Abstract
Capsule The recent population decline of Whinchats has accelerated, including core breeding areas of Britain. Contrasting patterns of change with Stonechat suggest a large-scale environmental driver is affecting the entire Whinchat population.
Aims To explore broad geographical and landscape related differences in long-term patterns of population change in the Whinchat and Stonechat across Britain to identify candidate mechanisms of change.
Methods The study uses 40 years of large-scale, long-term data from a series of three atlas studies to compare trends in range and abundance in Whinchats and Stonechats relative to landscape and weather variables.
Results For Whinchats there has been a long-term and accelerating decline in abundance, that includes stronghold areas of Britain. The Stonechat population has undergone a net gain in abundance with regional and altitudinal variations. These two very different patterns of change suggest the relative ubiquity of decline in Whinchats has a common source affecting the whole population.
Conclusions The scale and magnitude of decline in Whinchats should stimulate a revision of the species conservation status in Britain, with renewed focus on studying the species' ecology across its breeding and winter range in order to determine the likely large-scale drivers of its decline.
The Whinchat Saxicola rubetra is one of several sub-Saharan migrant species that breed in Europe that have suffered major long-term declines in abundance (Sanderson et al. Citation2006, Ockendon et al. Citation2012). The Whinchat has undergone an estimated 71% decline in abundance in Europe since the 1980 (EBCC Citation2012). In Britain, the species has declined by 60% in abundance since 1995 (Risely et al. Citation2013), though the true period of decline spans several decades (Holloway Citation1996). The cause of the decline in Whinchats in the last decade or so is poorly understood with changes in either land-use or climate in either Europe or in Africa being implicated (Vickery et al. Citation2014).
In Europe, the Whinchat is a species of open, invertebrate-rich grasslands, sometimes in the presence of light scrub or other perches such as Bracken Pteridium aquilinum (Stillman & Brown Citation1994, Fuller Citation2012, Grant & Pearce-Higgins Citation2012). In winter, in Africa, the species also uses open grassy steppes, wetlands and crops, such as maize (Hulme & Cresswell Citation2012). In both seasons their habitat is vulnerable to agricultural improvements, pesticide use and the loss of marginal habitats. In addition, in Europe, the Whinchat occupies a cool temperate range by occurring at higher densities in moist alluvial grassland habitats, meadows or bogs or lush upland landscapes (Hagemeier & Blair Citation1997, Müller et al. Citation2005). In Africa, low rainfall may contribute to higher Whinchat mortality (Dejaifve Citation1994), such that, in both seasons, Whinchats are potentially vulnerable to a drying climate.
In Britain, the broad geographic and topographic breeding range of Whinchats strongly overlaps with the closely related but less migratory Stonechat Saxicola torquata. The broad-scale habitats they occupy are frequently, superficially similar (Fuller & Glue Citation1977) though Stonechats will use drier components, heathlands and Mediterranean habitats (Hagemeier & Blair Citation1997). Unlike the Whinchat, the overall population trend for breeding Stonechats in Britain has shown a net increase over the last two decades (Risely et al. Citation2013; www.BTO/about-birds/birdtrends/2012). Their contrasting trends suggest there has been an opposing demographic response to common features of environmental change acting on both species in summer, or they have been exposed to different environmental drivers outside the breeding season. For either species, ubiquitous population change would imply that virtually the entire population has been affected at once, whereas regionally contrasting trends would imply context dependent exposure to environmental change by different sub-populations. Thus, a comparison of the direction and pattern of trends in these two species had the potential to offer clues on the drivers of change.
In Britain, two large-scale data sets are available for analysing population change in birds. First, periodic national atlas data are available from surveys carried out at approximately 20 year intervals. To date, three ‘atlas’ surveys of breeding birds have been conducted since 1968 with the latest Bird Atlas 2007–11 (Balmer et al. Citation2013) providing up-to-date data on range occupancy and relative abundance change for a 40 year and 20 year period, respectively, at a 10 km scale of resolution. Second, populations of common bird species are monitored annually by the BTO/JNCC/RSPB Breeding Bird Survey (BBS; Gregory et al. Citation2004), based on approximately 2500 1 km squares (Risely et al. Citation2013). Although this survey achieves light coverage in some remote habitats in Britain (Cook et al. Citation2011), it complements the geographic coverage of atlas studies with a more rigid sampling protocol at a higher spatial resolution (1 km scale). The BBS characteristics and finer scale of survey allowed an analysis of change to be measured in terms of elevation as well as latitude, though without the more complete spatial coverage of the atlas data. These two analyses complement each in analysing different factors, but also provide a second independent layer of evidence for assessing patterns of change emanating from the two different survey methods.
In Britain, it is also possible to match national bird data sets to landscape characteristics and weather patterns at large spatial scales. Land-class data (Institute of Terrestrial Ecology land classification; Bunce et al. Citation1998) and Land Cover Map data (Fuller et al. Citation2002) are objective characterizations (generalizations) of the British countryside. The data, along with weather data (Perry et al. Citation2009), provide the environmental context against which to analyse the population change characteristics of these bird species. Our analysis is restricted to Britain only, because comparable land classification data were unavailable for Ireland as a geographic entity.
This paper set out to identify broad geographical, altitudinal, weather and landscape related differences in the 40 year and 20 year patterns of population change observed in the Whinchat and Stonechat across Britain. On national and regional level in the last 20 years, the two species' broadly sympatric breeding ranges (Balmer et al. Citation2013) and habitat use suggest that contrasting populations trends are not readily explained by large-scale changes to habitat extent per se. Even competition between the two species has been raised as a potential source of change (Phillips Citation1970) but a more likely explanation is that of emerging climate niches that the birds may or may not be able to exploit (Jiguet et al. Citation2009) or other aspect of their contrasting life-history traits. Thus, interactions between warming weather patterns and habitat may favour the resident/short-distance migrant Stonechat, more so than in the Whinchat, due to the former's potential to exploit spring temperature amelioration, to extend the breeding season forward. Interactions with habitat might then be expected too, with geographic variations in population trends relating to local climate and landscape context. In contrast, as a late migrant to Britain, Whinchats are less able to exploit variations in spring temperatures and instead are exposed to external drivers operating across the entire migrant population until arriving Britain where habitats and summer conditions may vary and change over the long term. So it was important in this study to assess the extent of population change in relation to regional, landscape, latitude and elevation gradients, in order to understand the context behind population change in these two species. Key differences in the patterns of change in population in these two species were used to indicate plausible explanations for their contrasting population trends, which for Whinchats, in particular, may help direct conservation objectives.
