Abstract
Capsule The number of Dotterel breeding in the UK declined by 57% between 1987/88 and 2011, from 980 to 423 breeding males; there has been a contraction of the species’ geographical range as well as declining numbers on core sites.
Aims To estimate the number of Dotterel breeding in the UK in 2011, the changes since surveys in 1987–88 and 1999, and to document broad patterns of change in distribution.
Methods Two survey visits were carried out at montane sites (protected areas designated for Dotterel; and a stratified random sample of other sites >600 m altitude) across the Dotterel's breeding range using a standardized protocol as used in previous surveys. The first visit was to assess numbers in the pre-breeding period, and the second visit, comparable to those made in previous national surveys, provided data to calculate population estimates through extrapolation to unsurveyed sites and 95% confidence intervals by bootstrapping.
Results In 2011, the number of Dotterel in the UK was estimated to be 423 breeding males (95% CI 279–644), a decline of 57% since 1987/88 and 43% since 1999. As in previous surveys, the majority of the population (61%) was found in the East Highlands, but large declines were recorded in this region. Dotterels were largely absent from previously occupied sites in North and West Highlands.
Conclusions Numbers of Dotterel breeding in the UK declined by more than half between 1987 and 2011. Long-term monitoring data are rare for montane environments in the UK, but these findings may signify the occurrence of important ecological changes, with possible drivers including land-use/habitat change, nitrogen deposition and climate change.
The conservation status of birds associated with montane habitats is poorly understood in Europe and elsewhere (Chamberlain et al. Citation2012, but see Lehikoinen et al. Citation2014), reflecting the challenges of monitoring species in such remote locations. Developing a better understanding of their population trends would be informative, potentially offering wider insights into the effects of environmental stresses on their sensitive breeding habitats (Thompson & Whitfield Citation1993, Van der Wal et al. Citation2005, Welch et al. Citation2005). Climate change in particular may pose a substantial challenge to birds and other biota associated with montane environments (Chamberlain et al. Citation2012). Birds are expected to track shifts in suitable climate niches to higher altitudes in response to climate change (Huntley et al. Citation2008, Maggini et al. Citation2011, Lehikoinen et al. Citation2014), but opportunities for vertical migration may be restricted for those species (e.g. Ptarmigan Lagopus muta and Dotterel Charadrius morinellus) that occupy the higher reaches of several mountain systems, and lower sites may become unsuitable leading to range contraction (Sekercioglu et al. Citation2008, La Sorte & Jetz Citation2010). Chamberlain et al. (Citation2012) stressed that far greater emphasis needs to be placed on monitoring populations of vulnerable high-altitude bird species.
The Dotterel is a migrant shorebird that breeds in Arctic and montane habitats from northern Britain across Scandinavia and into Siberia (Cramp & Simmons Citation1983). In the UK, Dotterels occur on high mountain plateaux above 600 m, where they are particularly associated with moss–sedge (Racomitrium lanuginosum–Carex bigelowii) and Juncus trifidus heaths for both nesting and feeding (Thompson & Brown Citation1992, Galbraith et al. Citation1993a). Individuals breeding in the UK winter predominantly in north western Africa, while those breeding further east migrate predominantly to parts of north eastern Africa and the Middle East (Cramp & Simmons Citation1983, Whitfield et al. Citation1996, Whitfield Citation2002a). The Dotterel's unusual sex-role reversal in the breeding season means that males provide most of the parental care such that females can be sequentially polyandrous (Cramp & Simmons Citation1983, Kålås & Byrkjedal Citation1984, Holt et al. Citation2002). A considerable proportion of females are thought to leave Scotland after laying a first clutch to pursue additional mates in breeding areas further north and males have also been recorded in different countries within and between breeding seasons (Whitfield Citation2002a) illustrating the itinerant nature of the species.
The Dotterel is on Annex 1 of the European Communities’ Birds Directive (Council of the EC's Directive 2009/147/EC on the Conservation of Wild Birds) (Stroud et al. Citation2001) and the amber list of Birds of Conservation Concern in the UK (Eaton et al. Citation2009). Unusually amongst montane species, Dotterel in the UK have been the subject of substantial research and monitoring effort to understand their conservation status. There have been two previous national surveys of Dotterel. The first, in 1987 and 1988, estimated there to be 840 breeding males in Britain (Galbraith et al. Citation1993b), although this was later revised to 980 breeding males (Whitfield Citation2002b). The majority of the population was distributed across the Highlands of Scotland, with highest densities in the Central and Eastern Highlands, and small numbers breeding in northern England and southern Scotland. This first study enabled the designation of a suite of montane Special Protection Areas (SPAs) under the EC Birds Directive, which were estimated to support 56% of the breeding population (Stroud et al. Citation2001).
A second national survey in 1999 resulted in two population estimates: 755 males (95% CI: 590–970 males), calculated using only data from randomly selected sites; and a second estimate of 511 males (95% CL: 455–575), calculated using all survey data (including data from non-random survey coverage of designated sites) but ignoring regional differences (Whitfield Citation2002b). The large discrepancy between the two estimates, largely due to uneven distribution of SPA sites across regions, is discussed in detail in the survey paper (Whitfield Citation2002b). The authors took the midpoint of this range (630 males) as probably representing the best estimate and this figure has been reported subsequently (e.g. Musgrove et al. Citation2013). Population estimates from the 1999 survey data were lower than those from 1987/88 in all but two of the eight regions. The largest declines were recorded in the East and West Highlands of Scotland and the authors suggested that these declines may have indicated a contraction into a core area, although no obvious deterioration of site suitability was noted.
