Abstract
Capsule The breeding Woodcock population in Britain in 2013 was estimated at 55 241 males (95% CL: 41 806–69 004), suggesting a large-scale decline that is supported by 2 additional sources of data.
Aims To provide an updated estimate of the size of Britain's breeding Woodcock population, measure recent trends and identify spatial patterns of change.
Methods Displaying male Woodcock were surveyed at a stratified sample of 834 randomly selected sites. Population estimates were compared with a baseline survey conducted in 2003 and the trend with data from annual Woodcock counts (2003–13) and Bird Atlas 2007–11.
Results Woodcock were estimated to be present at 22% of 1 × 1 km squares containing ≥10 ha of woodland, compared to 35% in 2003. The British population estimate fell by 29% between 2003 and 2013. The Atlas suggests that presence at the 10 × 10 km scale has declined by 56% between 1970 and 2010. Both data sources suggest regional variation in the rate of decline, with losses greatest in the West and South.
Conclusion The Woodcock's population size and breeding range appear to be declining severely across Britain. Regional variation in the rate of decline might be explained by the distribution of large continuous woodlands.
An increasing body of research indicates recent changes in the composition of woodland bird communities in the UK (Amar et al. Citation2006, Hewson et al. Citation2007, Hopkins & Kirby Citation2007, Fuller et al. Citation2007a), with many species showing declines in range and abundance through the latter half of the 20th century (Fuller et al. Citation2005). These may be attributed to a range of hypotheses and their causes are likely to be species-specific (see Fuller et al. Citation2005 for an overview). The declines of several species have been linked to changes in woodland structure and floristic composition, especially reduced understorey density (Amar et al. Citation2006, Hopkins & Kirby Citation2007, Hewson & Noble Citation2009, Hewson et al. Citation2011), as well as reduced floristic diversity (Fuller et al. Citation2005) and the loss of open woodland habitats associated with early successional growth (Fuller et al. Citation2007a, Quine et al. Citation2007). An increasing trend in average woodland age since the large-scale post-war afforestation of the late 1940s and 1950s (Hopkins & Kirby Citation2007, Mason Citation2007), as well as modification, reduction or cessation of management in many woods (Fuller et al. Citation2005, Amar et al. Citation2006) are usually agreed to be important factors driving such changes. In many cases, these issues may be compounded by the impact of increased browsing pressure from rising deer populations (Gill & Fuller Citation2007, Holt et al. Citation2011) and climate change (Leech & Crick Citation2007).
Although the Eurasian Woodcock Scolopax rusticola (hereafter Woodcock) differs ecologically from most of the woodland birds considered in previous studies, it is likely to be susceptible to some of the factors thought to be responsible for their declines. Fuller et al. (Citation2005) were unable to identify any strong hypotheses to account for a decline in Woodcock but they listed disturbance, reduction of the field layer by deer, increasing dryness of woodland and changes in surrounding land management as potentially relevant. Breeding Woodcock require open rides and clearings for display and courtship and have specific habitat requirements during incubation and brood-rearing (Hoodless & Hirons Citation2007). Nesting Woodcock typically utilize woodland with open ground layer vegetation and patches of overhead cover (Hirons & Johnson Citation1987). When feeding, sites where trees are relatively small and close together with a dense shrub or herb layer are preferred (Hirons & Johnson Citation1987, Hoodless & Hirons Citation2007). Woodcock feed primarily on earthworms and other soil-dwelling invertebrates (Hoodless & Hirons Citation2007). During winter, they leave woodland to feed on open fields at night, but this becomes less frequent during the breeding season, when more time is spent foraging within woodland (Hoodless & Hirons Citation2007). This may make soil moisture and the availability of wet feeding areas within woodland an important consideration, particularly where summers have become drier (Smart et al. Citation2006). Because the habitat requirements of Woodcock appear to vary with different stages of the life cycle, heterogeneity of woodland habitats or the landscape as a whole, may have a significant effect on their abundance. Moreover, the Woodcock's status as a quarry species may subject it to additional pressure unique to the species.
The Woodcock is currently ‘amber-listed’ owing to its ‘SPEC 3’ classification as a Species of European Conservation concern (Eaton et al. Citation2009). This is the result of moderate, recent declines elsewhere in its European range, including in its Russian strongholds (Birdlife International Citation2004), though for many regions accurate data are lacking and those used for Britain are probably inaccurate (Hoodless et al. Citation2009). The British Trust for Ornithology's (BTO) Common Birds Census recorded a 74% decline in the UK during 1968–99 (95% CL −49% to −88%), but the estimate is based on only 20 plots in South-eastern Britain (Baillie et al. Citation2009). The Atlases of breeding birds in Britain and Ireland comprise three comprehensive surveys spanning a 40-year period and provide an important insight into changes in local occupancy. The Atlases indicate a clear contraction of −55% in the breeding range of the Woodcock in Britain and Ireland between 1968–72 and 2008–11 (Balmer et al. Citation2013).
Woodcock are nocturnal, exhibit cryptic behaviour and plumage and occur in comparatively low densities even in the strongholds of their range (Fokin et al. Citation2004, Hoodless et al. Citation2009). Consequently, observations tend to be infrequent and the conventional methods used to survey common birds are inadequate for representative sampling of Woodcock. The most suitable alternative relies upon observations of the birds’ crepuscular ‘roding’ display. At dawn and dusk, male Woodcock fly large circuits over woodland and intervening habitat advertising their presence to receptive females with a two-part call (Hirons Citation1980). Woodcock typically rode from late February to mid-July and during the peak of the season (May–June) do so consistently each evening unless inhibited by heavy rain or strong winds (Hoodless et al. Citation2006). This conspicuous display provides an opportunity for reliably observing Woodcock at sites where they occur and counts of the number of passes of roding males have been demonstrated, on the basis of spectrographic validation, to yield a measure of the local abundance of individuals (Hoodless et al. Citation2008).
In 2003, this survey method was employed to estimate Woodcock density, based upon the number of encounters recorded in an hour, in the first large-scale survey in Britain. Extrapolation from a stratified sample of 807 randomly selected 1 × 1 km squares provided regional and national estimates of abundance and the British breeding Woodcock population was estimated at 78 346 males (95% CL 61 717–96 493, Hoodless et al. Citation2009). This estimate was far larger than previous British estimates; Gibbons et al. (Citation1993) tentatively suggested a population of 8500–21 500 pairs in 1988–91, while Baker et al. (Citation2006) estimated 5000–12 500, but both of these sources recognized the difficulty of deriving accurate estimates for the species.
Given the continuing steep decline suggested by the maps in Balmer et al. (Citation2013), repeat surveys are important to assess ongoing status and to provide information that may help identify possible drivers of change. We present the results of the second national survey of breeding Woodcock in Britain conducted in 2013, enabling an evaluation of recent change in Woodcock abundance. We set this in the context of changes in Woodcock distribution over the last 40 years based on breeding atlas data, and alongside the results of annual surveys (2003–13), which provide short-term trend data and highlight the scale of annual fluctuations.