METHODS
Periodic national atlas monitoring: survey scale 2 km, analytical scale 10 km
Definitions and caveats
In Britain, three atlas surveys of breeding birds have been conducted since 1968, referred to here as: the ‘first atlas period’ – mid-point ‘1970’ (the 1968–1972 atlas; Sharrock Citation1976), the ‘second atlas period’ – mid-point ‘1990’ (the 1988–1991 atlas; Gibbons et al. Citation1993) and the ‘third atlas period’ – mid-point ‘2010’ (the Bird Atlas 2007–11; Balmer et al. Citation2013). The time between the first and second atlas is referred to as the ‘early 20 year period’ (broadly, 1970–1990), while the period between the second and third atlas is referred to as the ‘late 20 year period’ (broadly, 1990–2010). The survey methods are covered fully in each publication and summarized in Balmer et al. (Citation2013). In brief, survey volunteer observers chose one or more survey squares (a 2 km × 2 km ‘tetrad’) from a list. There they counted all birds seen or heard following standardized survey guidelines both for recording birds and for adding supporting information, such as breeding evidence. During the second and third atlas studies, observers followed self-determined transect routes, known as a Timed Transect Visit (TTV). Each TTV lasted for 1 hour and in some cases 2 hours. For the first atlas, birds were recorded on a ‘roving’ basis, with no set transect route, though the period of observation was recorded. During all atlases, visited tetrads received casual records made on an ad hoc basis outside of any allocated route or recording period. Casual records did not contribute to the relative abundance values because recording effort could not be taken into account (Balmer et al. Citation2013).
In Britain, atlas survey coverage is attempted in at least eight tetrads per visited 10 km square, and in virtually every 10 km square available. The survey coverage of 10 km squares during the first and the third atlas periods was broadly comparable and complete, but was lower during the second atlas period in some remote locations (Balmer et al. Citation2013). Thus, changes in occupancy between the second and third atlas periods may be, potentially, biased towards ‘gains’ in remote areas of northern Britain. Only records of ‘confirmed’ or ‘probable’ breeding status at either the beginning or end of any 20 year period of comparison were used in change statistics. However, the first and third atlases also required that birds were observed in suitable breeding habitat for breeding evidence to be considered as valid. As this stipulation was not applied to the second atlas, some apparent losses between the second and third atlases may be spurious, though this would mainly have affected transient individuals in coastal locations.
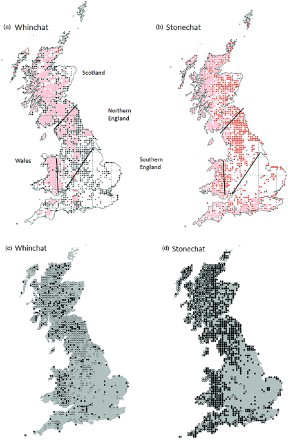
In this study it was convenient to divide Britain into four broad ‘regions’: Scotland, Wales, ‘Northern England’ (extending north and west of a line drawn between the Humber and the Severn estuaries) and ‘Southern England’ (land extending south and east of the same line, and including south-east and south-west England); see a.
Figure 1. A history of range occupancy of 10 km squares is shown for Whinchat (a) and Stonechat (b). The symbols represent: black triangles: large = loss since 1988–1991, small = old loss since 1968–1972; red triangles: large = gains since 1988–1991, small = gains since 1968–1972; pink shading = present in all atlases (maps reproduced from Balmer et al. Citation2013 with permission from the British Trust for Ornithology). The change in abundance per 10 km square between the 1988–1991 and 2007–2011 atlases is also shown for Whinchat (c) and Stonechat (d), respectively (black spots = gains, white spots = losses). Four analytical ‘regions’ were defined: Scotland, Wales, Northern and Southern England.
Range occupancy and relative abundance
Tetrad-level data outlined above were used to establish the presence or absence of birds within each 10 km square (‘range occupancy’), where presence in two consecutive atlas periods represented stable occupancy or no change. The tetrad-level data were also used to calculate a frequency index of ‘relative abundance’, from the proportion of occupied to unoccupied tetrads per 10 km square, including squares of stable range occupancy but of varying relative abundance. Change in the relative abundance between atlas periods was calculated only for 10 km squares with breeding evidence recorded in at least one atlas period, so eliminating very long-term, un-occupied squares from the analysis. In places where a species was very common, there could conceivably have been variation in density (within tetrads) without the frequency index dropping below 1, introducing some loss of sensitivity. However, for the two subject species in this paper, this scenario is considered a rare or unlikely scenario.
Change in range occupancy at the 10 km scale was analysed by χ2 tests, assuming an equal likelihood of gains to losses, and excluding range-stable squares (no change). Change in relative abundance was analysed by Generalized Linear Model logistic regression (SAS Incorporated Citation2002) testing both Poisson (log function) and binomial error structures (logit function; where 1 = positive change and 0 = negative change), to include as much information from range-stable squares as possible depending on the quality of the model fits. Parameter estimates are output as likelihood ratios (LR) with Type 3 probability values for partial effects accounting for other variables in the model, such as the environmental variables described below.
Superficially, the distribution of ‘gains’ in Whinchats appears close to random and is consistent with there being no clear regional or sub-regional patterns of colonization occurring against the national trend. Instead, only sporadic gains are apparent, perhaps as a consequence of stochastic variation in the detection of birds (following false absences during the early atlas period) or transient occurrences of territory settlement, or habitat change occurring at smaller spatial scales than the resolution of the analysis. These scenarios are more likely to occur within the current range of the species, but tend towards a random distribution of gains as squares switch between active or not (where no strong biological influence or systematic sampling bias was introduced between atlas survey periods). By comparison, redistributions of territories to patches of newly available habitat (perhaps with social attraction), would be expected to show stronger levels of aggregation than random. Regional or sub-regional clumping is expected where strong influences of land-use change and/or climate change occur.