Significant threats to Dotterel in the UK may include climate change, nitrogen deposition, predation and disturbance; outside of the UK, Dotterel may be impacted on migration or on the non-breeding grounds. Evidence for any particular factor directly driving changes in the number of Dotterel in the UK is, however, currently lacking. Montane habitats are likely to be particularly vulnerable to climate change: temperature and snowlines have risen over the last century and are predicted to continue to increase especially at higher altitudes, with summer precipitation and wind speeds also likely to increase (Hazeu et al. Citation2010). A combination of atmospheric nitrogen deposition and excessive sheep grazing have been shown to lead to degradation of moss–sedge heaths and their replacement by grasses and sedge-dominated communities (Van Der Wal et al. Citation2003). Such habitat deterioration in England and Wales has been suggested as a possible reason for the absence of breeding Dotterel south of the Scottish Highlands in recent decades (Watson Citation1988, Thompson & Whitfield Citation1993, Strowger Citation1998, Whitfield Citation2002b). In addition, impacts of disturbance and predation on Dotterel numbers and breeding success have been discussed by previous authors (Watson Citation1988, Thompson & Whitfield Citation1993, Strowger Citation1998, Whitfield Citation2002b), although there is little strong evidence for widespread effects. As an itinerant migratory species, numbers in the UK can be influenced by factors on southern non-breeding grounds but also conditions on other breeding areas especially Norway (Farmer & Wiens Citation1999, Whitfield Citation2002b).
This paper reports the findings of the third UK Dotterel survey, conducted in 2011. The aims of the paper are to present: the distribution of Dotterel in the UK during the pre-breeding and breeding period; the updated estimates of the number of Dotterel in the UK; and the changes in distribution and population size since previous surveys in 1987–88 and 1999, nationally, regionally and in protected areas.
Periodic national surveys carried out in single breeding seasons can face criticism that they only present a snap shot of the population, which can be greatly influenced by conditions in the survey year and may not reflect the status of the population more generally. Following the survey in 2011, monitoring has been carried out on a subset of sites each year (for an on-going research project) and results from 2012 and 2013 are presented here. This opportunity to place the national survey data in context is rare, particularly for a montane species, and allows an assessment of how representative the single-year survey data are of the status of the population.
While the main focus of the survey in 2011 was to update the estimates for the number of Dotterel breeding in the UK, an extra survey visit was added during the pre-breeding season (May). This was a first attempt to broaden our understanding of how Dotterel are distributed throughout the whole breeding season, including the period when birds have yet to settle on breeding sites and when some birds will still be migrating.
METHODS
Site selection
Sites with potential Dotterel breeding habitat were identified and digitized for the 1999 survey using 1:25 000 Ordnance Survey maps in conjunction with those of upland plant communities (Whitfield Citation2002b). We used these sites as the basis of the 2011 survey, as while there may have been changes in extent of vegetation types in these areas there was no reason to believe that the broad distribution of habitats would have changed since 1999. The sites are made up predominantly of Racomitrium lanuginosum-dominated heath, or open Juncus trifidus fells (Thompson & Brown Citation1992, Galbraith et al. Citation1993a). Site boundaries were drawn with the intention of excluding areas of steep ground and large (>25 ha) areas of montane bog or bog/Nardus stricta grassland mosaics, which are avoided by Dotterel (Galbraith et al. Citation1993a). Sites smaller than 25 ha and those composed entirely of dwarf Calluna vulgaris heath were also excluded. The majority of suitable montane habitat is in Scotland, but some suitable sites in the north of England and Wales were also identified. The resulting potential breeding habitat comprised 469 sites covering a total of 669 km2 (Whitfield Citation2002b). The majority of these sites were discrete mountains tops above 600 m elevation (area range 0.07–11.7 km2); in some cases these were larger continuous areas partitioned into a number of smaller survey units.
Montane sites were chosen for survey in 2011 using two separate selection procedures. First, we selected a non-random subset which included all sites within the network of protected sites with Dotterel as a designating feature (SPAs and four Sites of Special Scientific Interest (SSSIs) that lie outside the SPA suite) (n = 58, area = 153 km2; , ) (hereafter referred to as SPA/SSSI sites). This approach was taken as these sites held the bulk of the population in the previous national surveys and to satisfy the statutory requirement for the monitoring of designated protected areas. The second set of sites was a stratified random sample of montane sites from the remainder of the Dotterel's identified breeding range (n = 140, area = 184 km2). These sites were selected from the same random subset of sites as in 1999 (), thus maximizing repeat coverage of sites in 1999 and 2011 to allow like for like comparison. The sample of sites was stratified regionally to achieve an even geographical distribution of sites, which ensured better sample sizes for generating regional estimates; the eight regions are shown in . Overall, 198 sites (337 km2) were selected for survey, representing approximately 50% of the potential breeding area. The approach used to select sites for survey in 2011 differed from the two previous surveys. First, it is likely that during the 1987/88 national survey, sites were not sampled randomly. Second, in 1999, previously occupied sites that held birds in 1987/88 were preferentially selected for survey. However, subsequent analyses in the planning stages of the 2011 survey showed that selecting these previously occupied sites at the expense of surveying a greater number of randomly selected sites did not appear to have had a beneficial effect on estimate precision in 1999, so this approach was dropped for the 2011 survey.
Figure 1. The distribution of sites with suitable habitat for breeding Dotterel in Britain and regional boundaries used in analyses: (1) England & Wales, (2) South Scotland, (3) East Highlands, (4) Tayside Highlands, (5) Central Highlands, (6) SW Highlands, (7) West Highlands, (8) North Highlands. Unsurveyed sites, randomly selected sites and SPA/SSSI sites including the following: Ben Alder (SSSI & SPA); Beinn Dearg (SSSI & SPA); Lochnagar (SPA); Cairngorms (SSSI & SPA); Caenlochan (SSSI & SPA); Drumochter Hills (SSSI & SPA); Creag Meagaidh (SSSI & SPA); Ben Wyvis (SSSI & SPA); Monadhliath (SSSI); Fafernie (SSSI) and Beinn A’ Ghlo (SSSI). Glas Tulaichean was also included, as it is within the amended Cairngorms SPA although Dotterel is not a specific qualifying feature of the site.
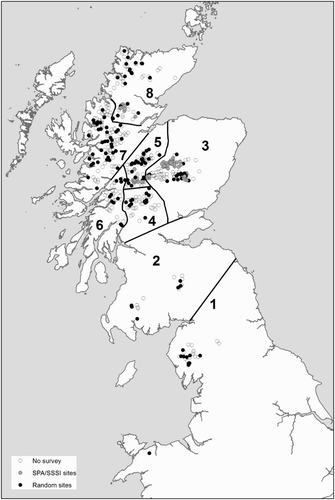
Table 1. Survey coverage of potential breeding habitat in Visit 1 and Visit 2 of the 2011 UK Dotterel survey.
