Abstract
Capsule Blackcap Sylvia atricapilla populations from the Azores archipelago show morphological differences to continental birds which are consistent with the ‘Island Rule’.
Aims The morphology of insular vertebrates is usually the result of the evolution in their particular environment and leads to predictable morphological patterns, according to the Island Rule. We test the predictions of the Island Rule, using the Blackcap of the Azores archipelago as our model.
Methods We compared morphological variation (body size and wing shape) of populations from the nine islands of the Azores to continental birds, using multivariate indexes. Also, we looked at the relationship between these patterns and possible insular ecological drivers of morphological divergence.
Results Our findings are concordant with Island Rule predictions, as in general birds from the Azores are larger than continental populations, especially birds from the most distant islands. Wing shape also differs significantly, as Azorean Blackcaps tend to have rounder wings than continental birds with a migratory-like phenotype.
Conclusion Overall, we conclude that the observed morphological patterns in Blackcap in the Azores conform in general to the Island Rule predictions.
Ever since Darwin (Citation1859) discussed the importance of islands in the process of speciation, island biogeography has been a classic topic in the study of evolution. Islands are usually colonized by species from the closest mainland areas and there they can evolve under different environmental constraints, such as smaller areas, different habitats, narrower spectrum of food resources or the lack of predators. These act as selective pressures driving the morphological evolution of colonizers, probably differently from what happens with individuals of continental populations. Islands are, therefore, important locations to study evolutionary processes and provide an opportunity to observe the results of colonizations with a range of histories, from recent to very old.
Although each island-mainland species interaction is supposed to be unique and shaped by its own environmental and evolutionary circumstances, Van Valen (Citation1973) proposed that patterns of morphological variation follow some predictable trends, what became known as the ‘Island Rule’. It postulates general trends in body size variation within groups of species: smaller animals have the tendency to become bigger than their mainland counterparts, while the opposite happens with larger species, converging towards an optimal size for that group of species (see Lomolino Citation2005, for a review on vertebrate data). This has been explained by the ecological release from mainland size-constraining selective pressures (e.g. predation, parasitism, mutualism and interspecific competition) that are more common on mainland habitats due to the higher species diversity and more complex species interactions. On the other hand, islands generally have high population densities, which could lead to higher intraspecific competition. This is positively correlated with the size of the island because smaller islands have fewer resources and more competition, and could promote both dwarfism (smaller individuals require less energy) and gigantism (larger sizes are beneficial in intraspecific disputes). Furthermore, immigrant selection acting mostly on smaller species could promote gigantism by selecting for larger individuals, which tend to disperse further and more easily.
Despite the large number of studies showing predictable patterns of body size change in island populations, some recent studies have found limited or no support for their occurrence (Meiri et al. Citation2006, Citation2008, McClain et al. Citation2013). These authors argue that trends are clade-specific, not size-specific and depend on ecological factors, species’ biology and the ecological and historical context in which the colonization took place. McNab (Citation2002), while conceding the generality of the application of the Island Rule, highlighted the need to look at resource availability and partitioning as factors determining vertebrate insular body size. The author also cited earlier studies suggesting that resource limitation on islands may lead to an increase in tolerance to conspecifics, instead of increased competition.
As highly mobile organisms, birds colonize islands more easily than other taxa. Once on islands, reduced gene flow with the mainland is frequently observed (Boessenkool et al. Citation2007, Dudaniec et al. Citation2011) and, even if panmixia occurs, divergence cannot always be excluded (Galligan et al. Citation2012). Therefore, birds are a good group to study patterns related to isolation on novel environments like islands. Studies on multiple-taxa bird morphological databases provide support for the Island Rule (Fitzpatrick Citation1998, Clegg & Owens Citation2002, Boyer & Jetz Citation2010). Studies centred on the morphological comparison between continental and insular populations of the same species, or group of species, show that slight, but appreciable, differences occur in these populations as a reflection of the particular eco-evolutionary characteristics of island habitats. The most coherent patterns have been found regarding wing shape and bill size: island birds tend to have more rounded wings (Förschler & Siebenrock Citation2007, Förschler et al. Citation2008, but see Komdeur et al. Citation2004, for an exception) and longer bills (Grant Citation1979, Scott et al. Citation2003, Förschler & Siebenrock Citation2007, Clegg et al. Citation2008, Mathys & Lockwood Citation2011, Wright & Steadman Citation2012). Wing pointedness, which is the increasing tendency for the wingtip to be defined by the tips of the outermost primaries, can vary in species in which migratory polymorphism occurs: pointed wings benefit the speed/energy cost ratio in migrants by minimizing drag, whereas resident populations tend to have rounder wings that aid manoeuvrability (Saville Citation1957, Rayner Citation1988, Mönkkönen Citation1995, Lockwood et al. Citation1998, Bowlin & Wikelski Citation2008). Thus, as island colonizers tend to become residents, rounder (or less pointed) wings are to be expected in comparison to continental migratory populations. The larger bills of island birds could provide the advantage of a larger array of feeding possibilities in the face of a lower quantity and quality of food resources, and decreased interspecific competition compared to the mainland. Overall, results of previous studies give further indication that simple generalizations may be inadequate when discussing the effect of insularity on morphological evolution, and more attention should be given to the specific organism-island system under study.