To examine whether or not the Whinchat gains were statistically random in distribution, we compared mean nearest neighbour distances (NND) between the centres of 255 gain squares (within a 300 km buffer) and 255 randomly distributed squares (within a 300 km buffer) selected from the entire breeding distribution of the species in the last 20 years (all gains, losses and stable occupancy squares, avoiding (40+ year) long-term historical absence). The 300 km buffer was imposed to effectively regionalize the analysis – to look for local rather than gross patterns of variation across Britain as a whole. NND mean and variance was compared between gain squares and between 20 re-iterations of randomly generated squares. For the gain squares mean and confidence intervals (CIs) were calculated by bootstrapping (999 iterations with replacement), and compared to the distribution of random squares, via t- and F-tests (Sokal & Rohlf Citation1995), for signs of similarity and aggregation (and normality). A similar analysis was run for Stonechat, but this time with emphasis on the distribution of losses rather than gains.
Associations with environmental variables
Gains relative to losses were analysed against time (early and late 20 year periods), weather variables and two landscape data sets: (a) Land Cover Map data (LCM2000; Fuller et al. Citation2002) and (b) land-class data (Bunce et al. Citation1998). LCM2000 satellite image variables (Fuller et al. Citation2002) quantify proportional estimates of land cover per 1 km square in Britain (here summarized for 10 broad land-use categories being: coastal (Coast), improved grassland (IG), semi-natural grassland (SNG), Moorland/Heather/Bracken (MHB), broad-leaved woodland (BLW), conifer woodland (CON), arable, sea, inland water and unclassed). By proportion, more than one land-use category may occur in any given 1 km square and that proportion may change between survey periods. The land-class system places every 1 km square in Britain into just one of 32–40 categories (32 this study) on a descriptive basis of its landscape and topographical character (Bunce et al. Citation1998). The land-class character of a square is less prone to change over time than LCM2000. In the absence of quantitative data on land-use change between all three atlas periods, we were limited to using landscape characteristics data representing the mid-point for the late 20 year period (LCM2000 in 1998).
Weather variables were included in the analyses as a potential influence on habitat condition or productivity (spring/summer variables), or for Stonechat alone, survival (winter variable). Weather variables were available as monthly values for every 5 km2 tile in the Britain (UK Meteorological Office). For our purposes, variables ‘total rainfall’, ‘mean temperature’ and ‘minimum temperature’ were first averaged per month at the 10 km2 scale. They were then averaged across months as mean summer rainfall (MSR: May, June, July), mean summer temperature (MST: May, June, July) and mean minimum temperature for spring (MMS: March, April, May).
The difference between the proportional distribution of all squares in which birds were recorded during the late 20 year period (gains, losses and stable occupancy) across the 32 land-classes and the actual proportional availability of each land-class was calculated as the response variable ‘Preferred’ (% occurrence − % availability). ‘Difference’ was calculated as the difference in the proportional distribution of gains to losses (% gains − % loss) between land-class categories. Significant differences in the distribution of response variables between land-classes was analysed by generalized linear models with a normal error structure. This analysis was intended to identify broadly favourable landscape characteristics between regions, for species occurrence and species relative gains in occurrence.
The relationship between change in bird occurrence and LCM2000 variables (per 10 km square) was run in a separate model to the land-class data because the two are related. In this analysis, the bird data modelled change as gains (score 1) or losses (score 0) using a binomial error structure and logit link function. The modelled structure analysed the probability that change = species (Whinchat or Stonechat) + land cover LCM200 variables (MHB + BLW + CON + IG + SNG + Coast) plus weather variables (MST + MSR + MMS). Interaction terms were added to identify species-specific effects of land cover or weather. Limited screening of variable permutations below the full model structure above was carried out, manually. Overall, this analysis was intended to identify important broad habitats and weather conditions that might help explain observed patterns of change across Britain in this species.
Assessment of changes in breeding densities and range from BBS monitoring (1 km scale)
The BTO/JNCC/RSPB BBS is an extensive volunteer survey used to monitor breeding bird populations in the UK every year since 1994. The BBS is undertaken on a random sample of 1 km squares, but stratified regionally to obtain representative coverage of habitats (Risely et al. Citation2013). The survey is structured in that each square is visited twice, once between April and mid-May (early visit), and once between mid-May and the end of June (late visit). Birds are recorded along two 1 km line transects with a recommended period of time spent on each transect and with sightings classified into three distance bands (0–25 m, 25–100 m, 100 m+). Each transect is split into 200 m sections, in which habitat is recorded using a hierarchical coding system (Crick Citation1992). To account for heterogeneity in detectability, we used a distance-sampling approach and fitted half normal distributions to the BBS count data (Buckland Citation2001, R Development Core Team Citation2009, Thomas et al. Citation2010). The habitat of each 200 m section and the visit (early or late visit during the breeding season) were included as covariates to account for their potential effect on detectability. From this model, we obtained an estimate of the average detection probability () in each surveyed square, which was used as an offset in the following analysis. Models were weighted by the inverse of the sampling effort within each region to account for spatial variation in the coverage of BBS squares across the country. Thus, squares from regions with low survey coverage received a greater weight in order to prevent the results being potentially biased by well-surveyed regions. However, data from Northern Ireland could not be included due to very low coverage in the early years of the BBS.
Using the maximum number of individuals detected (dependent variable) from either of the two annual visits in each year (1994–2011), for each 1 km square, species abundance was modelled using generalized additive models as a smooth function of northing, easting, elevation and year. We specified a logarithmic link function and quasi-Poisson error structure (McCullagh & Nelder Citation1989). The maximum degrees of freedom for the four-dimensional smooth were set to 16, to avoid over-fitting and keep the relationships unimodal. Heterogeneity in detectability was accounted for by using the log of the species-specific estimates of detection probability () as an offset (Renwick et al. Citation2011).