Survey methods
Survey methods used here largely followed the standardized protocol developed for the 1999 survey (Whitfield Citation2002b), although an additional survey visit was made in 2011. Surveys consisted of two visits to each site. The additional early visit, in May, was intended to monitor numbers of Dotterel during the pre-breeding period as knowledge of site occupation in this period is very poor. This period is of particular importance as it is when individuals migrating to northerly breeding grounds may still be present. The second visit, between mid-June and mid-July, aimed to estimate the number of Dotterel breeding in the UK. This latter visit corresponded to the period during which surveys were carried out in 1987/88 and 1999 and occurred during the Dotterel's chick-rearing period when breeding males are at their most conspicuous and survey efficiency is consequently at its highest (Whitfield Citation2002b).
Dotterel surveys involved walking to within, at most, 100 m of every point on the site, pausing periodically and scanning the surrounding ground with binoculars for birds every 50–100 m, depending on visibility. All sightings of Dotterel were recorded including: time, six-figure grid reference, sex, age (adult/juvenile), number, activity and breeding status, and were mapped. No time limit was set for surveys, as the amount of time required varied with both topography and prevailing weather (on average surveyors spent 3.8 hour/km2). Observers recorded the start and finish time for the survey period on site, as well as recording any time when the survey was suspended due to unsuitable weather. Sites were not visited during prolonged periods of heavy precipitation, low cloud cover or strong winds (>5 Beaufort).
DATA ANALYSES
Pre-breeding survey data
Dotterel are highly mobile during the pre-breeding period (Galbraith et al. Citation1993b, Whitfield Citation2002a, Citation2002b). For this reason, numbers of Dotterel presented here from the first survey visit are the maximum number of birds seen simultaneously by each observer on the first survey visit to each site. Unlike the second survey visit, we have no knowledge of detection rates in this pre-breeding period so we cannot attempt to correct counts to estimate the actual number of birds present. It is likely, nevertheless, that a larger proportion of birds were detected than on second survey visits, because birds are more likely to gather in groups at this time of the year and are more demonstrative in their behaviour, lacking discreet anti-predation breeding behaviours.
Population estimates
Female Dotterel can be sequentially polyandrous (Cramp & Simmons Citation1983, Kålås & Byrkjedal Citation1984, Holt et al. Citation2002), so as in previous Dotterel surveys the male, rather than a pair, is considered the most appropriate unit for estimates of breeding population size. Data from the second survey visit only were used to derive a population estimate for Dotterel in the UK. Sightings made during the breeding period were assigned to one of three breeding evidence codes based on information collected during observations. The criteria for a confirmed breeder were presence of a nest or brood, or records of distraction display or injury feigning. Probable breeding included observations of agitated behaviour or anxiety calls suggesting probable presence of a nest or a brood nearby. As in 1999, any observation of a single male in suitable nesting habitat was also considered to represent a probable breeding attempt (Whitfield Citation2002b). As this survey is during the chick-rearing period, any change in breeding failure rate over time could influence the results. To assess this, the proportion of individuals showing probable or confirmed breeding behaviour between 1999 and 2011 was compared. There was no evidence for a change between national surveys. Of all the observations of Dotterel in 2011 (n = 151) 58% provided confirmed, 24% probable and 19% possible breeding evidence. In 1999, between the same dates (n = 227), 63% observations were confirmed, 25% probable and 12% possible.
Preceding the 1999 survey, a calibration exercise (comprising 14 site surveys over 5 years) demonstrated that surveyors found, on average, only 42% of total Dotterel breeding attempts during single visits to sites, and a correction factor of 2.38 (1/0.42 = 2.38) was applied to counts to correct for undetected birds (Whitfield Citation2002b). The calibration exercise used 14 individual assessments of detectability to calculate the average correction factor but this does not take into account the variability inherent in these assessments themselves. In this study we attempt to account for this variation by bootstrapping the proportion of birds found during the calibration surveys. Bootstraps were calculated by resampling the calibration survey data set with replacement to create 999 replicates (Efron Citation1982, Greenwood Citation1991) and 2.5 and 97.5 percentiles of the replicates were taken to give confidence intervals around the mean correction factor (2.38, 95% CI: 1.7–3.4). The total number of individuals observed for each site was multiplied by the correction factor, and the estimated number of breeding males was taken as the nearest whole number.
Regional population estimates for Dotterel in 2011 and 1999 were calculated by first deriving the mean density of Dotterel (total count corrected as above and divided by the area surveyed) on randomly surveyed sites and extrapolating this across unsurveyed sites in each region. These extrapolated estimates from randomly sampled sites were then added to the corrected counts from the SPA/SSSI sites to provide overall regional population estimates. The national population estimate was calculated as the sum of these regional estimates.
For each region, counts from random sites were bootstrapped (as described above) to calculate 999 replicates of random site counts ( (a1–a999)). These uncorrected replicates were then multiplied by the correction factor replicates (b1–b999). Next, these corrected random count replicates (C1–C999) were extrapolated across the unsurveyed area (y) to give extrapolated corrected random count replicates (D1). The SPA/SSSI counts (x) were also multiplied by the correction factor replicates (b1–b999) to give corrected count replicates (E1–E999). Finally, both sets of corrected replicates are summed to give 999 population estimate replicates (F1–F999) and confidence intervals were taken as the 2.5 and 97.5 percentiles. Confidence intervals for the national population estimate were derived in the same way after summing the regional bootstrap replicates. It was considered necessary to recalculate population estimates and confidence intervals from 1999 survey data using the bootstrapped correction factor for comparability with results from 2011.
Table 2. Calculation of population estimate replicates using bootstraps of random counts and correction factor estimates. Process completed for each region separately and regional population estimate replicates summed to give national population estimate replicates.
