J., Lopez-Rodríguez, R. & Muñoz-Serrano, A. 2006. Heritability of dominant-aggressive behaviour in English cockler spaniels. Appl. Anim. Behav. Sci. 100: 219–227. doi: 10.1016/j.applanim.2005.11.005
- Réale, D., Reader, S.M., Sol, D., McDougall, P.T. & Dingemanse, N.J. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82: 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
- Roulin, A., Wink, M. & Salamin, N. 2009. Selection on a eumelanic ornament is stronger in the tropics than in temperate zones in the worldwide-distributed barn owl. J. Evol. Biol. 22: 345–354. doi: 10.1111/j.1420-9101.2008.01651.x
- Roulin, A., Altwegg, R., Jensen, H., Steinsland, I. & Schaub, M. 2010. Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecol. Lett. 13: 616–626. doi: 10.1111/j.1461-0248.2010.01459.x
- Sall, J. & Lehman, A. 1996. JMP Start Statistics: A Guide to Statistical and Data Analysis Using JMP and LMP in Software, 4th edn. Duxbury Press, London.
- Senar, J.C. 2006. Bird colours as intrasexual signals of aggression and dominance. In Hill, G.E. & McGraw, K.J. (eds.) Bird Colouration. 2. Function and Evolution, 125–193. Cambridge, MA: Harvard University Press.
- Sih, A., Bell, A.M., Johnson, J.C. & Ziemba, R.E. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79: 241–277. doi: 10.1086/422893
- Sternalski, A. & Bretagnolle, V. 2010. Experimental evidence of specialized phenotypic roles in a mobbing raptor. Behav. Ecol. Sociobiol. 64: 1351–1361. doi: 10.1007/s00265-010-0950-z
- Trut, L.N. 1999. Early canid domestication: the farm-fox experiment. Am. Sci. 87: 160–169. doi: 10.1511/1999.2.160
- Van den Brink, V., Dolivo, V., Falourd, X., Dreiss, A. & Roulin, A. 2012. Melanic colour-dependent anti-predator behaviour strategies in barn owl nestlings. Behav. Ecol. 23: 473–480. doi: 10.1093/beheco/arr213
- Wolf, M., van Doorn, G.S., Leimar, O. & Weissing, F.J. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447: 581–584. doi: 10.1038/nature05835