METHODS
Survey stratification
We used the same survey site stratification and sample of random 1 × 1 km squares in 2013 as used for the 2003 survey. To find all potential survey squares, every 1 × 1 km square containing at least 10 ha woodland was identified from the Land Cover Map 2000 (Fuller et al. Citation2002). We specified four woodland size classes (10–30, 31–50, 51–70 and 71–100 ha) and, using a GIS (MapInfo 7.5, MapInfo Corporation Citation2002), determined 11 custom geographic regions () such that each contained similar numbers of squares belonging to the four wood-size classes and, as near as possible, equal numbers of BTO members (potential surveyors). Survey squares were selected using a random number generator, ensuring that the centre of each new square was at least 3 km from the centres of all previously selected squares. Full details of the stratification and justification for our approach are given in Hoodless et al. (Citation2009). The total sample of 2677 squares was made available on the BTO online Woodcock survey application, where potential observers could view their locations and register to survey a square. The 807 random squares where surveys were conducted in 2003 were flagged as high priority to encourage repeat surveys of these sites in 2013.
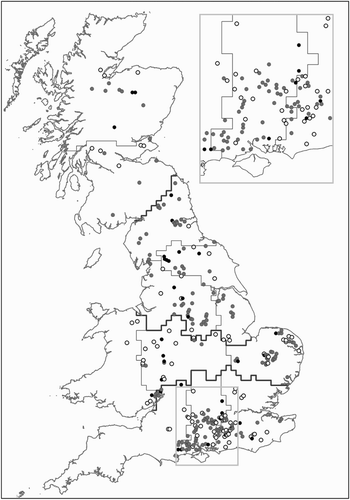
Figure 1. Changes in occupancy of randomly selected 1 km squares where surveys of breeding Woodcock were conducted in both 2003 and 2013. Sites are classified as having remained occupied (grey), lost Woodcock (white) or been colonized (black). Sites which were unoccupied in both years are not shown. Narrow boundary lines delineate the 11 regions used in the Breeding Woodcock Survey, whilst bold lines delineate the 4 aggregated regions used in the GLMMs. The inset map shows part of the Central South region at larger scale for greater clarity of this more intensively surveyed area.
Roding Woodcock surveys
The survey method employed in 2003 was based on Hoodless et al. (Citation2009). A preliminary visit was made in April to find an appropriate survey point within the largest wood in the square, at an open location where the observer's view was not obstructed by the tree canopy. Observers were permitted to select a point up to 400 m outside the allocated square if no other alternative was available. In such cases, the wood-size class was reclassified based on the new 1 × 1 km square. For sites surveyed in 2003, the same approximate count point was used in 2013 unless changes to the habitat had rendered it unsuitable. This meant 69% of repeated surveys were within 100 m of the original 2003 count point and 82% were within 200 m.
Three surveys were made during May–June 2013, each at least one week apart. Where no Woodcock were observed on the preliminary visit and the first two survey visits, the final survey visit was not compulsory. Surveys were not conducted on evenings with continuous rain or wind exceeding Beaufort force 3, so that roding activity and detection of displaying birds were not influenced by weather conditions (Hoodless et al. Citation2006). Surveys began 15 minutes before sunset and lasted for 75 minutes (15 minutes longer than in 2003). On each occasion that a Woodcock was seen or heard, it was recorded as a separate registration and time noted to the nearest minute.
Calculation of population estimates
For each visit, the number of registrations in the first 60 minutes was summed (for compatibility with the 2003 survey). The maximum number (R) of registrations across visits at each site was used to estimate the number of individual male Woodcock (N) using the equation N = 0.74R0.708 (Hoodless et al. Citation2008). Although this relationship has associated errors, the approach was considered better than simply basing analyses on the number of registrations because roding intensity is density-dependent and the relationship between registrations and the number of individuals is non-linear (Hoodless et al. Citation2008). Consequently, a decline in the number of registration does not represent a directly proportional decline in density. It is important to bear in mind, however, that the resulting population estimate should be viewed as an index and that independent evidence demonstrating how well the index correlates with the actual population trend is still required.
Estimates of density were derived from maximum counts, as they are considered to provide a better representation of the total number of males at a site than the mean (Hoodless et al. Citation2006, Citation2008). The estimated number of male Woodcock at each survey point was taken to be approximately equivalent to the density/km2 (Hoodless et al. Citation2009), given that the mean roding area of radio-tracked males was 88 ha (Hirons Citation1980).
A mean density of Woodcock/km2 (Ns) was calculated for each of the 44 strata (i.e. the 11 geographic regions subdivided by the 4 wood-size classes). A national (British) population estimate was generated aswhere Nnp is the national estimate and Ks is the number of 1 × 1 km squares within each stratum. Estimates for each of the 11 regions and for England, Scotland and Wales were obtained in a similar fashion. Confidence limits were produced by bootstrapping, using the ‘SVSTRATIFIED’ procedure in Genstat 16.1 (Lawes Agricultural Trust Citation2006). For each stratum, 1000 boot-strap samples were generated, each with a sample size equal to the number of squares surveyed. From these, confidence limits were calculated via the method described by Sarndal et al. (Citation1992) (see Hoodless et al. Citation2009 for a more detailed description).
The ‘SVSTRATIFIED’ procedure identifies the ten most influential squares by calculating the potential percentage change in the national population estimate when each square is omitted. To bring the influence of all squares below 5%, we increased the sample size for poorly represented strata by including 11 additional surveys conducted in 2014. These were conducted at sites taken from the original random list that had not been surveyed in 2013. We justified their inclusion on the basis that counts at these sites were not significantly different from those recorded in 2013 for their respective strata.
For all 11 regions (r), estimates of presence (Pr) and density in occupied squares (Dr) were calculated asand
where
is the proportion of occupied sites for each stratum in region r;
is the mean density in occupied squares per stratum and
is the stratum weight, calculated as the number of squares per region-wood-size stratum containing at least 10 ha of woodland divided by the total number of squares per region containing at least 10 ha of woodland. The standard error of regional density in occupied squares se(Dr) was calculated as
where se(
) is the standard error associated with
. Mean densities for England, Scotland, Wales and Britain were calculated by multiplying the density in occupied squares by the proportion of occupied squares for each region-wood-size stratum, and summing the values of the relevant strata.
We used a generalized linear model (GLM) with binomial errors and a logit link-function to examine whether occupancy at each survey site (‘present’ vs. ‘absent’) varied according to region and wood-size class. The maximum number of males recorded at each occupied site was examined in relation to region and wood-size class using a Kruskal–Wallis anova.
National and regional population estimates, the proportion of occupied sites per region and the mean number of registrations per region in 2003 and 2013 were compared to provide an indication of population trend over the ten-year period. Variation in mean Woodcock density between 2003 and 2013 was compared at the stratum level using a paired t-test in R (R Core Team Citation2014). More in-depth analysis of population change was confined to repeat surveys of the same sites, for which there were 544 squares. Change in Woodcock abundance based on repeat surveys was analysed using a generalized linear mixed model (GLMM) with a negative binomial distribution and logarithmic link-function. The dependent variable was the maximum number of registrations in 60 minutes, with year, region (aggregated as Scotland, Northern England, Central England and Wales, Southern England; ) and wood-size class as fixed effects and site as a random effect. First- and second-order interactions between each of these three fixed effects were also considered. GLMMs were conducted in R (R Core Team Citation2014) using the glmmADMB package (Skaug et al. Citation2014). A binomial GLM with a logit link-function was used to assess whether local extinction at each repeated survey site correlated with the number of Woodcock observed during the 2003 count.