Changes in population size
The percentage change in the estimated population size between national survey estimates from 1999 and 2011 was calculated from the corrected extrapolated estimates. The statistical significance of country and regional changes was assessed by calculating a ratio for each set of bootstrap replicates (e.g. 2011 estimate/1999 estimate), with the population estimates from the two surveys considered to be significantly different if the 95% confidence intervals of these ratios do not include one (i.e. P < 0.05).
As it is likely that sites were not truly randomly selected in the 1987/88 survey, both extrapolating from survey totals and the bootstrapping approach would be invalid and, therefore, comparisons between the first survey and 2011 use the national and regional population estimates calculated by Whitfield (Citation2002b). Matched pairs comparison tests (Wilcoxon matched-pairs signed-rank tests) were used to assess the degree of change across those sites that were surveyed in all national surveys i.e.1987/88 vs. 1999 vs. 2011.
Additional monitoring
A non-random subset of sites was surveyed in 2012 (29 sites) and 2013 (33 sites) during the second visit period using the same methodology as in 2011, providing an opportunity to assess the representativeness of results from the single-year national survey. In addition, a small number of sites were monitored more intensively with multiple visits in each breeding season (2012: 3 sites (n visits = 8, 11 and 16) and 2013: 3 sites, (n visits = 3, 12 and 13)) and in these cases population estimates are counts of minimum number of breeding males recorded. Matched pairs comparison tests were used as above to assess change on sites surveyed between 2011 and the subsequent two years. All data analyses were carried out in R version 3.1.0.
RESULTS
Survey coverage
During the 2011 Dotterel survey, a pre-breeding first visit survey was made to 158 separate montane sites (101 random and 57 SPA/SSSI sites). A second visit was made to each of these sites and to an additional 39 random sites and one extra SPA/SSSI site during the breeding period (, ). Just over half of the potential breeding habitat (337 km2) in the UK was surveyed during the breeding period (). Coverage was highest in East Highlands (68%) due to the concentration of SPA/SSSI sites within this region.
Distribution during pre-breeding and breeding season
Dotterel were recorded on 42 sites (27% of sites visited) during the first visit (); 57% of observations were of single birds or pairs, but groups of up to 17 individuals were also recorded. The first visit survey located a minimum of 159 individual Dotterel, nearly half of which were found on sites in the East Highlands (47%), with a further 30% in the Central Highlands. No Dotterel were found in England and Wales, South Scotland or Tayside Highlands during the pre-breeding first survey.
Figure 2. The distribution of sites occupied during the first visit (pre-breeding) and second visit (breeding) surveys.
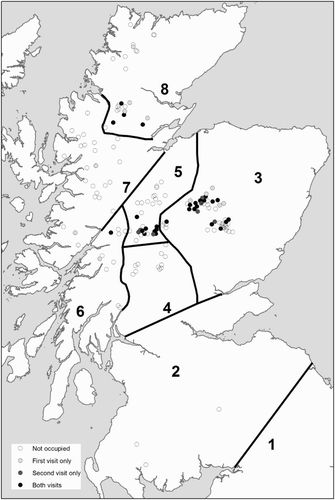
Of the 42 sites where Dotterel were recorded in the first visit in 2011, 67% had records of breeding birds in the second visit (). Site occupancy during the second visit survey (i.e. early chick-rearing period) was highest in the East and Central Highlands with evidence of breeding Dotterel recorded on over 30% of sites. Consistent with the first survey visits, there were no records of breeding Dotterel in England, Wales, the Tayside Highlands or South Scotland during the second survey visits.
Population estimate
A total of 129 breeding male Dotterel were recorded during second visit period in 2011, giving rise to a UK Dotterel population estimate of 423 males (95% CI: 279–644) (). The population was wholly confined to Scotland, with most (61%) in the East Highlands.
Data from the 1999 survey were reanalysed with analytical methods used for 2011 data for comparability. The revised population estimate for Dotterel in 1999 was 747 males (95% CI: 676–1232).
Change since 1987/88 and 1999
The proportion of sites on which Dotterel were recorded during the second visit survey was substantially lower in 2011 (19% of all sites surveyed; 10% of random sites and 41% SPA/SSSI sites) than both 1987/88 (57% of all sites surveyed) and 1999 (33% of all sites surveyed; 25% of random sites and 43% of SPA/SSSI sites) (a, ). Those areas with the greatest changes in occupancy include the West and Southwest Highlands, where rates across all sites surveyed declined from 56% and 43% in 1987/88 to 5% and 8% in 2011, respectively. Densities on occupied sites in 2011 were lower than in previous surveys in all regions, but particularly noticeably in East, Central and North Highlands (b).
Figure 3. Occupied and unoccupied sites surveyed in Scotland in each National survey; (a) 1987/88, (b) 1999 and (c) 2011.
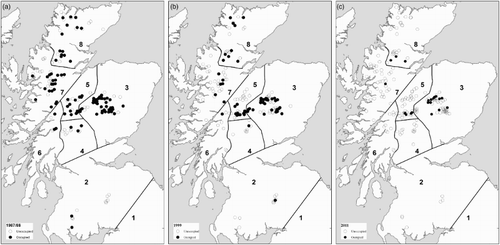
Table 3. Percentage of survey sites occupied (number sites surveyed in brackets) and regional densities of breeding Dotterel during each national survey (corrected number of breeding males/total area surveyed).
The 2011 population estimate for Dotterel was 43% lower than that for 1999 (P < 0.05, ) and 57% lower than 1987/88. Although all regions except West Highlands had lower population estimates in 2011 than for 1999, there was a significant difference (P < 0.05) in the East Highlands only; this was detectable due the greater sample size, and therefore statistical power, for this region. There were declines of between 50% and 60% in East, Central, West and South West Highlands between 1987/88 and 2011.
Table 4. Numbers of breeding male Dotterel (plus 95% confidence intervals) and estimates of population change from the three national surveys.
Pairwise comparison of sites surveyed in each of the national surveys (1987/88 and 1999 n = 105, 1987/88 and 2011 n = 93, 1999 and 2011 n = 145) shows that numbers of Dotterel breeding on these sites were significantly greater in 1987/88 and in 1999 than in 2011 (a). On the subset of 93 sites surveyed in 1987/88 and again in 2011 the total number of breeding males declined from 509 to 282 (−45%), and on the 145 sites surveyed in 1999 and again in 2011, total numbers declined from 445 to 258 (−42%). The decrease in numbers of Dotterel on this subset of sites is at a similar scale to the national decline.