The Blackcap Sylvia atricapilla is one of the most abundant and cosmopolitan passerines in the Western Palearctic, inhabiting forests and other habitats where shrubs are present (Shirihai et al. Citation2001). Across its distribution range, populations present a wide diversity of migratory strategies, from completely sedentary to long-distance migrants (Berthold & Helbig Citation1992, Pérez-Tris et al. Citation2004, Fiedler Citation2005). The Blackcap is also one of the predominant forest passerines in the North Atlantic volcanic islands of the Macaronesia, where populations are sedentary (archipelagos of Azores, Madeira, Canaries and Cape Verde; Shirihai et al. Citation2001). These Atlantic populations show little genetic differentiation to Western continental birds (Pérez-Tris et al. Citation2004, Dietzen et al. Citation2008) and, in the case of Azorean Blackcaps, there was probably only a single colonization event (from the mainland or Madeira), indicating reduced or absent current gene flow with the mainland (Rodrigues Citation2012). These populations can, therefore, provide a good opportunity to test the effects of island colonization on bird morphology.
Previous comparisons of the morphology of Blackcap from several European and Atlantic island populations reveal mixed patterns: Fiedler (Citation2005) analysed wing morphology and body mass from birds of Madeira and Cape Verde Islands, and found conflicting patterns with regard to body size in insular populations. In a comparison of wing length between several insular and continental bird populations (Fitzpatrick Citation1998), continental residents of many species had longer wings in general but the opposite was found for the Blackcap. In this study, our objective was to assess the occurrence of patterns of size and shape variation associated with insularity in Blackcap populations from the Azores, by comparing them to continental populations. Populations from the Azores are especially suited for this, since this species is present in all of the nine islands of the archipelago, each of them with different biotic and abiotic characteristics. This makes it possible to analyse how different size and shape patterns arise in different ecological backgrounds. If Azorean Blackcaps conform to the Island Rule we predict that they will: (1) be larger than continental birds; (2) present patterns of wing shape associated with sedentary behaviour (rounder wings) and (3) show increased island-like morphological patterns in association with specific descriptors of physical and biological geography (e.g. distance to the mainland or island area).
METHODS
Sampling sites
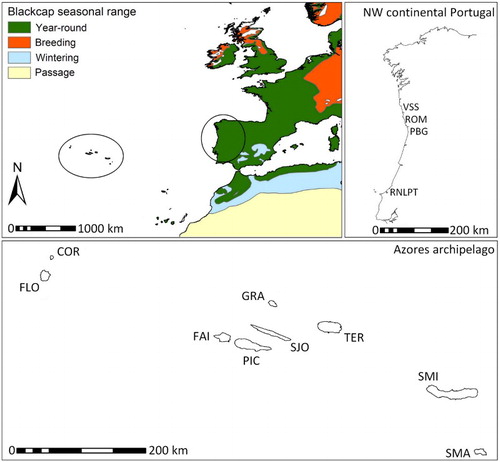
In the Azores, nine major islands cluster in three geographical groups (): the Eastern Group (Santa Maria and São Miguel), the Central Group (Terceira, Graciosa, Faial, Pico and São Jorge) and the Western Group (Flores and Corvo). The archipelago's approximate distances to the European continent are between 1898 and 1343 km, for the most western and most eastern islands, respectively. Several locations on each island were sampled, in the interface between native or production forests and pasture fields. On continental Portugal, birds were sampled at four sites along the Northwestern Portuguese Atlantic coast () in habitats close to riparian corridors and shrubs, usually with mixed autochthonous and exotic plant species.