Annual counts of roding Woodcock
Given that the 2013 Breeding Woodcock Survey was only the second of its kind, a population trend derived from these surveys is limited to two points in time. Surveying sites annually helps validate the trend observed in the main data set and give an indication of the scale of annual fluctuations.
Annual counts were undertaken between 2003 and 2013 at a selection of random survey squares and additional observer-selected sites. Not all sites were covered for the entire 10-year period; the number visited ranged from 18 to 48 per year and averaged 26. Sites were spread across 24 counties and roding Woodcock surveys were conducted using a methodology identical to that used in the 2003 survey. Although these data come from a non-random sample that contains mostly sites with above average Woodcock densities, they provide an insight into year-on-year population change.
The results of the annual counts were analysed using a Poisson GLM, with maximum number of Woodcock registrations at each site as the dependent variable and year as an explanatory variable. Site was included as a second explanatory variable to account for the fact that not all sites were visited every year. Index values, representing annual change and standard errors, were calculated from the model coefficients. A regression line was fitted in R using the model coefficients to provide an average annual rate of decline.
Atlas data
Three atlases covering Britain and Ireland have mapped the distribution of breeding birds during the periods 1968–72, 1988–91 and 2007–11 (hereafter referred to as ‘1970’, ‘1990’ and ‘2010', respectively, Sharrock Citation1976, Gibbons et al. Citation1993, Balmer et al. Citation2013). Full accounts of these are given in Bird Atlas 2007–11 (Balmer et al. Citation2013). We used Atlas data to assess changes in Woodcock distribution and examine whether local losses occurred at random or exhibited a geographic pattern; the latter potentially indicating a retraction of the species’ range. Not only did the use of Atlas data allow analysis of distributional trends over a longer time period than the Breeding Woodcock Survey data, but also presented a more complete picture given that every complete 10 × 10 km square in mainland Britain was surveyed at least once in each Atlas period (Gibbons et al. Citation1993, Balmer et al. Citation2013).
Despite their typically elusive behaviour, it is unlikely that breeding Woodcock were often falsely reported as absent at the 10-km square scale. The Atlas establishes species presences based on ‘roving records’ in which observers were encouraged to record all species present, without time restrictions being imposed. In the latest Atlas, observers were encouraged to conduct dusk visits, when the male's distinctive roding call should have made the presence of Woodcock obvious. If Woodcock were overlooked, it is most likely this would have occurred in earlier Atlases without dusk visits and, therefore, a decline detected by this analysis is unlikely to represent a Type I error. It may, however, result in an underestimate of the rate of decline and so our estimates ought to be viewed as cautious minima.
We assessed the presence of Woodcock at the 10 × 10 km square scale from all three Atlas periods for England, Wales, the Isle of Man and most of Scotland. Data gathered in the Republic of Ireland and Northern Ireland were excluded, as coverage was not as comprehensive as that achieved for Britain. Shetland and Orkney were excluded as there are no recent records of Woodcock breeding in these regions in any of the three Atlases (Balmer et al. Citation2013) and the absence of suitable habitat all but eliminates the possibility. Atlas surveys were conducted during the breeding season (April–July). This analysis only included records of Woodcock where breeding was categorized as ‘probable’ or ‘confirmed’ (see Gillings et al. Citation2013 for explanation of categories), rejecting records of ‘possible’ breeding to avoid inadvertently including observations of non-breeding migrant birds which may remain in the UK as late as mid-April (Hoodless & Coulson Citation1994).
For each Atlas period, Kolmogorov–Smirnov tests and cumulative frequency plots were used to visually compare the distribution of occupied 10 × 10 km squares with that of a spatially random pattern on both a South–North and West–East gradient. Kolmogorov–Smirnov tests and plots were produced in R (R Core Team Citation2014) using the spatstat package (Baddeley & Turner Citation2005).
Change in distribution between 1970 and 1990 and between 1990 and 2010 was examined by classifying 10 × 10 km squares occupied in the first atlas as ‘persisted’ or ‘lost’ depending on whether Woodcock were still present in the latter atlas. A binomial GLM with a logit link-function, using British National Grid X and Y co-ordinates as explanatory variables, was used to determine whether the frequency of local extinctions at the 10 × 10 km square level varied according to South–North and West–East gradients. GLMs were fitted in R (R Core Team Citation2014) using the lme4 package (Bates et al. Citation2012). Separate binomial GLMs were run to examine relationships between change in status of unoccupied squares and their geographic location, with squares classed as ‘remaining unoccupied’ or having ‘gained’ Woodcock.
RESULTS
Data set attributes
In 2013, 823 random squares were surveyed. Eleven extra squares were covered in 2014, giving a grand total of 834 (). Of these, 544 were repeats of squares surveyed in 2003. Discounting the preliminary visit, 88% of squares surveyed received a minimum of 2 visits. Of those where Woodcock were detected, 99% received 2 visits and 89% received the recommended 3 visits. At occupied sites, the number of visits was independent of wood-size class (Fisher's Exact Test P = 0.239) and region (with regions pooled as Scotland, Northern England, Wales and the Midlands, and Southern England, Fisher's Exact Test P = 0.380).
Table 1. Presence of roding Woodcock, mean numbers of registrations (based on maximum count per square) and mean densities in occupied squares in 2003 and 2013 (all weighted by the number of available 1 km squares within each region-wood-size class stratum).
The 44 strata were not surveyed in the same ratios that they occurred in the randomly selected sample (, P < 0.001). Greater than expected coverage was achieved in the South and East of Britain; coverage in the Central South and East Anglia regions, in particular, were proportionally greater than anticipated. Generally, the smallest wood-size class (representing 10–30 ha woodland/km2) was under-represented in the final data set, with the exception of Northern Scotland.
Woodcock distribution and population estimates
Woodcock were recorded at 273 (33%) of the random survey squares. When weighted by the availability of suitable squares within each of the 44 strata, Woodcock presence was estimated at 22% for all 1 × 1 km squares containing ≥10 ha of woodland. Presence varied significantly between regions (GLM , P < 0.001). Northern and Eastern regions held a greater percentage of occupied squares; the three regions with highest rates of occupancy were North Scotland (50%), Northern England (44%) and Eastern England (33%). The lowest occupancy was recorded in Wales (5%), with occupancy also low in South West England (6%) and South Midlands (7%). Likelihood of occupancy differed between the 4 wood-size classes, increasing progressively as woodland area within the 1 × 1 km square increased, from national averages of 17% (10–30 ha woodland/km2) up to 36% (71–100 ha woodland/km2) (GLM
, P < 0.001).