Table 5. Pairwise comparisons of estimated numbers of breeding Dotterel on sites surveyed (a) in each national survey 1987/88, 1999 and 2011 and (b) in 2011 national survey and in ongoing monitoring 2012 and 2013. Z-scores, two tailed probability values and effect size (r) are shown above the diagonal (number of sites in brackets), the year with the greater number of Dotterel below the diagonal (if P< 0.05).
Sites within the 8 SPAs designated for Dotterel in the UK now support an estimated 241 breeding males (57% of the population). Calculating this SPA population estimate, using the 95% confidence intervals around the correction factor, gives a range of 179–340 breeding males, representing between 40% and 80% of the population. A similar percentage were found on these sites in 1987/88 and 1999 (56% (38–79%) and 52% (38–76%), respectively; Stroud et al. Citation2001), indicating that the population is declining within the SPA suite as well as outside of it (by 40% since 1999 and by 49% since 1987/88). Outside of the 59 sites within SPA/SSSIs, occupancy was very low with only 10% of randomly selected sites occupied across the range.
Additional monitoring
A total of 37 sites were surveyed for breeding Dotterel in at least 2 seasons between 2011 and 2013 inclusive. The total estimated number of breeding records of Dotterel in 2011 (breeding males = 203, sites n = 36), 2012 (breeding males = 140, sites n = 29) and 2013 (breeding males = 204, sites n = 34) showed some degree of variation across this subset of sites. Numbers reported in 2012 were substantially lower, but pairwise comparison of sites surveyed in 2011, 2012 and 2013 showed no statistical evidence of difference in numbers between years (b).
DISCUSSION
The number of Dotterel breeding in the UK declined by over half between 1987/88 and 2011. The population estimate of 423 breeding males in 2011 represents a significant decline of 43% since 1999. While all regions except the West Highlands had lower numbers in 2011 than in 1999, only in the East Highlands was there a significant difference. However, owing to the large proportion of sites surveyed in the East Highlands, we had greater power to detect change in that region. Other regions had higher percentage changes but comparisons had low statistical power and so were not significant. No Dotterel were recorded during the survey outside of Scotland in 2011 and, within Scotland, nearly 80% of the population was found in only two regions. We are aware, however, of a breeding record in Northern England in 2011 and one in Galloway in 2008 from fieldwork for the Bird Atlas 2007–11 (Balmer et al. Citation2013).
In each national survey there have been differences in methods of site selection and population estimation which have implications for comparing estimates. Our intention was to maximize our ability to detect change by reanalysing the 1999 national survey data with the methods used for the 2011 survey. Further analysis of the 1987/88 data set (Galbraith et al. Citation1993b) was limited by the fact that it cannot be assumed that sites were selected randomly (see also Whitfield Citation2002b). This precludes statistical comparison with subsequent surveys and the use of the 2011 population estimate methodology. Declining numbers of Dotterel on sites surveyed in all national surveys suggests that there has been a true decline since 1987/88.
Pre-breeding surveys
For the first time, the national survey in 2011 included pre-breeding survey visits over a large number of sites. The trial nature of these surveys mean that results should be interpreted with some caution but results show that sites used during the breeding season are used in a similar way during the pre-breeding season. During these surveys, 47% and 30% of birds located were found in East and Central Highlands, respectively. This suggests that the places where Dotterel breed in the UK are also used by birds during the pre-breeding season, when some individuals will be re-fuelling for onward migration. Numbers recorded during this survey were, however, considerably lower than implied by counts within the same period of the breeding season, albeit at fewer sites, in the 1980s and 1990s (P Whitfield unpubl. data). Weather conditions during the survey period may be one reason why counts were lower than expected. The month of May 2011, when these pre-breeding surveys were carried out, was exceptionally wet (Met Office UK 2011), and approximately 40% of first survey visits were carried out in very poor survey conditions (high winds and heavy rain). These conditions could have had an impact on the ability of surveyors to detect birds. Dotterel behaviour, distribution and settlement decisions may have also been influenced particularly in relation to individuals choosing to stay in Scotland or to continue to more northerly breeding grounds. This study does not provide evidence to do more than speculate on the factors that may influence the decision whether to migrate further or to stay and breed, but there is likely to be substantial annual (e.g. weather and prey availability) and individual (e.g. condition, timing of arrival and competition) variability in these factors. Collaboration at an international scale, as well as detailed studies of colour-ringed individuals and turnover rates through repeated surveys on sites across years, would go some way to illuminating this.
Population estimates
Annual variation in weather conditions during the pre-breeding period may influence settlement decisions by birds and impact on the timing of breeding attempts (Tulp et al. Citation2009). This in turn will affect Dotterel counts carried out during the chick-rearing phase; the presence and behaviour of birds during this period will depend on timing of breeding as well as timing and cause of failures (e.g. extreme weather events such as late snow falls).
While single-year surveys may only provide a snapshot estimate of population abundance, additional results from ongoing monitoring in 2012 and 2013, presented here, put the 2011 national survey results into context. There does appear to be some variation between years (2011–13), but overall the data give us greater confidence that numbers were representative of the current UK Dotterel population. Ongoing monitoring such as this also provides evidence that the national survey methodology is robust and counts are repeatable across years.
Changes in UK Dotterel population
The long-term decline of the extent and condition of Racomitrium moss heath in the UK is well documented over the last 50–60 years (Thompson et al. Citation1987, Thompson & Brown Citation1992, Van Der Wal et al. Citation2003, Citation2005). These studies have outlined how overgrazing and levels of atmospheric nitrogen interact, resulting in changes to the composition and extent of montane heaths. A frequent prey of both adult and juvenile Dotterel is Tipulid larvae (Galbraith et al. Citation1993a) which require dense mats of moss vegetation (Smith et al. Citation2001) and Racomitrium moss heath has been shown to provide important foraging opportunities for Dotterel of all ages (Thompson & Brown Citation1992, Galbraith et al. Citation1993a, Citation1993b). Changes in composition and extent of Racomitrium heath could result in reduced prey availability and could potentially impact upon settlement decisions as well as breeding success for Dotterel. Restoration and enhancement of Racomitrium heath has been recommended as a priority for montane conservation in the UK (Thompson & Brown Citation1992), namely in order to expand the breeding range for Dotterel. Without a better understanding of the drivers of Dotterel decline, however, it is not clear how effective this would be in increasing numbers of breeding Dotterel in the UK.