Three reference values were calculated along the latitudinal and elevation gradients of each species. The three values were located: (1) at the point on each gradient where population density peaked (PD), (2) at the leading edge of each gradient (i.e. the northern front for latitude or higher limit for elevation) and (3) at the trailing edge of each gradient (i.e. the southern tail – for latitude or lower limit for elevation). The reference values for leading and trailing edges were defined according to where the population density equalled PD * exp(−0.5) (Heegaard Citation2002, Maggini et al. Citation2011). We used the model to quantify shifts in latitude or elevation for the three reference values, between 1994 and 2011, with 95% CIs estimated by bootstrapping (n = 200). The same model could also estimate the change in population density for each species over time, irrespective of any gradient shifts in latitude or elevation.
RESULTS
National atlases
Occupancy of range
The number of 10 km squares in which Whinchats were recorded with breeding evidence declined from 1484 to 696 between 1970 and 2010, a change of −53.1% over 40 years. There was a significantly higher proportion of losses to gains during both the early and later 20 year periods for Britain as a whole (, P < 0.05 and
, P < 0.01 respectively; a), which increased from a 66% (n = 548) to 82.6% (n = 586) between the early and later 20 year periods (
, P < 0.01; a). The results indicate an accelerated loss of occupancy of 10 km squares across Britain since 1990 (also highly significant in all regions:
for Scotland, Northern England, Southern England, Wales, respectively (P < 0.01); a). Apparent stable range occupancy of 10 km squares in Britain accounted for 38% of all squares (gains 12.6% and losses 49.4%; a).
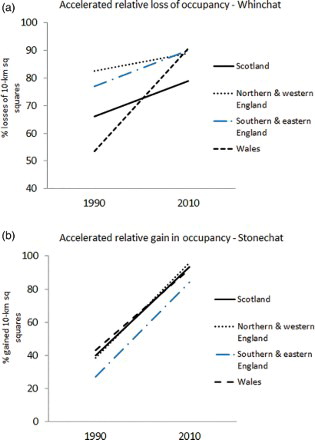
Figure 2. Change in the relative percentage of losses and gains of 10 km square occupancy between second and third atlases (broadly, 1990–2010) for Whinchat (a) and Stonechat (b) for four ‘regions’ of Britain.
For Stonechat, the number of occupied 10 km squares with breeding evidence increased from 1060 to 1634 between 1970 and 2010 (54%; b). The proportion of gains to losses was 37% (i.e. <50% and a net loss) during the early period, but increased to 89% during the late period, indicating an accelerated gain in the occupancy of 10 km squares across Britain since 1990 (also significant in all regions: for Scotland, Northern England, Southern England and Wales, respectively (P < 0.01); b). For Stonechat, apparent stable range occupancy of 10 km squares in Britain was mainly concentrated into milder north-west Scotland and coastal areas elsewhere (b).
Relative abundance
Across the Whinchat's breeding range, including squares of stable range occupancy, the relative percentage of losses to gains in abundance between 1990 and 2010 was 70%, 85%, 65% and 96% of squares, respectively, for Scotland, Northern England, Wales and Southern England (a mean of 79% losses for the whole of Britain, , P < 0.05; c). Within the range of the species the gains squares (mean NND = 119.8; CI = 7.6) were significantly closer than the ‘expected’ random distribution (mean NND = 166.2 km, CI = 19.6; F-test: F
255 = 2.2, P < 0.01) and likewise when compared to a distribution of randomly selected losses squares (mean NND = 167.2 km, CI = 26.2, n = 255 squares; F
255 = 2.2, P < 0.01). Thus, the apparent sporadic distribution of gains () masks, localized, small-scale aggregations (re-distributions) of birds.
For Stonechat, the relative percentage of gains to losses in abundance between 1990 and 2010 was 70%, 92% and 77% and 75% of squares, respectively, for Scotland, North and West England, Wales and South and East England; a mean of 78.4% gains across Britain (, P < 0.0001; d). For Stonechat, the mean NND for losses was significantly closer than the mean NND for randomly placed squares (151.0 km; CI = 6.1 and 170.3 km; CI = 17.8; respectively; F
356 = 3.8, P < 0.01), indicative of stronger than ‘expected’ aggregation among losses.
Associations with environmental variables and inter-specifics
The distribution of abundance gains and losses by landscape variables is summarized in . Note, the landscape analyses were restricted to only those land-classes present in each region and to squares that were occupied during the second or third atlas periods (i.e. gains and losses), in this way excluding 40 year, long-term absences. Thus, for both the Whinchat and Stonechat there were significant differences in the proportional distribution of occupied squares between land-classes (). Also, there were significant differences in the proportional distribution of gains and losses between land-classes. For Whinchats, ‘occupied’ squares were over-represented on hill slopes and plateaux but under-represented in coastal habitats and lowland plains, except for calcareous hills in the southern and eastern England region. Whinchat gains were associated with landscape characteristics supporting agriculturally marginal habitats, but some losses were associated with these habitats too (hillsides and slopes) as well as lowland plains (). Stonechat occupancy fitted a broader profile, including hill slopes, ‘complex valleys’ and coastal habitats. Gains were especially well represented in hilly areas and losses with all landscape types but especially lowland plains and coastal areas (significant negative relationships in ).
Table 1. A regional summary of the distribution of Whinchat and Stonechat atlas change values by land-class category. ‘Preference’ is the proportional occurrence (gains + losses) across land-classes relative to the proportional availability of each land-class category. ‘Difference’ refers to the difference between the proportions of gains to losses associated with each land-class category. The significance of the overall model for each category of ‘Preference’ or ‘Difference’ is given in column 5 with probabilities presented as **P < 0.001, ***P < 0.0001, meaning that significant differences were detected between land-class codes. Columns 2 and to 4 identify which land-class codes contributed to that overall result with a significant positive (+) or negative association (−) with the bird data, where P < 0.05. For those in parenthesis, P < 0.1. A summary description is given to help interpret which land-class characteristics emerged as contributing factors. For official definitions of each land-class code, see Bunce et al. (Citation1998).