In Britain, the mean density of Woodcock in occupied squares was 3.1 males/km2 (). The highest densities were recorded in Eastern England (4.22 ± 1.23) and the Northern Midlands (4.20 ± 1.26) whilst the lowest densities were recorded in South West England (1.63 ± 0.34) and Wales (1.87 ± 0.52). Regional variation was close to statistical significance (Kruskal–Wallis , P = 0.052). Amongst occupied survey squares, only 19% held densities that exceeded 5 males/km2.
The British Woodcock population was estimated at 55 241 males (95% CL: 41 806–69 004), comprising 24 229 males in England (95% CL 17 563–32 239), 30 098 males in Scotland (95% CL 19 664–41 015) and 914 males in Wales (95% CL: 119–1900) (). The North Scotland region accounted for the majority of the estimated Scottish population (23 913 males, 95% CL 14 796–33 478). Of the English population, 59% resided within the three northernmost regions.
Table 2. Woodcock population size estimates (males) with 95% confidence intervals and percentage change between 2003 (Hoodless et al. Citation2009) and 2013.
Population change
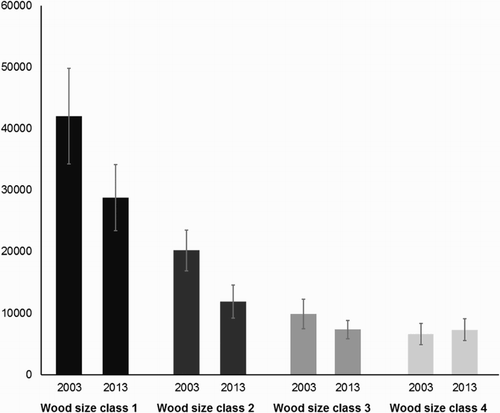
Between 2003 and 2013, the British population estimate dropped from 78 346 males (95% CL 61 717–96 493) to 55 241 males (95% CL 41 806–69 004), representing an overall decline of 29%. Declines were recorded in all 11 regions (), though the scale of decline in North Scotland was less than 1%. Amongst the other regions, declines ranged from −18% in Northern England to −59% in South Scotland. The South West England, East Anglia, Wales, South East England and Central South regions all experienced declines greater than 40%. Population estimates for each of the woodland size classes decreased in all but the 71–100 ha class ().
Figure 2. Estimates of the British population size in 2003 and 2013 for the 4 woodland size classes (1 = 10–30 ha, 2 = 31–50 ha, 3 = 51–70 ha and 4 = 71–100 ha) ±1 se. Estimates and standard errors were produced by bootstrapping.
The percentage of wooded 1 × 1 km squares where Woodcock were present has declined from 35% to 22%. Considering occupied sites only, and accounting for survey stratification, mean density was 3.1 males/km2 in 2013 compared to 2.8 males/km2 in 2003. The mean densities of Woodcock in each of the 44 strata did not vary significantly between 2003 and 2013 (paired t-test t43 = 0.57, P = 0.570).
Amongst repeat survey sites, the percentage of sites at which Woodcock were seen decreased from 47% in 2003 to 37% by 2013 (). These figures are higher than the averages derived from the entire data set because a smaller proportion of squares from the smallest wood-size class were included in the repeat surveys. Of the 258 sites occupied in 2003, 86 sites (33%) recorded zero counts in 2013. There were only 34 sites (12% of sites unoccupied in 2003) where gains were recorded. The loss of Woodcock from once-occupied squares was significantly more likely where the 2003 survey recorded a lower number of registrations (GLM , P = 0.001).
Examination of the change in mean number of registrations revealed a significant three-way interaction between year, the four aggregated regions and wood-size class (GLMM , P = 0.005). The mean number of registrations declined between 2003 and 2013 in all region-wood class strata except in the 10–30 ha category in Scotland, where the maximum number of registrations increased. This was due to large increases in the maximum number of registrations in the North Scotland region, though this is based on small sample sizes, especially in 2003.
Annual trend since 2003
The mean number of registrations for sites visited annually was 14.0 (±1.5) in 2003 and 9.4 (±1.8) in 2013, compared to British national survey averages of 4.0 (±0.2) and 2.7 (±0.2), respectively. The difference is explained by the fact that annual counts were focused on occupied sites (only 9% of sites recorded no Woodcock in any year). The overall trend in the mean number of registrations over the ten-year period was a significant decline (GLM , P < 0.001). The average annual decline was 4.9%, equivalent to a decline of 39.8% over 10 years ().
Figure 3. Trend in the number of Woodcock registrations at annual survey sites between 2003 and 2013. Index values are based on the mean number of registrations observed in 2003 and are back-transformed year coefficients from a Poisson GLM (error bars ± 1 se). A regression line is fitted through the index values.
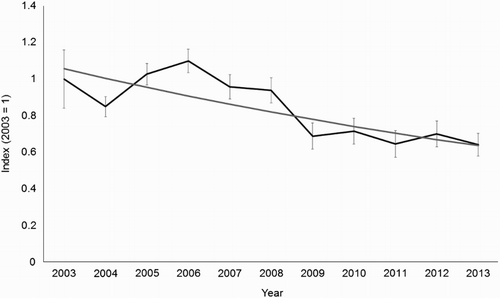
There was high variation in mean counts between years, with annual change ranging from +21% (2004–05) to −27% (2008–09). All of the mean counts prior to 2009 were higher than those after 2009, and the steep decline between 2008 and 2009 makes a large contribution to the downward trend seen over the ten-year period.
Long-term distributional change
Between 1970 and 1990, the number of 10 × 10 km atlas squares where Woodcock were present fell from 1439 to 917, a decline of 36%. By 2010, the number of occupied squares had fallen to 632, a further decline of 31%. Over the whole 40-year period this represents a decline of 56% nationally. The proportion of occupied squares in the West of Britain was lower than would be expected if a completely spatially random distribution existed (Kolmogorov–Smirnov tests 1970: D = 0.1369, P < 0.001; 1990: D = 0.1595, P < 0.001; 2010: D = 0.1889, P < 0.001). On a North–South axis occupancy was lower than expected in the South (Kolmogorov–Smirnov tests 1970: D = 0.0985, P < 0.001; 1990: D = 0.0776, P < 0.001; 2010: D = 0.1451, P < 0.001). These patterns become more pronounced with each subsequent Atlas ().
Figure 4. Chronological maps of Woodcock presence on a 10-km square scale in each of the 3 Atlas periods (1968–72, 1988–91 and 2007–11). Where evidence of breeding was only categorized as ‘possible’, records have been omitted to avoid inadvertent inclusion of migrant Woodcock.
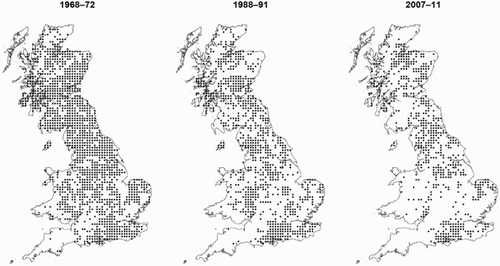
In the 1970 Atlas, Woodcock were generally widespread, with birds absent only from Cornwall, West Devon and the extreme South-west of Wales (). Presence was uniformly high in a region that stretches from Derbyshire and Nottinghamshire northwards to central Scotland and there were relatively high concentrations of occupied 10 × 10 km squares in Northern Scotland, separated from those to the South by the highest peaks of the Cairngorms. West Sussex and Hampshire showed the highest proportion of occupied 10 × 10 km squares in the South of England, with other clusters of occupied squares in Eastern parts of Wales and the Welsh borders (i.e. The Forest of Dean northwards) and East Anglia.