Figure 1. Geographic placement of continental Portugal and the Azores archipelago in Southwestern Europe, with information on the phenology of Blackcap populations in this region. The Azores archipelago is composed of three main groups of islands, the Western (FLO – Flores and COR – Corvo), Central (FAI – Faial; PIC – Pico; GRA – Graciosa; SJO – São Jorge and TER – Terceira) and Eastern groups (SMI – São Miguel and SMA – Santa Maria) each successively closer to the European continent. Sampling was done on all nine islands. Maps were obtained from BirdLife International & NatureServe (Citation2011) and Instituto Hidrográfico da Marinha (Citation2006).
Biometric data collection
Blackcaps were captured with mist nets (Ecotone, Poland) and ringed with individual metal rings in the Azores (n = 299, February–October from 2008 to 2011), and continental Portugal (n = 300, September 2012–April 2015) (see supplementary Table A1 for more details). Biometrical measures were recorded according to Svensson (Citation1992), unless otherwise noted: tarsus length (±0.1 mm, measured from the notch of the intertarsal joint to the lower edge of the last scale), tail length (±1 mm, from the base of the two central tail feathers to their tips), wing length (±1 mm, measured as the maximum length of the flattened and straightened wing), length of primaries 1–9 (±1 mm), numbered descendantly (from the outermost, excluding the outermost vestigial primary), each measured with a pin-ruler on its outer web from the point where it enters the skin, except for primary 9 which was measured on the inner web (Jenni & Winkler Citation1989) and distances of primaries 1 and 9 to the wingtip (±1 mm, measured on a flattened wing in continental birds, for morphotype discrimination). No measurements were taken on birds with feathers that were heavily worn or growing and all wing measurements were taken on the bird's right wing. Birds that showed signs of stress were released without measuring. All measurements on continental birds were taken by PA, while PR measured birds from the Azores. Both were trained under the Portuguese ringing scheme, using the same standardized measuring techniques.
Morphological data analysis
Principal component analysis (PCA) on ln-transformed linear measurements were used since they are able to account for the dimensions of all the variables and reduce them to a number of orthogonal principal components (Chandler & Mulvihill Citation1988, Rising & Somers Citation1989). To derive an index of overall body (or structural) size, a PCA with wing, tail and tarsus length as variables was used. The first principal component (sizePC1) explained more than 50% of the overall variation and each univariate variable shows a significant strong positive correlation (P < 0.001) with sizePC1 (). Consequently, it was interpreted as an index of structural size. To analyse patterns of ecomorphological shape we studied variation in wing shape using primary feather lengths as variables in PCA. First, we used the first component of this PCA as a proxy of size (wingsizePC1, ). To compare wing shape without the effect of size, the original measurements were regressed with wingsizePC1 scores and only the residuals were used as variables for a second PCA to derive indices of wing shape (Drovetski et al. Citation2006). As expected, the first component (wingshapePC1, ) recovered a component of wing shape, namely pointedness: proximal primary lengths (P1 to P5) have a negative loading on this component, while distal primary lengths (P6 to P9) have positive loadings. The second component (wingshapePC2, ) had negative loadings associated with primaries P4 to P7, which we interpret as a component of wing concavity.
Table 1. Factor loadings, eigenvalues and percentage of total variance for the first principal component of a PCA with ln-transformed values of the lengths of wing, tail and tarsus (n = 556) are shown. The significance level between each factor loading and the original ln-transformed biometrical variable is also shown.
Table 2. Factor loadings for principal components of a PCA to obtain indexes of wing shape (n = 345). Ln-transformed values of primary feather lengths were regressed against the first component of a previous PCA using ln-transformed feather lengths (wingsizePC1), and the residuals were used in a new PCA to obtain wingshapePC1 and wingshapePC2. Eigenvalues, the percentage of total variance for each principal component and the significance level between each factor loading and the original ln-transformed biometrical variable are shown. Primaries were numbered descendantly.
Continent-island morphological comparison
In a preliminary analysis, we tested for differences in morphology for the three multivariate indices of size and shape described previously for different age (birds up to their first primary moult vs. adults) and sex (male vs. female) categories. Since no differences were detected, we pooled all individuals captured for the following analysis.