Late period abundance change among Whinchats was not significantly associated with any land cover LCM2000 variable, bar a negative association with IG and CON (all tree-age categories combined; ). Although some young growth stage conifer plantations will have supported Whinchats (Bibby et al. Citation1985, Calladine & Bray Citation2012), appropriate data on woodland age classes were not available and hence the over-riding observation is for avoidance of forests. For Stonechat, there was a negative association (more losses than gains) with MHB category, and a positive association with SNG (implying some net colonization of the grassier upland mosaics).
Table 2. An analysis of the association between Land Cover Map and weather variables and the change in abundance of Whinchats and Stonechats in Britain between second and third atlas periods (broadly, 1990–2010). The statistics are Type 3, χ2 effect values and probabilities P. Model fit: dev./df = 1.02.
For Whinchats, there was no significant association with weather variables but for Stonechat there was a positive relationship with mean minimum spring temperature ().
There was no clear evidence of Stonechats contributing to the decline in Whinchats. Though the population trends of the two species have diverged in the last 20 years (), the proportion of losses to gains in abundance for Whinchat was lower in squares with Stonechat gains or presence than for squares with Stonechat losses or absence (LR: , P < 0.001) except in Wales where there was no significant relationship (LR:
, P = 0.77).
Assessment of changes in breeding density and range from annual BBS monitoring (1 km scale)
The BBS modelling of the Whinchat's distribution (mean = 73 occupied squares per year, 137 individuals) showed a strong and significant decline in density between 1994 and 2011. At both the northern (leading) and southern (trailing) edges of the distribution, the abundance declined by −2.7 birds/km2 (P < 0.01; 95% CI = –5.2 to −1.5 for the southern and −5.3 to −1.5 for the northern edge) and at the peak centre of the distribution (centred on northern England) by −4.5 birds/km2 (P < 0.01; 95% CI = −8.7 to −2.5). No significant shift in latitude was detected (a). A general gain in altitude was apparent but was marginally non-significant (the optimal point shifted by +92 m, P = 0.08, ns; 95% CI = −6 to 206 m, and the upper edge centre point shifted by +92 m, P = 0.09; 95% CI = −4 to 205 m; b). Although the model fit was not very strong (deviance = 0.24) the data imply a decline of abundance rather than a decline in range, and an indication of a marginal shift to higher altitudes.
Figure 3. Density change between 1994 and 2011 for Whinchat and Stonechat. The dashed line shows the modelled 1994 distribution and the solid line shows the modelled 2011 distribution, in relation to (a and c) northing and (b and d) elevation. The triangles represent the peak densities for each species and the circles define the leading and trailing edge of each distribution. For the Whinchat, the relative position of the reference points between the two distributions indicates a decline in density but no significant shift in latitude or altitude (marginal change). For the Stonechat, densities imply a general increase, especially at higher latitudes and higher altitudes, where the altitudinal optimum has shifted to a much higher elevation.
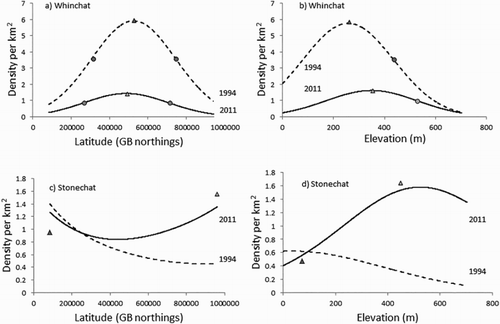
For Stonechat, the model distribution was very different from Whinchat. As the latitudinal gradient of its density is rather flat, no latitudinal reference point could be reliably located. Even the location of its peak (or optimum) density was estimated with CIs that spanned across the whole latitudinal range of Britain (c). On the other hand, the Stonechat showed a significant upward shift of its optimal altitudinal point by 490 m, although uncertainty around this estimate was also high (CI = 157–637 m; d). There was a significant increase in density by 1.0 birds/km (P < 0.01; 98% CI = 0.7–1.8) at the optimal altitudinal point. Although the model fit was weak (deviance = 0.18) the data imply an expansion of range in the north and into higher altitudes, upland landscapes for Stonechat.
DISCUSSION
Over the 40 years since 1970, the pattern of change in the status of breeding Whinchats has been characterized by a strong and accelerating decline in abundance throughout the species' core breeding range, including stronghold areas of northern and western Britain in later years. This conclusion may be in contrast to perceptions that the species has maintained stability within habitats and landscapes that support higher breeding densities (Gibbons et al. Citation1993, Eaton et al. Citation2011). But with new atlas data increasing the temporal and geographic scope of such analyses, data reveal strong abundance declines rather than range shift for this species. Only a patchy distribution of abundance gains was evident, with no clear regional geographic bias beyond the current breeding range of the species and losses were also associated with important hillside habitats too. This pattern of gains reflects either sampling stochasticity or small-scale redistributions of birds into newly created habitats, principally on hillside slopes and possibly in habitats such as new young-stage planation forest. This pattern of change is primarily unrelated to regional variations in breeding habitat or weather, that may be evident for other long-distance migrant species, such as the Willow Warbler Phylloscopus trochilus (cf. Morrison et al. Citation2010, Balmer et al. Citation2013). For Whinchat, the only strong habitat association with change was with IG (an expected negative result for a highly sub-optimal breeding habitat). However, a weak non-significant, but nevertheless negative relationship with SNG (potentially optimal breeding habitat; Grant & Pearce-Higgins Citation2012) supports the broader view that the species has declined even in ‘preferred’ habitats. A negative relationship with conifer plantations suffered from there being no distinction available between plantations age classes. Whinchats will use the grassy matrix of very young trees but are ultimately displaced by ageing forest plantations (Grant & Pearce-Higgins Citation2012).