Over the course of the next 40 years these patterns become more exaggerated and fragmentary (). As the number of occupied sites in Southern Scotland declined, the populations of central Scotland and Northern England became more poorly connected and declines in the English Midlands led to the scattered strongholds in the South appearing more distinct. There were large-scale declines in presence in Wales and the South West. Presence in East Anglia declined between 1970 and 1990, then between 1990 and 2010 the number of occupied squares remained broadly the same but the East Anglian population became more isolated from those elsewhere in Southern and central England. Bucking the general North–South trend, the number of occupied 10 × 10 km squares remained high in parts of Hampshire, West Sussex and Surrey. By far the largest cluster of occupied squares South of the Pennines is found in an area encompassing the New Forest National Park, the Western sides of the South Downs National Park and the Surrey Hills Area of Outstanding Natural Beauty.
For the period 1970–1990, there was a significant relationship between the frequency of local extinction (i.e. losses of occupied squares) and longitude (binomial GLM , P = 0.041), indicating that Woodcock were ‘lost’ from a higher proportion of 10 km squares in the West of Britain. For the period 1990–2010, a similar, more pronounced relationship was observed (
, P < 0.001). Between 1990 and 2010, a significant latitudinal trend was also detected (
, P < 0.001), suggesting that losses were greater in the South of Britain. Amongst squares that were not occupied in 1970, the proportion of ‘gains’ by 1990 increased significantly on a West to East gradient (
, P < 0.001), and to a lesser extent from South to North (
, P = 0.008). These patterns were present and more pronounced in the 1990–2010 data (South–North
, P < 0.001, West–East
, P < 0.001).
DISCUSSION
The two Breeding Woodcock Surveys and three Atlases indicate large-scale national declines in site occupancy, ranging from 31% in the Atlas (1990–2010) to 37% in the Breeding Woodcock Survey (2003–13). The same broad geographic trends were observed in both surveys, including low levels of occupancy in Wales, South West England and the South Midlands, and higher than average levels in Northern and Eastern England and Scotland. Changes in regional population estimates demonstrate a decline that is severe but showing regional variation, with Woodcock numbers in the North of England and Northern Scotland generally declining at a lower rate than those in most of Southern England and Wales. Overall, there is clear evidence for a decline that increases in intensity towards the South-west of Britain.
Historical accounts suggest that the Woodcock's British range has always exhibited a bias towards the North and East (Holloway Citation1996). The Western boundary of the Woodcock's main European range lies within the British Isles and abundance appears to increase across Britain, eastwards, with distance from the range edge. Given that breeding Woodcock have only rarely been recorded in Cornwall and South-west Wales in the past (Holloway Citation1996), range is not thought to be physically constrained by the islands’ limits but could be determined by climatic or other geographic factors. This is supported by the fact that the range edge continues through France, and Woodcock are absent as a breeding species from Brittany, Western Normandy and most of Pays-de-la-Loire (Ferrand et al. Citation2008). Some small sedentary Woodcock populations do survive much further South and West than this on the Azores, Madeira and the Canary Islands, but these populations are small with restricted distributions largely confined to mountainous regions (Machado et al. Citation2008). The same is true of small breeding populations in Northern Spain (Braña et al. Citation2013). This tendency to favour upland areas, where soils are moister at more southerly latitudes, suggests that climate probably does, in some instances, limit distribution. The Woodcock's diet of ground-dwelling woodland invertebrates may mean that soil moisture or soil type, in particular, are determining factors.
As far as Britain is concerned, however, such a theory is contradicted by the fact that breeding Woodcock are present in Southern and Eastern regions that experience drier summers than most of the South-west. Since spring and summer rainfall is greater in South-west Britain than, for example, East Anglia, where breeding Woodcock occur, a simple climatic relationship is not immediately apparent. Nevertheless, a more complex explanation that also considers drainage, topography and soil acidity could potentially account for some regional differences in Woodcock presence and this may present a possible line of enquiry for future research.
Current declines appear to be exaggerating the Woodcock's uneven distribution in Britain and increasing fragmentation, as well as the apparent retraction from the South-west, may be due to recent climatic changes increasing the extent of areas in which conditions are unsuitable. If this is so, and British declines are part of a European change in Woodcock abundance at the edges of the species’ range, we may expect to see the same pattern in France. Ferrand et al. (Citation2008), however, state that the French breeding population for the period 1994–2003 remained stable, with an estimated size of 10 000–30 000 pairs (Birdlife International Citation2004). This may not eliminate the possibility of wider declines due to changing climate but could suggest that the negative impact is greater in Britain, preceding future declines elsewhere in Europe, owing to the additive effect of secondary factors specific to Britain or the geographic isolation of Britain's Woodcock populations. It may also be that monitoring in France failed to detect localized declines as there was no consideration of regional variations in population trend and Woodcock surveys were conducted only at sites where extensive woodland was available (squares in the French survey are approximately 2.8 km2 and only those with at least 90% woodland cover are surveyed).
It is important to consider declines at the range edge in relation to trends across the range as a whole (Fuller et al. Citation2007b) and comparison with roding Woodcock surveys conducted in Western Russia, thought to be the species’ European stronghold, sets British declines in a European context. Trend data from Russia are available only over a short time frame and are based on roding ‘intensity’ rather than an estimate of true Woodcock density. Annual Woodcock surveys conducted in Western Russia since 1999 had initially suggested a slight decline (Fokin et al. Citation2004) and this provided the basis of the Woodcock's ‘amber-listed’ status in Europe. Continuation of these surveys, however, points towards a Western Russian population trend that exhibits a high degree of annual variation but is broadly stable between 2000 and 2010 (Fokin & Blokhin Citation2013). These continental Woodcock winter in Britain, and their numbers tend to fluctuate annually according to the severity of weather on the continent, with higher numbers seen in cold winters. However, the long-term trend from records of Woodcock shot indicates that migrant numbers visiting Britain have been stable or even increased during the period of decline of residents (Aebischer & Davey Citation2010).
Like those conducted in Russia, annual surveys in Britain show a large annual variation in breeding Woodcock abundance, which is likely to be related to environmental conditions and their effects on productivity and adult survival. Given that Woodcock feed predominantly on soil invertebrates by probing, extended cold spells with frozen ground in winter and dry summers, that render soil impenetrable, may both detrimentally affect Woodcock numbers in subsequent years. Our annual counts showed a sharp decline between 2008 and 2009 which made a large contribution to the overall downward trend seen in the ten-year period. This decline coincides with a cold winter in 2008/09, followed by similarly cold winters in 2009/10 and 2010/11 which may have continued to suppress the breeding population. This may have exaggerated the rate of decline observed in the ten years between 2003 and 2013 but, based upon all the evidence, is unlikely to be solely responsible for it. Continuation of these annual counts to accumulate a longer data series should, in the future, enable separation of the effects of stochastic weather events on annual productivity and over-winter survival from the underlying trend.