To assess the occurrence of morphological differences between continental and insular Blackcaps, anova and manova (generalized linear models (GLM)) were used to test for significant differences in the multivariate indices of size and wing shape, with a three-level approach. First, we grouped all birds caught in the Azores, and all birds sampled on the mainland, to assess overall differences between insular and continental birds. Since our full continental sample includes birds captured during the entire year, and thus probably wintering individuals from multiple source populations along Western Europe (Cantos Citation1995), we repeated the previous test comparing Azorean birds to continental birds that were caught only during breeding season. Third, we looked for finer patterns: Azorean birds were grouped according to their island of origin, while continental birds were grouped as either continental ‘residents’ or continental ‘wintering/migrant’. The last two groups were used to discern between continental birds with either a sedentary or migratory-like morphology, to give a better reference to which Azorean patterns could be compared. Blackcaps captured in Iberia can be separated as either migratory (including wintering birds from Central European breeding grounds) or sedentary, based on morphological characters: P8 length, tail/wing ratio, tarsus length and a summary index of wing shape, according to a discriminant function (Morganti et al. Citation2015). This classification was possible for 250 individuals (50 wintering/migratory and 200 residents). anova and manova tests were followed by Tukey's post hoc multiple comparison tests to identify pairs of groups with significant differences for each variable.
Additionally, to assess the relationship between island characteristics and morphology, Kendall's τ non-parametric rank correlations were performed to test the significance of the relationships between values of the multivariate indices of size and wing shape for each individual island (n = 9, the mean value of the variable from birds of each island was used as data), and the following descriptors of island physical and biological geography: island area (km2), approximate distance to the nearest continent (km), approximate distance to the nearest island (km), maximum elevation (m), percentages of urban, whole forest, native forest and agriculture cover, ratio of agriculture to whole forest cover and ratio of native forest to other types of forest (data for each island taken from Cardoso et al. Citation2010, DRRF Citation2007, see supplementary table A2). Prior to analysis, absolute values and ratios were ln-transformed, while percentages were arcsine-transformed.
RESULTS
The comparison of mean values of the multivariate size index sizePC1 revealed a significant difference in size between Azorean and continental Blackcaps, both for our full sample (F1,554 = 41.834, P < 0.001) and for our reduced continental sample including only breeding populations (F1,314 = 26.314, P < 0.001; , Table A3 in supplementary material). This was verified in a more detailed analysis comparing populations of each island with continental sedentary and wintering/migratory morphotypes (F10,510 = 14.384, P < 0.001; , a, Table A3 in supplementary material). Tukey's pairwise comparisons indicated that, in general, birds from the Azores had higher structural size values than both continental groups; the exceptions were birds from Graciosa, Terceira and Santa Maria, which did not differ significantly from continental birds, and Faial with the wintering/migratory birds. The larger sizes were especially pronounced in birds from the more distant Western Group islands, which tended to have higher values than Blackcaps from other islands, particularly birds from Flores, which were larger than all other populations except those from Corvo and São Jorge (Central Group).
Figure 2. Comparison of Blackcap Sylvia atricapilla populations (see the legend of for island acronyms) from the Azores Western (white circles), Central (grey circles) and Eastern groups (black circles), and Iberian continental birds classified as having resident (R) or wintering/migratory-like (W) morphology, for three multivariate measures of morphology: overall structural size (sizePC1, a), wing pointedness (wingshapePC1, b) and wing concavity (wingshapePC2, c). Graphics show, for each population/variable, the mean value of the observations and 95% confidence intervals, in relation to the distance of each island to the mainland.
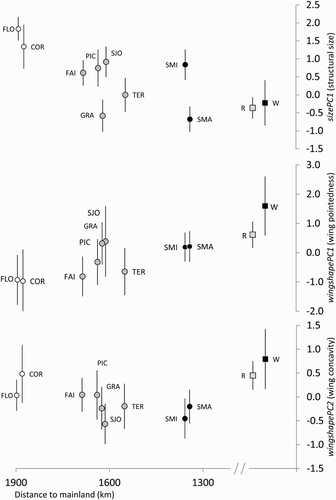
Table 3. Results of an anova and manova (GLM) to determine significant differences in multivariate indices of structural size and wing shape between Blackaps from the Azores and continental Portugal (a full sample from around the year, or just from the breeding season), and also between each island and continental birds classified as having a ‘sedentary’ or ‘migratory’-like morphotype. Azores archipelago: FLO – Flores; COR – Corvo; FAI – Faial; PIC – Pico; GRA – Graciosa; SJO – São Jorge; TER – Terceira; SMI – São Miguel and SMA – Santa Maria. Continental Portugal: R – continental ‘residents' and W – continental ‘wintering/migratory’.