Predation of Dotterel eggs by Ravens Corvus corax has been described previously as having the potential for causing localized declines; lower return rates have been reported for adult male Dotterel after clutch loss by predation (Whitfield Citation2002b). The period of decline in Dotterel is coincident with an increase in range, and 36% increase in abundance, of Ravens in Scotland (Balmer et al. Citation2013, Harris et al. Citation2014). Whether there is a causal relationship between the Raven population expansion and the Dotterel decline warrants further investigation, however, previous work found no significant negative associations between Raven numbers and upland wader populations (Amar et al. Citation2010, Madden et al. Citation2015). In addition, impacts of disturbance from increasing numbers of walkers on Dotterel have previously been discussed (Whitfield Citation2002b, Thompson et al. Citation2003) although there is little strong evidence for widespread effects, despite negative impacts of such activities on heath condition.
SPAs have supported between 50% and 60% of the population since designation. Designation was based on results from the 1987/88 survey, however, three of the five sites selected as SPAs solely on the basis of the importance for Dotterel now hold less than the 1% breeding population threshold required for designation. The decline in numbers of Dotterel within and outwith the SPA network is of concern, but in terms of site occupancy, sites in SPA/SSSIs were more likely to be occupied than those outside the protected area network. Indeed, protected area designation has been shown to have a positive effect on species persistence for a group of northern species at the trailing edge of their distribution in the UK, although this effect decreased at higher latitudes and altitudes (Gillingham et al. Citation2015).
The importance of considering changes in the number of Dotterel breeding in the UK in the context of the wider European population was emphasized by Whitfield (Citation2002b). This is of particular interest due to the Dotterel's itinerant nature, and breeding dispersal of females as well as some males (Whitfield Citation2002a). In, Finland, Pulliainen & Saari (Citation1996) observed that most females left their study area after egg-laying and hypothesized that this was in order to secure more mates further north. Lucker et al. (Citation2011) found evidence for higher rates of shared incubation by females at the more northern extent of the species’ breeding range than those breeding further south, providing some evidence to support this hypothesis. Historic declines in Dotterel in Finland are described by Saari (Citation1995), who estimated the population there to be no more than 10% of former numbers in the early 1900s. The author hypothesizes that hunting in the early 20th century, and possibly overgrazing by reindeer on the breeding grounds, may be responsible for this decline. Since the 1960s the tree line has advanced and large areas of the mountain heath are covered by scattered Scots Pines Pinus sylvestris, making the habitat largely unsuitable for Dotterel. Pesticides and hunting on the wintering grounds were also attributed as possible factors in the decline. Given the vast areas of montane habitat elsewhere in the Dotterel's global breeding range, population monitoring is largely reliant on sample-based transect surveys that are not directly comparable to estimates of population size and trends presented here. The small number of records of Dotterel on such transects make robust trends difficult to calculate and the only current data we are aware of comes from southern Swedish Lapland (Svensson & Andersson Citation2013) which reports no change in the population there between 1972 and 2011. This is in contrast to a significant positive trends reported in the same study for Golden Plover Pluvialis apricaria, Ptarmigan Lagopus muta, Dunlin Calidris alpina, Redshank Tringa totanus and Whimbrel Numenius phaeopus breeding in the same area of Sweden. Increases in numbers of breeding waders in this area of Sweden over this period coincide with a period of warming April temperature (+2°C 2002–11) but at present no causal connection has been demonstrated. It is possible that the decline in numbers in the UK, reported from this survey, could be due to redistribution of the population away from the UK but without a flyway approach to Dotterel monitoring it is not possible to distinguish between this and population scale decline.
Upland species such as Dotterel are potentially vulnerable to changes in climate due to being cold-adapted and breeding in highly seasonal locations where potential for negative impacts of climate-driven changes in prey availability appears to be high (Chamberlain & Pearce-Higgins Citation2013). The direct effects of changes in climate on the availability of adult Tipulid prey has been shown to account for fluctuations in a Golden Plover population between years (Pearce-Higgins et al. Citation2010). Given the importance of tipulid larvae in the diet of Dotterel, the possibility of climate warming reducing the size of cranefly populations should also be considered as a factor in driving population change in Dotterel. However, Whitfield (Citation2002b) speculated that it was unlikely that climate change was a key driver in the decline of Dotterel in Scotland between 1987 and 1999, as changes in numbers between these two surveys were most marked in two climatically different regions, the oceanic west Highlands and the more continental eastern Highlands. Changes in Dotterel numbers reported here, with a significant decline only in the East Highlands, appear to show a similar spatial pattern of change.
CONCLUSIONS AND RECOMMENDATIONS FOR FUTURE SURVEYS
The 2011 Dotterel survey clearly shows the decline in numbers of Dotterel breeding in the UK and contraction to core sites in the East and Central Highlands since the late 1980s.
Drivers of the decline observed in Dotterel may include factors on the breeding and non-breeding grounds and the impacts of climate change throughout the migration cycle. However, further detailed autecological work is required to understand the mechanisms driving the observed population trends of this species in the UK before any potential threats can be definitely excluded. In conjunction, identification of processes occurring elsewhere on migration will be necessary. These may include changes in abundance on wintering, passage and northerly breeding sites. Advancing our understanding of the complex interactions between the large-scale drivers of environmental change discussed here, and their impact on Dotterel numbers, requires ongoing commitment to long-term monitoring and research projects. Future surveys would benefit from adopting the same site selection protocols and population estimation methods used for the 2011 survey in order to ensure comparability.