Irrespective of other factors, the Woodcock's uneven distribution in Britain appears to be partly linked to the availability of large areas of woodland. Analysis of the paired survey data indicates that 1 × 1 km squares suffering local extinctions were usually sites with low Woodcock densities in 2003, and that this was more common within the smaller wood-size classes. According to the Atlas and the Breeding Woodcock Survey, Hampshire, West Sussex and Surrey do not conform to the general North–South distribution. The region in which occupancy remained high coincides with an area that is well wooded – not just in the provision of large continuous blocks of woodland, as seen in the New Forest, but also in areas with numerous, medium-sized woods that are relatively well connected, as in parts of the South Downs National Park.
Generally, recent losses have exaggerated an apparent association with large woods. Several declining woodland specialists, such as Pied Flycatcher Ficedula hypoleuca (Huhta et al. Citation1998), Marsh Tit Poecile palustris (Broughton & Hinsley Citation2015) and Western Capercaillie Tetrao urogallus (Dolman et al. Citation2007) also show a preference for heavily wooded landscapes with large forested patches and high levels of connectivity.
The specificity of the Woodcock's habitat requirements are not fully understood, but the species’ once broad range, as shown by the 1968–72 Atlas, suggests some adaptability. Moreover, unlike many woodland specialists, Woodcock do not rely on mature forest and appear to require areas with at least some young growth or clearings. Perhaps habitat complementarity is a more important consideration (Dunning et al. Citation1992) as radio-tracking studies suggest that habitats associated with nesting and chick-rearing differ (Hirons Citation1988, Hoodless & Hirons Citation2007). It may be that larger woods usually offer a greater diversity of stand type and ages as well as a more diverse range of micro-climates, ensuring the availability of wet feeding areas throughout the summer.
This hypothesis is consistent with recent trends recorded in Wales and Southern Scotland where, despite extensive areas of woodland, Woodcock have declined significantly. In these areas, woods exhibit a low diversity of age and stand type despite their large size as they comprise mostly commercial, coniferous forestry which is only utilized by Woodcock in its early stages (Shorten Citation1974). Beyond the thicket stage, reached at 15–20 years, it is no longer suitable and regional declines may be due to an increase in mean woodland age as the large areas of forestry planted during 1935–85 mature (Hopkins & Kirby Citation2007).
The respective influences of geographic location, climate, wooded area and woodland diversity are difficult to separate, given that they are so intrinsically linked and inevitably influenced by a range of additional secondary factors. There are areas of Southern England, for instance, where Woodcock have declined in spite of the fact that relatively well-wooded landscapes remain (e.g. South-eastern Weald in Kent and the Chilterns). In such regions, causes of decline are likely to be complex and could include factors operating on a local scale or interacting at different spatial scales.
Rising deer numbers may affect breeding Woodcock by reducing the extent of understorey vegetation, resulting in inferior nesting habitats and drier soils, and in extreme cases, by increasing the likelihood of disturbance and trampling (Gill & Fuller Citation2007). The impacts of deer upon woodland birds have been documented both in Europe and North America (Allombert et al. Citation2005, Chollet & Martin Citation2013), but the vast majority of existing research focuses on passerines, particularly those that nest and feed in understory vegetation. Although Holt & Fuller (Citation2013) found that intensive deer browsing had no significant effect on the local distribution of Woodcock at Bradfield Woods, their study focused on Woodcock abundance during winter when habitat requirements are less specific than those during the breeding season.
The significance of recreational disturbance has been demonstrated for other ground-nesting species such as European Nightjars Caprimulgus europaeus and Wood Larks Lullula arborea (Langston et al. Citation2007), which makes the recreational use of woodlands, particularly by dog-walkers, another potential cause for concern. Changes in woodland management have been implicated in the loss of open areas within woodland (Fuller et al. Citation2007a, Quine et al. Citation2007) and these are an important requirement of the Woodcock's breeding display. Hunting may have a local effect on over-winter survival (Duriez et al. Citation2004), but the impact is hard to quantify given that hunting in Britain occurs in winter when migrant Woodcock are also present and outnumber residents by about ten to one (Hoodless et al. Citation2013).
Survey accuracy and future considerations
Despite a large sample size overall, several of the 44 strata were poorly represented in both 2003 and 2013, meaning that these stratum estimates are calculated from relatively few surveys. Since survey coverage is dependent on the distribution of potential observers, low survey uptake was expected in less densely populated areas but this is problematic as coverage is lowest in areas where Woodcock densities are thought to be highest. Northern Scotland, which we predict may hold 43% of the national breeding population, has an estimate based on 5% of the completed surveys and Northern England may be similarly, though less acutely, affected. The bias in coverage is greatest in the 2003 Breeding Survey, meaning that the 2013 estimate represents an improvement in accuracy of the population estimate, but comparison of the two figures should be treated with some caution. Particular effort was made to recruit volunteers in regions where low coverage was expected, and this appears to have been effective, but it may be wise for future surveys to pre-empt the shortfall and hire professional fieldworkers to ensure minimum adequate coverage. It should also be borne in mind that our calculations rely on a somewhat artificial link between the maximum number of contacts with roding Woodcock in 60 minutes and an unknown number of males present in the 1 × 1 km square, and hence the estimate is best used as an index of population size for comparison between surveys. Nevertheless, we feel that the range contraction since 1970 makes an attempt to estimate of the size of the British breeding Woodcock population necessary in order to gauge the urgency with which conservation actions are required. Independent evidence to gauge how accurately changes in our population estimates correlate with actual population trend would help justify this methodology.
Demographic modelling of the breeding Woodcock population in the UK presents an attractive prospect for future research, but is not currently possible owing to very limited data for birds ringed in the breeding season. Mark-recapture models could provide a valuable insight into spatial heterogeneity in Woodcock survival rates (Péron et al. Citation2013) and we would encourage the establishment of a network of sites where attempts are made to ring and recapture Woodcock in the breeding season. Whether this would result in a viable study, however, remains to be seen as obtaining sufficient data, particularly in areas experiencing declines, might require an unfeasibly high catching effort.
Notwithstanding these issues, this survey illustrates the importance of understanding species-specific behaviour in order to develop effective and accurate survey methodologies for species of conservation importance. Whilst large-scale atlas and multi-species monitoring projects can provide long-term data on general trends and distribution, bespoke methods are required to gather reliable information on densities and fine-scale distribution change.
ACKNOWLEDGEMENTS
We are grateful to all the volunteers who conducted the surveys and to the Regional Representatives who encouraged participation. We thank Dan Chamberlain and Julie Ewald for their advice and help with survey design and stratification in 2003; Nicki Read for undertaking the data entry in 2003 and Iain Downie for implementing the online data capture in 2013. We appreciate the constructive comments made by Guillaume Péron and an anonymous referee on an earlier draft of the manuscript.