For the analysis of wing shape, a manova with wing pointedness (wingshapePC1) and concavity (wingshapePC2) indices revealed significant differences in wing shape between Azorean and the full continental bird sample (Wilk's lambda = 0.940, F2,342 = 10.993, P < 0.001), the restricted breeding sample (Wilk's lambda = 0.958, F2,304 = 10.993, P < 0.01) and also among the more detailed study groups (Wilk's lambda = 0.826, F20,666 = 3.333, P < 0.001). Tukey's pairwise comparisons revealed, for wing pointedness, that continental birds had more pointed wings than birds from the Azores (, b, Table A3 in supplementary material). Looking at more detailed comparisons, there were generally non-significant differences between island birds and continental ‘residents’, and significantly lower wing pointedness values in the three more distant insular populations of Flores, Corvo and Faial, compared to ‘migrants’. Birds from the Eastern Group islands (Santa Maria and São Miguel) tended to have more pointed wings than most other island groups, albeit non-significantly. Regarding wing concavity, results were similar to wing pointedness in that continental birds had significantly more concave wings than island birds (, c, Table A3 in supplementary material). When we analysed birds from the several islands separately, however, the only significant differences were between São Miguel birds and ‘residents’; the latter had more concave wings.
Looking at the correlations between mean values of the multivariate indices of size and wing shape of Blackcaps and physical and biological geography descriptors for each of the nine islands of the Azores, we found significant correlations between: distance to the mainland and overall structural size (rτ = 0.50, n = 9, P = 0.038) and wing pointedness (rτ = −0.56, n = 9, P = 0.022); ratio of native forest to other types of forest and structural size (rτ = 0.56, n = 9, P = 0.022) and wing concavity (rτ = 0.50, n = 9, P = 0.03). All other correlations were not significant.
DISCUSSION
In our 11 study groups, birds from the Azores tended to have higher structural size (sizePC1 scores) than continental birds, especially the birds from the Western Group, which is in accordance to other studies (Fitzpatrick Citation1998, Clegg & Owens Citation2002, Lomolino Citation2005, Boyer & Jetz Citation2010). Our results also indicate a significant positive correlation between distance to mainland and structural size of Blackcaps. Highlighting these trends is the highly significant difference in size between continental birds with sedentary-like morphology and birds from most Azores islands. The latter have higher values of sizePC1. This is especially relevant because all are non-migratory populations from similar latitudes, which is a good case study to compare morphological differences in relation to the colonization of island habitats, as Iberian Blackcaps are closely related to both western European migrants and Atlantic island populations (see Pérez-Tris et al. Citation2004, Dietzen et al. Citation2008, Rodrigues Citation2012). Our results provide evidence to support the Island Rule in relation to the structural body size of Blackcaps for the Azores. The islands of the Western Group are the furthest from continental Portugal, so the pattern of increasing size with increasing distances to the mainland seems to fit predictions stating that poorer communities on islands (a consequence of isolation) might lead to a decrease in interspecific competition. Bird communities in the Azores are much less diverse when compared to the mainland (Catry et al. Citation2010, Rodrigues et al. Citation2010), which could lead to a decrease in interspecific competition when compared to continental habitats, and thus larger overall sizes. Even within the Azores archipelago, the Western group islands are those with the least diverse passerine communities: 9 species breed regularly in Flores and 8 in Corvo, compared to 9–12 in the Central group and 10–13 in the Eastern group. Some possible competitors with the Blackcap are absent from the Western group, namely the Robin Erithacus rubecula and the Goldcrest Regulus regulus (absent from Corvo). An increase in size was also correlated with an increase in the ratio of native forests to other types of forest, which suggests an influence of habitat composition in the size of Blackcaps in the Azores. It has been suggested that the roles of ecological release and niche expansion in determining island passerine body size may be less important than has been generally thought (Scott et al. Citation2003), and better environmental conditions, such as a wider variety of food resources, may also play a role. This could explain why birds on islands with better preserved native habitats tend to be larger, as Azores Blackcaps are known to prefer habitats with more diverse fruiting plant species, which are not found in some exotic forest habitats (Ceia et al. Citation2009). On the other hand, evidence from phenotypic plasticity studies in island birds indicates that morphology is mostly determined by a genetic component (Merilä & Sheldon Citation2001).