For Stonechat, a complex change in distribution follows an entirely different pattern to Whinchat. For Stonechat, there have been both regional and altitudinal differences in change involving a northward and west-coastal expansion of range (increase in density) and a detectable change in altitude. Unlike the Willow Warbler, for example (Morrison et al. Citation2010), there is no detectable shift of the southern trailing edge of the species' breeding distribution in Britain which may be centred much further south in mainland Europe. Still, regional differences in the pattern of change combined with a net northward population expansion over the last 40 years implies the species may have benefited from long-term ameliorations of winter or spring temperatures. This conclusion was supported in the present study by a correlation between atlas change in abundance and spring temperatures. Unlike, the Whinchat, the Stonechat's life-history strategy as a short-distance migrant and earlier breeding species allows it to benefit from milder winters and springs by exploiting emerging climate niches (Jiguet et al. Citation2009, Zollinger Citation2011). If anything, the climate niche of Whinchat in hillside grassland mosaics may be being squeezed (Calladine & Bray Citation2012).
There was no evidence of competition between Whinchats and Stonechat (Phillips Citation1970) driving the population decline in Whinchats. Behavioural dominance does not imply competitive exclusion and there has been no convincing demonstration of competitive exclusion operating in these species. These species' broadly, superficially similar breeding ranges mask important fine-scale differences in habitat preference and patterns of change undoubtedly reduce competition (Stonechat: taller, drier scrub and ruderal vegetation; Whinchat: more open, even humid grassland, Bracken often with light scrub; Urquhart & Browley Citation2002). Instead, the patterns of change in the two species appear entirely independent of one another. They are more consistent with their respective life-history traits than competition or a common cause of change pertaining to the breeding population (such as predation) as plausible drivers of large-scale population change.
In Britain, these two species are broadly sympatric in distribution and in habitat occupancy. Their contrasting population trends and different patterns of change do not suggest the influence of a large-scale (national or regional) common driver of change in landscape structure or suitability (such as forestry) on the breeding grounds in the last 20 years, because this would have affected both species. The comparison between the two species also controls for any possibility of systematic biases in atlas coverage contributing to the observed patterns of change. Still, the ubiquity of decline in Whinchats indicates a large-scale environmental driver is operating across the majority of the population at once, not least in core areas of the breeding range in Britain, with formerly high densities. This pattern of change is not consistent with a pattern of range contraction into core habitats, and the sporadic gains suggest that only localized re-distributions of the population have occurred. Whinchats are a late breeding species with a short breeding season, otherwise exposed to environmental complexities in Africa and during migration over long distances (Vickery et al. Citation2014). Given the geographic extent of the decline, which has included core breeding habitats and landscapes (unlike Stonechat), reduced survival during the winter or on migration tends to emerge as the strongest recent candidate source of population ‘pressure’. This is not to say that change could not have been exacerbated by changes in habitat suitability during the breeding season given subtle differences in habitat use between Whinchats and Stonechats that could be contributing to their contrasting trends. For example, Whinchats may be more vulnerable to disruptions in peak summer abundances of biomass on the breeding grounds than Stonechat due to a short breeding season. The Stonechats' longer breeding season may buffer against pressure points caused by food shortage or even predation. Also the Whinchats' finer grassland habitat use may be more vulnerable to grazing intensification and grassland improvement measures than the courser, drier, ruderal vegetation used by Stonechats (Fuller & Gough Citation1999).
The Whinchats' present relationship, in Britain, with incidentally ‘protected’ grassland mosaics, among Bracken, less accessible hillside scrub mosaics such as ffridd in Wales (Fuller et al. Citation2006), or within young plantations, emphasizes an increasing reliance upon rare habitats, protected mainly by circumstances of topography (difficult terrain), legislation (protected or military sites or habitats) or fencing (forest plantations). Also on mainland Europe, the species persists where strict agri-environment schemes (Birrer et al. Citation2007) or mowing controls (Müller et al. Citation2005, Grüebler et al. Citation2008, Broyer et al. Citation2014) are imposed. Such change may not have been detected in the present study due to the bird (atlas) data not matching directly to the historical landscape data. Moreover, Whinchats can still occur at outstanding densities (60 pairs/ha) in lowland wet grasslands under ‘traditional’ low intensity management (M. Tome, pers. comm.). Their persistence in such high quality habitats implies that large scale, low input grassland habitats still offer legitimate conditions for viable breeding populations, with evidence of this effect on Salisbury Plain in lowland England where a 400-pair, strong population persists on a very large, extensively managed and largely protected grassland (Henderson Citation2011). Yet, with the species is now disappearing from previously occupied and apparently suitable habitats in Britain (Brown & Grice Citation2005), and in contrast to Stonechats, the winter or stop-over ecology of Whinchats emerges again as a convincing source of recent population decline in this species. If this is true then we can expect the current declining population trend to affect even the strongest existing breeding populations and especially in small and/or increasingly isolated locations.
Whinchats are exposed to potential migratory and African obstacles (Vickery et al. Citation2014). In at least one context there is evidence from Nigeria of conditions currently being favourable for this species (Hulme & Cresswell Citation2012), shifting the focus for population decline on to the stop-over ecology of this species. Unfortunately, no ecological work has yet been done on the British population of Whinchats in Africa that probably lies mainly to the west of Nigeria. There is an urgent need to identify the wintering range of the species breeding in different regions of Europe to help rule out or pinpoint key determinants of survival operating on different sub-populations.
At the beginning of the 20th century breeding Whinchats were common and widespread throughout the British lowlands (Witherby et al. (Citation1938–Citation1941) Citation1943, Alexander & Lack Citation1944). An initial phase of large-scale decline (Parslow Citation1973) was recognized by the time of the first national atlas of birds in 1968–1970 (Sharrock Citation1976) and was concurrent with large-scale, post-war programmes of land conversion for agriculture. Similar patterns of decline and range change have been observed in other countries such as Switzerland (Britschgi et al. Citation2006), Germany (Fischer et al. Citation2013) and France (Archaux Citation2007, Broyer Citation2009). Evidently, gross habitat loss has probably shaped the broad distribution of this species in Britain, and both habitat availability and predation pressure are known to impart local and potentially exacerbating constraints on colonization and productivity (Grant & Pearce-Higgins Citation2012). A reversal of trend may be possible locally by re-installing suitable habitat management methods at a suitable, probably large, spatial scale. But once again, the ubiquity of the decline in Whinchats in the last 20 years suggests a common external driver operating on virtually the entire population, simultaneously. Whinchats have become all but extinct as a breeding bird in most lowland regions (Balmer et al. Citation2013) of southern Britain and on this evidence will remain vulnerable to rapid decline especially on remaining relatively isolated colonies. On the strength of its decline, the species was moved from the green to the amber list of conservation concern (Eaton et al. Citation2011). However the scale and magnitude of decline appears more acute than assumed, which perhaps should stimulate a revision of the species conservation status.