FUNDING
The 2003, 2013 and annual Woodcock surveys were funded by the Game & Wildlife Conservation Trust, the Shooting Times Woodcock Club and an anonymous English charitable trust. Bird Atlas 2007–11 was organized by the British Trust for Ornithology (BTO), BirdWatch Ireland and the Scottish Ornithologist's Club and funded by legacies, charitable trusts and individual donations through a BTO appeal.
REFERENCES
- Aebischer, N. & Davey, P. 2010. National Gamebag Census: data back to Darwin. Game & Wildlife Conservation Trust Review of 2009, pp. 30–33.
- Allombert, S., Gaston, A.J. & Martin, J. 2005. A natural experiment on the impact of overabundant deer on songbird populations. Biol.Conserv. 126: 1–13. doi: 10.1016/j.biocon.2005.04.001
- Amar, A., Hewson, C.M., Thewlis, R.M., Smith, K.W., Fuller, R.J., Lindsell, A., Conway, G., Butler, S. & MacDonald, M.A. 2006. What's Happening to Our Woodland Birds? Long Term Changes in the Populations of Woodland Birds. BTO Research Report No. 169 and RSPB Research Report No.19. BTO/RSPB, Thetford/Sandy.
- Baddeley, A. & Turner, R. 2005. spatstat: An R package for analyzing spatial point patterns. J. Stat. Software 12: 1–42. doi: 10.18637/jss.v012.i06
- Baillie, S.R., Marchant, J.H., Leech, D.I., Joys, A.C., Noble, D.G., Barimore, C., Grantham, M.J., Riseley, K. & Robinson, R.A. 2009. Breeding birds in the winder countryside: their conservation status (1972–1996). A report of the BTO's integrated population monitoring. British Trust for Ornithology Research Report 516.
- Baker, H., Stroud, D.A., Aebischer, N.J., Cranswick, P.A., Gregory, R.D., McSorley, C.A., Noble, D.G. & Rehfisch, M.M. 2006. Population estimates of birds in Great Britain and the United Kingdom. Br. Birds 99: 25–44.
- Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. & Fuller, R.J. 2013. Bird Atlas 2007–11: The Breeding and Wintering Birds of Britain and Ireland. BTO Books, Thetford.
- Bates, D., Maechler, M. & Bolker, B. 2012. lme4: Linear mixed-effects models using S4 classes. URL http://CRAN.R-project.org/package=lme4. R package version 0.999999–0.
- Birdlife International. 2004. Birds in Europe: Populations Estimates, Trends and Conservation Status. Birdlife International, Cambridge.
- Braña, F., González-Quirós, P., Prieto, L. & González, L. 2013. Spatial distribution and scale-dependent habitat selection by Eurasian Woodcocks Scolopax rusticola at the south-western limit of its continental breeding range in northern Spain. Acta Ornithol. 48: 27–37. doi: 10.3161/000164513X669973
- Broughton, R. & Hinsley, S. 2015. The ecology and conservation of the marsh tit in Britain. Br. Birds 108: 12–28.
- Chollet, S. & Martin, J. 2013. Declining woodland birds in North America: should we blame Bambi? Diversity Distrib. 19: 481–483. doi: 10.1111/ddi.12003
- Dolman, P.M., Hinsley, S.A., Bellamy, P.E. & Watts, K. 2007. Woodland birds in patchy landscapes: the evidence base for strategic networks. Ibis 149: 146–160. doi: 10.1111/j.1474-919X.2007.00748.x
- Dunning, J.B., Danielson, B.J., & Pulliam, H.R. 1992. Ecological processes that affect populations in complex landscapes. Oikos 65: 169–175. doi: 10.2307/3544901
- Duriez, O., Eraud, C., Barbraud, C. & Ferrand, Y. 2004. Factors affecting population dynamics of Eurasian Woodcocks wintering in France: assessing the efficiency of a hunting free reserve. Biol. Conserv. 122: 89–97. doi: 10.1016/j.biocon.2004.07.002
- Eaton, M.A., Brown, A.F., Noble, D.G., Musgrove, A.J., Hearn, R.D., Aebischer, N.J., Gibbons, D.W., Evans, A. & Gregory, R.D. 2009. Birds of Conservation Concern 3: the population status of birds in the United Kingdom, Channel Islands and Isle of Man. Br. Birds 102: 296–341.
- Ferrand, Y., Gossmann, F., Bastat, C. & Guénézan, M. 2008. Monitoring of the winter and breeding Woodcock populations in France. Rev. Catalana d'ornitol. 24: 24–52.
- Fokin, S. & Blokhin, Y. 2013. Monitoring of the Woodcock population in European Russia (1996–2010). In Ferrand, Y. (ed.) Proc. Seventh European Woodcock and Snipe Workshop, 29–35. Office National de la Chasse et de la Faune Sauvage, Paris.
- Fokin, S., Blokhin, Y., Zverev, P., Kozlova, M. & Romanov, Y. 2004. Spring migration of the Woodcock, Scolopax rusticola, and roding in Russia in 2004. Woodcock & Snipe Specialist Group Newslett. 30: 4–8.
- Fuller, R.M., Smith, G.M., Sanderson, R.A., Hill, A.G., Thompson, A., Cox, R., Brown, N.J., Clarke, R.T., Rothery, P. & Gerald, F. 2002. Countryside Survey 2000. Module 7, Land Cover map 2000. Centre for Ecology and Hydrology, Dorset.
- Fuller, R.J., Noble, D.G., Smith, K.W. & Vanhinsbergh, D. 2005. Recent declines in populations of woodland birds in Britain. Br. Birds 98: 116–143.
- Fuller, R.J., Smith, K.W., Grice, P.V., Currie, F.A. & Quine, C.P. 2007a. Habitat change and woodland birds in Britain: implications for management and future research. Ibis 149: 269–268.
- Fuller, R.J., Gaston, K.J., & Quine, C.P. 2007b. Living on the edge: British and Irish woodland birds in a European context. Ibis 149: 53–63. doi: 10.1111/j.1474-919X.2007.00734.x
- Gibbons, D.W., Reid, J.B. & Chapman, R.A. 1993. The New Atlas of Breeding Birds in Britain and Ireland: 1988–1991. Poyser, London.
- Gill, R.M.A. & Fuller, R.J. 2007. The effects of deer browsing on woodland structure and songbirds in lowland Britain. Ibis 149: 119–127. doi: 10.1111/j.1474-919X.2007.00731.x
- Gillings, S., Balmer, D.E., Caffrey, B.J. & Swann, R.L. 2013. Survey methods and data sources. In Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. & Fuller, R.J. (eds) Bird Atlas 2007–11: The Breeding and Wintering Birds of Britain and Ireland, 33–45. BTO Books, Thetford.