Similarly to other studies (Chandler & Mulvihill Citation1988, Lockwood et al. Citation1998), after the removal of the shape-related variation from wing size, the first component is related to an increase in wing pointedness (wingshapePC1). This trait is significantly related to island distance to the mainland, as populations in closer islands tended to have higher values of wingshapePC1. When we analysed the scores of this index for the 11 study groups, birds from the 3 more distant islands (Flores, Corvo and Faial), had significantly less pointed wings than continental birds with migrant-like morphology. Blackcaps in the Azores are thought to be sedentary, which seems to be confirmed by genetic data (Rodrigues Citation2012), so the generally rounder wings of these birds are expected if individuals do not face long-distance migration. However, birds from the closer islands tended to have more pointed wings, which is unexpected if all populations are sedentary. Since genetic data have consistently precluded significant amounts of gene flow with continental Blackcaps, these relatively high pointedness values could be better explained by differences in habitat. Birds that inhabit open habitats tend to have more pointed wings (Rayner Citation1988), so if birds from these eastern islands had to adapt to less forested habitats this could have resulted in differences in wing morphology. Some studies already have pointed towards unusual behaviour in insular Blackcap: Bourne (Citation1955) noticed a marked tendency for Cape Verde Blackcaps to forage on the ground, which is unusual in their continental conspecifics which are found mostly in shrub habitats (favouring rounder wings); Buxton (Citation1960) recorded similar behaviour in birds from Madeira. However, relationships between wing morphology and several descriptors of habitat structure were, in general, non-significant for our Azores sample, with the exception of higher values of wing concavity with increases in the ratio of native forest to other types of forest. Previous research (Spurgin et al. Citation2014) has shown that colonization history could have a stronger effect on insular bird morphology than geographical differences between populations, which could explain our generally non-significant results. Furthermore, data on the structure and composition of habitats in the Azores is preliminary for a study of this scope. More work is needed to better understand the relationships between the insular environment and the patterns of morphological change in Macaronesian Blackcap populations.
SUPPLEMENTAL DATA
Supplemental data for this article can be accessed http://dx.doi.org/10.1080/00063657.2015.1077780.
Supplementary material
Download MS Word (105.5 KB)ACKNOWLEDGEMENTS
The authors would like to thank Instituto da Conservação da Natureza e das Florestas (ICNF) for the permission to capture birds in continental Portugal (permits 152/2011 and 175/2013); Direção Regional do Ambiente dos Açores for the permit to capture Azorean birds (permit 119/2011/DRA). GVC – Anilhagem Científica de Aves and Helder Cardoso for providing the equipment with which the majority of continental birds were captured. To the great number of volunteers who participated during fieldwork. Tiago Rodrigues, Javier Pérez-Tris and Michelangelo Morganti contributed with many suggestions towards data analysis and presentation. All the conducted experiments comply with the current laws of Portugal.
Additional information
Funding
REFERENCES
- Berthold, P. & Helbig, A. 1992. The genetics of bird migration: stimulus, timing, and direction. Ibis 134: 35–40. doi: 10.1111/j.1474-919X.1992.tb04731.x
- BirdLife International & NatureServe. 2001. Bird Species Distribution Maps of the World. BirdLife International, Cambridge, UK & NatureServe, Arlington, USA.
- Boessenkool, S., Taylor, S.S., Tepolt, C.K., Komdeur, J. & Jamieson, I.G. 2007. Large mainland populations of South Island robins retain greater genetic diversity than offshore island refuges. Conserv. Genet. 8: 705–714. doi: 10.1007/s10592-006-9219-5
- Bourne, W.R.P. 1955. The birds of Cape Verde islands. Ibis 97: 508–556. doi: 10.1111/j.1474-919X.1955.tb04981.x
- Bowlin, M.S. & Wikelski, M. 2008. Pointed wings, low wingloading and calm air reduce migratory flight costs in songbirds. PlosOne 3: e2154. doi: 10.1371/journal.pone.0002154
- Boyer, A.G. & Jetz, W. 2010. Biogeography of body size in Pacific island birds. Ecography 33: 369–379.
- Buxton, E.J.M. 1960. Winter notes from Madeira. Ibis 102: 127–129. doi: 10.1111/j.1474-919X.1960.tb05101.x
- Cantos, F.J. 1995. Migración e invernada de la curruca capirotada (Sylvia atricapilla). Ecología 9: 425–433.
- Cardoso, P., Arnedo, M.A., Triantis, K.A. & Borges, P.A.V. 2010. Drivers of diversity in Macaronesian spiders and the role of species extinctions. J. Biogeogr. 37: 1034–1046. doi: 10.1111/j.1365-2699.2009.02264.x
- Catry, P., Costa, H., Elias, G. & Matias, R. 2010. Aves de Portugal, ornitologia do território continental. Assírio & Alvim, Lisboa.
- Ceia, R., Heleno, R. & Ramos, J.A. 2009. Summer abundance and ecological distribution of passerines in native and exotic forests in São Miguel, Azores. Ardeola 56: 25–39.