ACKNOWLEDGEMENTS
Thank you to all volunteers and organizers contributing to the BTO/JNCC/RSPB Breeding Bird Survey and the Bird Atlas 2007–11, which was a joint project between BTO, BirdWatch Ireland and the Scottish Ornithologists' Club. Maps are reproduced with permission from the British Trust for Ornithology.
FUNDING
The study was funded by the British Trust for Ornithology.
REFERENCES
- Alexander, W.B. & Lack, D. 1944. Changes in status among British breeding birds. Br. Birds 38: 62–69.
- Archaux, F. 2007. Are mountains refuges for farmland bird species? A case study in the northern French Alps. Bird Study 54: 73–79.
- Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. & Fuller, R.J. 2013. Bird Atlas 2007–11: The Breeding and Wintering Birds of Britain and Ireland. BTO Books, Thetford.
- Bibby, C.J., Philips, B.N. & Seddon, A.J.E. 1985. Birds of restocked conifer plantations in Wales. J. Appl. Ecol. 22: 619–633.
- Birrer, S., Spiess, M., Herzog, F., Jenny, M., Kohli, L. & Lugrin, B. 2007. The Swiss agri-environment scheme promotes farmland birds: but only moderately. J. Ornithol. 148: 295–303.
- Britschgi, A., Spaar, R. & Arlettaz, R. 2006. Impact of grassland farming intensification on the breeding ecology of an indicator insectivorous passerine, the Whinchat Saxicola rubetra: lessons for overall Alpine meadowland management. Biol. Conserv. 130: 193–205.
- Brown, A.F. & Grice, P.V. 2005. Birds in England. T. & A.D. Poyser, London.
- Broyer, J. 2009. Whinchat Saxicola rubetra reproductive success according to hay cutting schedule and meadow passerine density in alluvial and upland meadows in France. J. Nat. Conserv. 17: 160–167.
- Broyer, J., Sukhanova, O. & Mischenko, A. 2014. Mowing management and density dependence in meadow passerine hatching success. Bird Study 61: 394–403.
- Buckland, S.T., Anderson, D.R., Burnham, K.P., Laake, J.L., Borchers, D.L. & Thomas, L. 2001. Introduction to Distance Sampling, Oxford University Press, Oxford.
- Bunce, R.G.H., Barr, C.J., Clarke, R.T., Howard, D.C. & Lane, A.M.J. 1998. ITE Land Classification of Great Britain 1998. NERC-Environmental Information Data Centre. doi:10.5285/971671a6-98b4-4d80-b165-21dace7373b9
- Calladine, J. & Bray, J. 2012. The importance of altitude and aspect for breeding Whinchats Saxicola rubetra in the uplands: limitations of the uplands as a refuge for a declining, formerly widespread species? Bird Study 59: 43–51.
- Cook, M., Waltho, C., Evans, I. & Wernham, C. 2011. Why bird monitoring in Scotland needs more volunteers. In Marrs, S.J., Foster, S., Hendrie, C., Mackey, E.C. & Thompson, D.B.A. (eds) The Changing Nature of Scotland, 139–144. TSO Scotland, Edinburgh.
- Crick, H.Q.P. 1992. A bird-habitat coding system for use in Britain and Ireland incorporating aspects of land-management and human activity. Bird Study 39: 1–12.
- Dejaifve, P.A. 1994. Ecology and behaviour of a palearctic migrant in Africa – the wintering of the Whinchat Saxicola rubetra in the Zaire and its winter distribution in Africa. Rev. Ecol. (Terre Vie) 49: 35–52.
- Eaton, M.A., Balmer, D.E., Cuthbert, R., Grice, P.V., Hall, J., Hearn, R.D., Holt, C.A., Musgrove, A.J., Noble, D.G., Parsons, M., Risely, K., Stroud, D.A. & Wotton, S. 2011. The State of the UK's Birds 2011. RSPB, BTO, WWT, CCW, JNCC, NE, NIEA and SNH, Sandy, Bedfordshire.
- EBCC. 2012. European Bird Census Council. Available from: http://www.ebcc.info
- Fischer, K., Busch, R., Fahl, G., Kunz, M. & Knopf, M. 2013. Habitat preferences and breeding success of Whinchats (Saxicola rubetra) in the Westerwald mountain range. J. Ornithol. 154: 339–349.
- Fuller, R.J. 2012. Avian responses to transitional habitats in temperate cultural landscapes: woodland edges and young-growth. In Fuller, R.J. (ed.) Birds and Habitats: Relationships in Changing Landscapes, 125–149. Cambridge University Press, Cambridge.
- Fuller, R.J. & Glue, D.E. 1977. The breeding biology of the Stonechat and Whinchat. Bird Study 24: 215–228.
- Fuller, R.J. & Gough, S.J. 1999. Changes in sheep numbers in Britain: implications for bird populations. Biol. Conserv. 91: 73–89.
- Fuller, R.M., Smith, G.M., Sanderson, J.M., Hill, R.A. & Thomson, A.G. 2002. The UK Land Cover Map 2000: construction of a parcel-based vector map from satellite images. Cartogr. J. 39: 15–25.
- Fuller, R.J., Atkinson, P.W., Garnett, M.C., Conway, G.J., Bibby, C.J. & Johnstone, I.G. 2006. Breeding bird communities in the upland margins (ffridd) of Wales in the mid-1980s. Bird Study 53: 177–186.