ACKNOWLEDGEMENTS
We thank the BTO for provision of 2007–2011 Atlas data. Thanks go to the field staff who were employed on the survey: Alisdair Boulton, James Gordon, Ben Hayes, Kristin Keyes, Gareth Marshall, Ewan Munro, Fiona Newcombe and Robert Potter. In particular we must pay tribute to the large amount of fieldwork undertaken by volunteers. Thanks to the following for provision of data for 2011 as well as ongoing monitoring: Natural Research Projects (Cairn Lochan) and the National Trust for Scotland Mar Lodge (Beinn A'Bhuird). Thanks also to SNH staff and William George, Paul Britten and Sharolyn Parnham in the RSPB Conservation Data Management Unit for GIS production of maps. We thank the many landowners and managers, on whose land surveying took place. We are grateful to Jeremy Wilson and two anonymous referees for comments on earlier drafts of this manuscript.
FUNDING
The 2011 Dotterel Survey was carried out under the Statutory Conservation Agencies/RSPB Annual Breeding Bird Survey (SCARABBS) programme and was funded by the RSPB and SNH.
REFERENCES
- Amar, A., Redpath, S., Sim, I. & Buchanan, G. 2010. Spatial and temporal associations between recovering populations of common raven Corvus corax and British upland wader populations. J. Appl. Ecol. 47: 253–262. doi: 10.1111/j.1365-2664.2010.01772.x
- Balmer, D., Gillings, S., Caffrey, B., Swann, R., Downie, I. & Fuller, R. 2013. Bird Atlas 2007–11: The Breeding and Wintering Birds of Britain and Ireland. BTO Books, Thetford.
- Chamberlain, D. & Pearce-Higgins, J. 2013. Impacts of climate change on upland birds: complex interactions, compensatory mechanisms and the need for long-term data. Ibis 155: 451–455. doi: 10.1111/ibi.12070
- Chamberlain, D., Arlettaz, R., Caprio, E., Maggini, R., Pedrini, P., Rolando, A. & Zbinden, N. 2012. The altitudinal frontier in avian climate impact research. Ibis 154: 205–209. doi: 10.1111/j.1474-919X.2011.01196.x
- Cramp, S. & Simmons, K. 1983. The Birds of the Western Palearctic., Vol. 3. Oxford University Press, Oxford.
- Eaton, M., Brown, A., Noble, D., Musgrove, A., Hearn, R., Aebischer, N., Gibbons, D., Evans, A. & Gregory, R. 2009. Birds of conservation concern 3: the population status of birds in the United Kingdom, Channel Islands and the Isle of Man. Br. Birds 102: 296–341.
- Efron, B. 1982. The Jackknife, the Bootstrap and Other Resampling Plans. SIAM, Philadelphia.
- Farmer, A.H. & Wiens, J.A. 1999. Models and reality: time-energy trade-offs in pectoral sandpiper (Calidris melanotos) migration. Ecology 80: 2566–2580.
- Galbraith, H., Murray, S., Duncan, K., Smith, R., Whitfield, D.P. & Thompson, D.B.A. 1993a. Diet and habitat use of the Dotterel Charadrius morinellus in Scotland. Ibis 135: 148–155. doi: 10.1111/j.1474-919X.1993.tb02826.x
- Galbraith, H., Murray, S., Rae, S., Whitfield, D.P. & Thompson, D.B.A. 1993b. Numbers and distribution of Dotterel Charadrius morinellus breeding in Great Britain. Bird Study 40: 161–169. doi: 10.1080/00063659309477179
- Gillingham, P.K., Bradbury, R.B., Roy, D.B., Anderson, B.J., Baxter, J.M., Bourn, N.A.D., Crick, H.Q.P., Findon, R.A., Fox, R., Franco, A., Hill, J.K., Hodgson, J.A., Holt, A.R., Morecroft, M.D., O'Hanlon, N.J., Oliver, T.H., Pearce-Higgins, J.W., Procter, D.A., Thomas, J.A., Walker, K.J., Walmsley, C.A., Wilson, R.J. & Thomas, C.D. 2015. The effectiveness of protected areas in the conservation of species with changing geographical ranges. Biol. J. Linnean Soc. 115: 707–717. doi: 10.1111/bij.12506
- Greenwood, J. 1991. Estimating the total number and its confidence limits. Appendix to: Shrubb, M. & Lack, PC The numbers and distribution of Lapwings V. vanellus nesting in England and Wales in 1987. Bird Study 38: 20–37. doi: 10.1080/00063659109477063
- Harris, S.J., Risely, K., Massimino, D., Newson, S.E., Eaton, M.A., Musgrove, A.J., Noble, D.G., Procter, D. & Baillie, S.R. 2014. The Breeding Bird Survey 2013. BTO Research Report 658. British Trust for Ornithology, Thetford.
- Hazeu, G.W., Roupioz, L.F.S. & Perez-Soba, M. 2010. Europe's Ecological Backbone: Recognising the True Value of Our Mountains. EEA Report No 6/2010. European Environment Agency, Copenhagen.
- Holt, S., Whitfield, D.P. & Gordon, J. 2002. Potential reproductive rates in the Eurasian dotterel Charadrius morinellus. Bird Study 49: 87–88. doi: 10.1080/00063650209461248
- Huntley, B., Collingham, Y.C., Willis, S.G. & Green, R.E. 2008. Potential impacts of climatic change on European breeding birds. PlosOne 3: e1439. doi: 10.1371/journal.pone.0001439
- Kålås, J.A. & Byrkjedal, I. 1984. Breeding chronology and mating system of the Eurasian Dotterel (Charadrius morinellus). Auk 101: 838–847. doi: 10.2307/4086911
- La Sorte, F.A. & Jetz, W. 2010. Projected range contractions of montane biodiversity under global warming. Proc. Roy. Soc. B: Biol. Sci. 277: 3401–3410. doi: 10.1098/rspb.2010.0612
- Lehikoinen, A., Green, M., Husby, M., Kålås, J.A. & Lindström, Å. 2014. Common montane birds are declining in northern Europe. J. Avian Biol. 45: 3–14. doi: 10.1111/j.1600-048X.2013.00177.x
- Lucker, L., Kraatz, S. & Kraatz, B. 2011. Field notes on the breeding biology of the Dotterel Charadrius morinellus in arctic Norway. Ornis Svec. 21: 109–118.