- Hewson, C.M. & Noble, D.G. 2009. Population trends of breeding birds in British woodlands over a 32 year period: relationships with food, habitat use and migratory behaviour. Ibis 151: 464–486. doi: 10.1111/j.1474-919X.2009.00937.x
- Hewson, C.M., Amar, A., Lindsell, J.A., Thewlis, R.M., Butler, S., Smith, K. & Fuller, R.J. 2007. Recent changes in bird populations in British broadleaved woodland. Ibis. 149: 14–28. doi: 10.1111/j.1474-919X.2007.00745.x
- Hewson, C.M., Austin, G.E., Gough, S.J. & Fuller, R.J. 2011. Species-specific responses of woodland birds to stand-level habitat characteristics: the dual importance of forest structure and floristics. Forest Ecol. Manag. 261: 1224–1240. doi: 10.1016/j.foreco.2011.01.001
- Hirons, G. 1980. The significance of roding by Woodcock Scolopax rusticola: an alternative explanation based on observations of marked birds. Ibis 122: 350–354. doi: 10.1111/j.1474-919X.1980.tb00888.x
- Hirons, G. 1988. Habitat use by Woodcock (Scolopax rusticola) during the breeding season. In Havet, P. & Hirons, G. (eds) Proc. Third European Woodcock and Snipe Workshop, 42–47. IWRB, Slimbridge.
- Hirons, G. & Johnson, T.H. 1987. A quantitative analysis of habitat preferences of Woodcock, Scolopax rusticola, in the breeding season. Ibis 129: 371–381. doi: 10.1111/j.1474-919X.1987.tb03181.x
- Holloway, S. 1996. The Historical Atlas of Breeding Birds in Britain and Ireland 1875–1900. Poyser, London.
- Holt, C.A. & Fuller, R.J. 2013. An experimental assessment of the effect of deer on use of young coppiced woodland by Eurasian Woodcocks Scolopax rusticola in winter. Wader Study Group Bull. 120: 124–127.
- Holt, C.A., Fuller, R.J. & Dolman, P.M. 2011. Breeding and post-breeding responses of woodland birds to modification of habitat structure by deer. Biol. Conserv. 144: 2151–2162. doi: 10.1016/j.biocon.2011.05.004
- Hoodless, A.N. & Coulson, J.C. 1994. Survival rates and movements of British and Continental Woodcock Scolopax rusticola in the British Isles. Bird Study 41: 48–60. doi: 10.1080/00063659409477197
- Hoodless, A.N. & Hirons, G.J.M. 2007. Habitat selection and foraging behaviour of breeding Eurasian Woodcock Scolopax rusticola: a comparison between contrasting landscapes. Ibis. 149: 234–249. doi: 10.1111/j.1474-919X.2007.00725.x
- Hoodless, A., Lang, D., Fuller, R.J., Aebischer, N. & Ewald, J. 2006. Development of a survey method for breeding Woodcock and its application to assessing the status of the British population. In Ferrand, Y. (ed) Proc. 6th European Woodcock and Snipe Workshop, 48–54. Wageningen, The Netherlands: Wetlands International.
- Hoodless, A.N., Inglis, J.G., Doucet, J-P. & Aebischer, N.J. 2008. Vocal individuality in the roding calls of Woodcock Scolopax rusticola and their use to validate a survey method. Ibis 150: 80–89. doi: 10.1111/j.1474-919X.2007.00743.x
- Hoodless, A.N., Lang, D., Aebischer, N.J. Fuller, R.J. & Ewald, J.A. 2009. Densities and population estimates of breeding Eurasian Woodcock Scolopax rusticola in Britain in 2003. Bird Study 56: 15–25. doi: 10.1080/00063650802674768
- Hoodless, A.N., Powell, A., Ferrand, Y., Gosler, A.G., Fox, J., Newton, J. & Williams, O. 2013. Application of new technologies to the study of Eurasian Woodcock migration. In Ferrand, Y. (ed) Proc. Seventh European Woodcock and Snipe Workshop, 29–35. Office National de la Chasse et de la Faune Sauvage, Paris.
- Hopkins, J.J. & Kirby, K.J. 2007. Ecological change in British broadleaved woodland since 1947. Ibis. 149: 29–40. doi: 10.1111/j.1474-919X.2007.00703.x
- Huhta, E., Jokimäki, J. & Rahko, P. 1998. Distribution and reproductive success of the Pied Flycatcher Ficedula hypoleuca in relation to forest patch size and vegetation characteristics; the effect of scale. Ibis 140: 214–222. doi: 10.1111/j.1474-919X.1998.tb04382.x
- Langston, R.H.W., Wotton, S.R., Conway, G.J., Wright, L.J., Mallord, J.W., Currie, F.A., Drewitt, A.L., Grice, P.V., Hoccom, D.G. & Symes, N. 2007. Nightjar Caprimulgus europaeus and Woodlark Lullula arborea – recovering species in Britain? Ibis 149: 250–260. doi: 10.1111/j.1474-919X.2007.00709.x
- Lawes Agricultural Trust. 2006. Genstat 9 Release 1. Reference Manual. Oxford University Press, Oxford.
- Leech, D.I. & Crick, H.Q.P. 2007. Influence of climate change on the abundance, distribution and phenology of woodland bird species in temperate regions. Ibis. 149: 128–145. doi: 10.1111/j.1474-919X.2007.00729.x
- Machado, A.L., Brito, J.C., Mederios, V., Leitao, M., Moutinho, C., Jesus, A., Ferrand, Y. & Goncalves, D. 2008. Distribution and habitat preference of Eurasian Woodcock Scolopax rusticola in S. Miguel Island (Azores) during the breeding season. Wildlife Biol. 14: 129–137. doi: 10.2981/0909-6396(2008)14[129:DAHPOE]2.0.CO;2
- MapInfo Corporation. 2002. MapInfo Professional. MapInfo Corporation, New York, NY.
- Mason, W.L. 2007. Changes in the management of British forests between 1945 and 2000 and possible future trends. Ibis. 149: 41–52. doi: 10.1111/j.1474-919X.2007.00696.x
- Péron, G., Ferrand, Y., Leray, G. & Gimenez, O. 2013. Waterbird demography as indicator of wetland health: the French-wintering common snipe population. Biol. Conserv. 164: 123–128. doi: 10.1016/j.biocon.2013.04.015
- Quine, C.P., Fuller, R.J., Smith, K.W. & Grice, P.V. 2007. Stand management: a threat or opportunity for birds in British woodland? Ibis 149: 161–174. doi: 10.1111/j.1474-919X.2007.00742.x
- R Core Team. 2014. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. URL http://www.R–project.org/
- Sarndal, C., Swenssion, B. & Wretman, J. 1992. Model Assisted Survey Sampling. Springer-Verlag, New York, NY.
- Sharrock, J.T.R. 1976. The Atlas of Breeding Birds in Britain and Ireland. Poyser, Calton.
- Shorten, M. 1974. The European Woodcock (Scolopax rusticola). A search of the literature since 1940. Game Conservancy Report No. 21. 95 pp.
- Skaug, H., Fournier, D.A., Ancheta, J., Ianelli, J., Magnusson, A., Maunder, M., Nielsen, A. & Sibert, J. 2014. Generalised linear mixed models using AD model builder. R Package version 0.8.0.
- Smart, J., Gill, J.A., Sutherland, W.J. & Watkinson, A.R. 2006. Grassland-breeding waders: identifying key habitat requirements for management. J. Appl. Ecol. 43: 454–463. doi: 10.1111/j.1365-2664.2006.01166.x