- Gibbons, D.W., Reid, J.B. & Chapman, R.A. 1993. The New Atlas of Breeding Birds in Britain and Ireland: 1988–1991. T.& A.D. Poyser, London.
- Grant, M.C. & Pearce-Higgins, J.W. 2012. Spatial variation and habitat relationships in moorland bird assemblages: a British perspective. In Fuller, R.J. (ed.) Birds and Habitat. Relationships in Changing Landscapes, 207–236. Cambridge University Press, Cambridge.
- Gregory, R.D., Baillie, S.R. & Bashford, R.I. 2004. Monitoring breeding birds in the United Kingdom. Bird numbers 1995. In: A. Anselin, ed. Proceedings of the international conference and 13th meeting of the European Bird Census Council, Pärnu, Estonia. Bird Census News, 13 (2000): 101–112.
- Grüebler, M.U., Schuler, H., Müller, M., Spaar, R., Horch, P. & Naef-Daenzer, B. 2008. Female biased mortality caused by anthropogenic nest loss contributes to population decline and adult sex ratio of a meadow bird. J. Ornithol. 146: 14–23.
- Hagemeier, W.J.M. & Blair, M.J. (eds) 1997. The EBCC Atlas of European Breeding Birds: Their Distribution and Abundance. Poyser, London.
- Heegaard, E. 2002. The outer border and central border for species-environmental relationships estimated by non-parametric generalised additive models. Ecol. Model. 157: 131–139.
- Henderson, I.G. 2011. Fighting for Whinchats on Salisbury plain. BTO News, 293, pp. 10–11.
- Holloway, S. 1996. The Historical Atlas of Breeding Birds in Britain and Ireland 1875–1900. T. & A.D. Poyser, London.
- Hulme, M.F. & Cresswell, W. 2012. Density and behaviour of Whinchats Saxicola rubetra on African farmland suggest that winter habitat conditions do not limit European breeding populations. Ibis 154: 680–692.
- Jiguet, F., Gregory, R.D., Devictor, V., Green, R.E., Voříšek, P., Van Strien, A. & Couvet, D. 2009. Population trends of European common birds are predicted by characteristics of their climatic niche. Global Change Biol. 16: 497–505.
- Maggini, R., Lehmann, A., Kéry, M., Schmid, H., Beniston, M., Jenni, L. & Zbinden, N. 2011. Are Swiss birds tracking climate change? Detecting elevational shifts using response curve shapes. Ecol. Model. 222: 21–32.
- McCullagh, P. & Nelder, J.A. 1989. Generalized Linear Models. Chapman & Hall, London.
- Morrison, C.A., Robinson, R.A., Clark, J.A. & Gill, J.A. 2010. Spatial and temporal variation in population trends in a long-distance migratory bird. Diversity Distrib. 16: 620–627.
- Müller, M., Spaar, R., Schifferli, L. & Jenni, L. 2005. Effects of changes in farming of subalpine meadows on a migrant bird, the Whinchat (Saxicola rubetra). J. Ornithol. 146: 14–23.
- Ockendon, N., Hewson, C.M., Johnston, A. & Atkinson, P.W. 2012. Declines in British-breeding populations of Afro-Palaearctic migrant birds are linked to bioclimatic wintering zone in Africa, possibly via constraints on arrival time advancement. Bird Study 59: 111–125.
- Parslow, J. 1973. Breeding Birds of Britain and Ireland: A Historical Survey. T.& A.D. Poyser, Berkhamsted.
- Perry, M., Hollis, D. & Elms, M. 2009. The Generation of Daily Gridded Datasets of Temperature and Rainfall for the UK. Climate Memorandum 24. National Climate Information Centre, Exeter.
- Phillips, J.S. 1970. Inter-specific competition in Stonechat and Whinchat. Bird Study 17: 320–324.
- R Development Core Team. 2009. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Renwick, A.R., Massimino, D., Newson, S.E., Chamberlain, D.E., Pearce-Higgins, J.W. & Johnston, A. 2011. Modelling changes in species' abundance in response to projected climate change. Divers. Distrib. 18: 121–132.
- Risely, K., Massimino, D., Newson, S.E., Eaton, M.A., Musgrove, A.J., Noble, D.G., Procter, D. & Baillie, S.R. 2013. The Breeding Bird Survey 2012. BTO Research Report 645. British Trust for Ornithology, Thetford.
- Sanderson, F.J., Donald, P.F., Pain, D.J., Burfield, I.J. & van Bommel, F.P.J. 2006. Long-term population declines in Afro-Palearctic migrant birds. Biol. Conserv. 131: 93–105.
- SAS. 2002. Statistical Analysis System: Version 9.1. SAS Institute, Cary, NC.
- Sharrock, J.T.R. 1976. The Atlas of Breeding Birds in Britain and Ireland. T. & A. D. Poyser, Berkhamsted.
- Sokal, R.R. & Rohlf, F.J. 1995. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd edn. W.H. Freeman, New York.
- Stillman, R.A. & Brown, A.F. 1994. Population sizes and habitat associations of upland breeding birds in the south Pennines, England. Biol. Conserv. 69: 307–314.
- Thomas, L., Buckland, S.T., Rexstad, E.A., Laake, J.L., Strindberg, S., Hedley, S.L., Bishop, J.R.B., Marques, T.A., Burnham, K.P. 2010. Distance software: design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 47: 5–14.
- Urquhart, E. & Bowley, A. 2002. Stonechats. A Guide to the Genus Saxicola. Christopher Helm, London.
- Vickery, J.A., Ewing, S.R., Smith, K.W., Pain, D.J., Bairlein, F., Škorpilova, J. & Gregory, R.D. 2014. The decline of Afro-Palaearctic migrants and an assessment of potential causes. Ibis 156: 1–22.
- Witherby (Ed), H.F., Jourdain, F.C.R., Ticehurst, N.F., Tucker, B.W. (1936–1941). 1943. The Handbook of British Birds. H.F. & G. Witherby Ltd, London.
- Zollinger, J.L. 2011. L'expansion du Tarier pâtre Saxicola torquatus au pied du Jura vaudois. Nos Oiseaux 58: 127–144.