- Madden, C.F., Arroyo, B., Amar, A. 2015. A review of the impacts of corvids on bird productivity and abundance. Ibis 157: 1–16. doi: 10.1111/ibi.12223
- Maggini, R., Lehmann, A., Kéry, M., Schmid, H., Beniston, M., Jenni, L. & Zbinden, N. 2011. Are Swiss birds tracking climate change? Detecting elevational shifts using response curve shapes. Ecol. Model. 222: 21–32. doi: 10.1016/j.ecolmodel.2010.09.010
- Musgrove, A., Aebischer, N., Eaton, M., Hearn, R., Newson, S., Parsons, M., Risely, K. & Stroud, D. 2013. Population estimates of birds in Great Britain and the United Kingdom. Brit. Birds 106: 64–100.
- Pearce-Higgins, J.W., Dennis, P., Whittingham, M.J. & Yalden, D.W. 2010. Impacts of climate on prey abundance account for fluctuations in a population of a northern wader at the southern edge of its range. Glob. Change Biol. 16: 12–23. doi: 10.1111/j.1365-2486.2009.01883.x
- Pulliainen, E. & Saari, L. 1996. Pre- and non-breeding biology of Dotterel Charadrius morinellus in Värriötunturi fell area, NE Finland. Wader Study Group Bull 81: 54–58.
- Saari, L. 1995. Population trends of the Dotterel Charadrius morinellus in Finland during the past 150 years. Ornis Fenn. 72: 29–36.
- Sekercioglu, C.H., Schneider, S.H., Fay, J.P. & Loarie, S.R. 2008. Climate change, elevational range shifts, and bird extinctions. Conserv. Biol. 22: 140–150. doi: 10.1111/j.1523-1739.2007.00852.x
- Smith, R.M., Young, M.R. & Marquiss, M. 2001. Bryophyte use by an insect herbivore: does the crane-fly Tipula montana select food to maximise growth? Ecol. Entomol. 26: 83–90. doi: 10.1046/j.1365-2311.2001.00297.x
- Stroud, D.A., Chambers, D., Cook, S., Buxton, N., Fraser, B., Clement, P., Lewis, P., McLean, I., Baker, H. & Whitehead, S. 2001. The UK SPA Network: Its Scope and Content. JNCC, Peterborough, ON.
- Strowger, J. 1998. The status and breeding biology of the Dotterel Charadrius morinellus in northern England during 1972–95. Bird Study 45: 85–91. doi: 10.1080/00063659809461081
- Svensson, S. & Andersson, T. 2013. Population trends of birds in alpine habitats at Ammarnäs in southern Swedish Lapland 1972–2011. Ornis Svec. 23: 81–107.
- Thompson, D.B.A. & Brown, A. 1992. Biodiversity in montane Britain – habitat variation, vegetation diversity and some objectives for conservation. Biodivers. Conserv. 1: 179–208. doi: 10.1007/BF00695915
- Thompson, D.B.A. & Whitfield, D.P. 1993. Research progress reports. Scottish Birds 17: 1–8.
- Thompson, D.B.A., Galbraith, H. & Horsfield, D. 1987. Ecology and resources of Britain's mountain plateaux: landuse conflicts and impacts. In Bell, M. & Bunce, R. (eds.) Agriculture and Conservation in the Hills and Uplands Terrestrial Ecology (NERC) Symposium, 22–31. Institute Terrestrial Ecology (ITE Symposium), Grange-over-Sands.
- Thompson, D.B.A., Whitfield, D.P., Galbraith, H., Duncan, K., Smith, R.D., Murray, S. & Holt, S. 2003. Breeding bird assemblages and habitat use of alpine areas in Scotland. In Nagy, L., Grabherr, G., Körner, C. & Thompson, D.A. (eds.) Alpine Biodiversity in Europe, 327–338. Springer, Berlin.
- Tulp, I., Schekkerman, H., Klaassen, R.H., Ens, B.J., & Visser, G.H. 2009. Body condition of shorebirds upon arrival at their Siberian breeding grounds. Polar Biol. 32: 481–491. doi: 10.1007/s00300-008-0543-8
- Van Der Wal, R., Pearce, I., Brooker, R., Scott, D., Welch, D. & Woodin, S. 2003. Interplay between nitrogen deposition and grazing causes habitat degradation. Ecol. Lett. 6: 141–146. doi: 10.1046/j.1461-0248.2003.00407.x
- Van der Wal, R., Pearce, I.S. & Brooker, R.W. 2005. Mosses and the struggle for light in a nitrogen-polluted world. Oecologia 142: 159–168. doi: 10.1007/s00442-004-1706-0
- Watson, A. 1988. Dotterel Charadrius morinellus numbers in relation to human impact in Scotland. Biol. Conserv. 43: 245–256. doi: 10.1016/0006-3207(88)90118-8
- Welch, D., Scott, D. & Thompson, D.B.A. 2005. Changes in the composition of Carex bigelowii–Racomitrium lanuginosum moss heath on Glas Maol, Scotland, in response to sheep grazing and snow fencing. Biol. Conserv. 122: 621–631. doi: 10.1016/j.biocon.2004.09.016
- Whitfield, D.P. 2002a. Dotterel Charadrius morinellus. In Wernham, C.V., Toms, M.P., Marchant, J.H., Clark, J.A., Siriwardena, G.M. & Baillie, S.R. (eds.) The Migration Atlas: Movements of the Birds of Britain and Ireland, 281–283. T. & A.D. Poyser, London.
- Whitfield, D.P. 2002b. Status of breeding Dotterel Charadrius morinellus in Britain in 1999. Bird Study 49: 237–249. doi: 10.1080/00063650209461271
- Whitfield, D.P., Duncan, K., Pullan, D. & Smith, R.D. 1996. Recoveries of Scottish-ringed Dotterel Charadrius morinellus in the non-breeding season: Evidence for seasonal shifts in wintering distribution. Ring. Migr. 17: 105–110. doi: 10.1080/03078698.1996.9674125