- Chandler, C.R. & Mulvihill, R.S. 1988. The use of wing shape indices: An evaluation. Ornis Scand. 19: 212–216. doi: 10.2307/3676561
- Clegg, S.M. & Owens, I.P.F. 2002. The ‘island rule’ in birds: medium body size and its ecological explanation. Proc. R. Soc. B: Biol. Sci. 269: 1359–1365. doi: 10.1098/rspb.2002.2024
- Clegg, S.M., Frentiu, F.D., Kikkawa, J., Tavecchia, G. & Owens, I.P.F. 2008. 4000 Years of phenotypic change in an island bird: heterogeneity of selection over three microevolutionary timescales. Evolution 62: 2393–2410. doi: 10.1111/j.1558-5646.2008.00437.x
- Darwin, C. 1859. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life, 1st edn. John Murray, London.
- Dietzen, C., Garcia-Del-Rey, E., Castro, G.D. & Wink, M. 2008. Phylogenetic differentiation of Sylvia species (Aves: Passeriformes) of the Atlantic islands (Macaronesia) based on mitochondrial DNA sequence data and morphometrics. Biol. J. Linnean Soc. 95: 157–174. doi: 10.1111/j.1095-8312.2008.01005.x
- Drovetski, S.V., Rohwer, S. & Mode, N.A. 2006. Role of sexual and natural selection in evolution of body size and shape: a phylogenetic study of morphological radiation in grouse. J. Evol. Biol. 19: 1083–1091. doi: 10.1111/j.1420-9101.2006.01097.x
- DRRF. 2007. Inventário Florestal da Região Autónoma dos Açores. Secretaria Regional da Agricultura e Florestas – SRAF.
- Dudaniec, R.Y., Schlotfeldt, B.E., Bertozzi, T., Donnellan, S.C. & Kleindorfer, S. 2011. Genetic and morphological divergence in island and mainland birds: informing conservation priorities. Biol. Conserv. 144: 2902–2912. doi: 10.1016/j.biocon.2011.08.007
- Fiedler, W. 2005. Ecomorphology of the external flight apparatus of blackcaps (Sylvia atricapilla) with different migration behavior. Ann. N.Y. Acad. Sci. 1046: 253–263. doi: 10.1196/annals.1343.022
- Fitzpatrick, S. 1998. Interspecific variation in wing length and male plumage coloration with migratory behaviour in continental and island populations. J. Avian Biol. 29: 248–256. doi: 10.2307/3677107
- Förschler, M.I. & Siebenrock, K.H. 2007. Morphological differentiation of mainland Citril Finches, Carduelis [citrinella] citrinella and insular Corsican (Citril) Finches, Carduelis [citrinella] corsicanus. Bonner zoologische Beiträge 55: 159–162.
- Förschler, M.I., Siebenrock, K.H. & Coppack, T. 2008. Corsican Finches have less pointed wings than their migratory congeners on the mainland. Vie et Milieu 58: 277–281.
- Galligan, T.H., Donnellan, S.C., Sulloway, F.J., Fitch, A.J., Bertozzi, T. & Kleindorfer, S. 2012. Panmixia supports divergence with gene flow in Darwin's small ground finch, Geospiza fuliginosa, on Santa Cruz, Galápagos Islands. Mol. Ecol. 21: 2106–2115. doi: 10.1111/j.1365-294X.2012.05511.x
- Grant, P.R. 1979. Evolution of the chaffinch, Fringilla coelebs, on the Atlantic Islands. Biol. J. Linnean Soc. 11: 301–332. doi: 10.1111/j.1095-8312.1979.tb00042.x
- Instituto Hidrográfico da Marinha. 2006. World Vector Shoreline, version 1.3. Instituto Hidrográfico da Marinha [Download]. Retrieved from http://www.hidrografico.pt/download-gratuito.php.
- Jenni, L. & Winkler, R. 1989. The feather-length of small passerines: a measurement for wing-length in live birds and museum skins. Bird Study 36: 1–15. doi: 10.1080/00063658909476996
- Komdeur, J., Piersma, T., Kraaijeveld, K., Kraaijeveld-Smit, F. & Richardson, D.S. 2004. Why Seychelles Warblers fail to recolonize nearby islands: unwilling or unable to fly there? Ibis 146: 298–302. doi: 10.1046/j.1474-919X.2004.00255.x
- Lockwood, R., Swaddle, J.P. & Rayner, J.M.V. 1998. Avian wingtip shape reconsidered: wingtip shape indices and morphological adaptations to migration. J. Avian Biol. 29: 273–292. doi: 10.2307/3677110
- Lomolino, M.V. 2005. Body size evolution in insular vertebrates: generality of the island rule. J. Biogeogr. 32: 1683–1699. doi: 10.1111/j.1365-2699.2005.01314.x
- Mathys, B.A. & Lockwood, J.L. 2011. Contemporary morphological diversification of passerine birds introduced to the Hawaiian archipelago. Proc. R. Soc. B: Biol. Sci. 278: 2392–2400. doi: 10.1098/rspb.2010.2302
- McClain, C.R., Durst, P.A.P., Boyer, A.G. & Francis, C.D. 2013. Unravelling the determinants of insular body size shifts. Biol. Lett. 9(1): doi:10.1098/rsbl.2012.0989.
- McNab, B.K. 2002. Minimizing energy expenditure facilitates vertebrate persistence on oceanic islands. Ecol. Lett. 5: 693–704. doi: 10.1046/j.1461-0248.2002.00365.x
- Meiri, S., Dayan, T. & Simberloff, D. 2006. The generality of the island rule reexamined. J. Biogeogr. 33: 1571–1577. doi: 10.1111/j.1365-2699.2006.01523.x
- Meiri, S., Cooper, N. & Purvis, A. 2008. The island rule: made to be broken? Proc. R. Soc. B: Biol. Sci. 275: 141–148. doi: 10.1098/rspb.2007.1056
- Merilä, J. & Sheldon, B.C. 2001. Avian quantitative genetics. In Nolan, V. Jr & Thompson, C.F. (eds) Current Ornithology, Vol. 16: 179–255. Kluwer Academic/Plenum, New York, NY.
- Mönkkönen, M. 1995. Do migrant birds have more pointed wings? A comparative study. Evol. Ecol. 9: 520–528. doi: 10.1007/BF01237833
- Morganti, M., Åkesson, S. & Pulido, F. 2015. Decoupling of behavioural and morphological differentiation in a partially migratory bird population. Bird Study 62: 29–38. doi: 10.1080/00063657.2014.971703
- Pérez-Tris, J., Bensch, S., Carbonell, R., Helbig, A.J. & Tellería, J.L. 2004. Historical diversification of migration patterns in a passerine bird. Evolution 58: 1819–1832. doi: 10.1554/03-731
- Rayner, J.M.V. 1988. Form and function in avian flight. In Johnston, R.F. (ed.) Current Ornithology, Vol. 5: 1–66. Plenum, New York, NY.
- Rising, J.D. & Somers, K.M. 1989. The measurement of overall body size in birds. Auk 106: 666–674. doi: 10.2307/4087777
- Rodrigues, P. 2012. Phylogeography and genetic diversity of the Azores passerines. PhD Thesis, Universidade dos Açores.
- Rodrigues, P., Bried, J., Rodebrand, S. & Cunha, R. 2010. Aves.. In Borges, P.A.V., Costa, A., Cunha, R., Gabriel, R., Gonçalves, V., Martins, A.F., Melo, I., Parente, M., Raposeiro, P., Rodrigues, P., Santos, R.F., Santos, L., Vieira, P. & Vieira, V. (eds) A List of the Terrestrial and Marine Biota from the Azores, 219–221. Princípia, Cascais.
- Saville, D.B.O. 1957. Adaptive evolution in the avian wing. Evolution 11: 212–224. doi: 10.2307/2406051
- Scott, S.N., Clegg, S.M., Blomberg, S.P., Kikkawa, J. & Owens, I.P.F. 2003. Morphological shifts in island-dwelling birds: the roles of generalist foraging and niche expansion. Evolution 57: 2147–2156. doi: 10.1111/j.0014-3820.2003.tb00392.x
- Shirihai, H., Gargallo, G. & Helbig, A.J. 2001. Sylvia Warblers: Identification, Taxonomy and Phylogeny of the Genus Sylvia. Christopher Helm, London.
- Spurgin, L.G., Illera, J.C., Jorgensen, T.H., Dawson, D.A. & Richardson, D.S. 2014. Genetic and phenotypic divergence in an island bird: isolation by distance, by colonization or by adaptation? Mol. Ecol. 23: 1028–1039. doi: 10.1111/mec.12672
- Svensson, L. 1992. Identification Guide to European Passerines. Lars Svensson, Stockholm.
- Van Valen, L. 1973. Pattern and the balance of nature. Evol. Theor. 1: 31–49.
- Wright, N.A. & Steadman, D.W. 2012. Insular avian adaptations on two neotropical continental islands. J. Biogeogr. 39: 1891–1899. doi: 10.1111/j.1365-2699.2012.02754.x
