ABSTRACT
Capsule: The Red-backed Shrike Lanius collurio and the Barred Warbler Sylvia nisoria had similar habitat preferences and their territories often overlapped. However, we found that Red-backed Shrikes were more flexible in habitat choice whilst Barred Warblers had more specific requirements.
Aim: We aimed to analyse and compare distribution and habitat preferences of Red-backed Shrikes and Barred Warblers breeding sympatrically in semi-natural landscape in a wetland/farmland mosaic.
Methods: We examined habitat availability and use by the two species within their breeding territories to identify differences in habitat selection.
Results: Territories of both species were similar in habitat composition and used levees, bushes, fallow areas and single trees. However, the spatial characteristics of the territories differed between species. Red-backed Shrikes used a wider range of sizes and shapes of habitat patches, whilst Barred Warblers preferred a more complex landscape structure and a higher diversity of habitat types. We also found that areas of 71% of Barred Warbler and 34% of Red-backed Shrike territories overlapped.
Conclusion: Whilst both species showed similar habitat choices, they appeared to differ significantly in terms of landscape structure: Red-backed Shrikes were more flexible and less selective than Barred Warblers in their habitat choice.
Natural resources (such as habitat or food) and inter-specific interactions (e.g. competition and predation) have been recognized as the main factors influencing size and density of bird populations (Newton Citation1998). Birds choose their breeding habitats based on various environmental traits, suitable for their species-specific requirements, in regard to nesting, singing, avoiding predation and foraging (Cody Citation1985). Since quality of breeding habitat may affect breeding success, optimal areas (e.g. with high nest site availability, high food richness, small number of predators etc.) attract many individuals, resulting in high densities of breeding pairs (Fretwell Citation1972).
Habitat selection depends not only on habitat features per se but also on inter- and intra-specific interactions (Krebs Citation1997). Whilst species may compete for various resources, factors limiting competition, such as differences in habitat use or diet, are crucial to enable co-existence (Krebs Citation1997). Protective nesting associations provide an example of such co-existence (Richardson & Bolen Citation1999; Quinn & Ueta Citation2008), where less aggressive species (e.g. small passerines) use a protective umbrella of more aggressive heterospecifics (often larger species, such as birds of prey), nesting in close proximity (Bang et al. Citation2005). The association of two species with similar body size and similar level of aggression is less frequent (Isenmann & Fradet Citation1995; Richardson & Bolen Citation1999). Nevertheless, there are species pairs that are similar to each other in various aspects but which still manage to co-exist. The Red-backed Shrike Lanius collurio and the Barred Warbler Sylvia nisoria are a good example of such a pair of co-nesting species (Schmidt Citation1981; Kuźniak & Tryjanowski Citation2003; Goławski & Kuźniak Citation2015a, Citation2015b).
Small passerines generally avoid nesting close to Red-backed Shrikes due to its aggression to potential rivals, nest predation (Cramp Citation1992) and predation of small birds (Lefranc & Worfolk Citation1997). Despite that, the smaller Barred Warbler frequently nests close to the Red-backed Shrike. Kuźniak et al. (Citation2001) suggested that close breeding of the two species is solely a result of similar habitat preferences. Polak (Citation2014) classified it as a facultative mutualism, showing that both species benefit from nesting in association. Indeed, both species have significantly higher reproductive success when nesting close to each other than when nesting in isolation (Neuschulz Citation1988; Goławski Citation2007; Polak Citation2014). The advantages of breeding in close proximity result from the high level of aggression of both Red-backed Shrikes and Barred Warblers towards potential nest predators and active defence of broods (Cramp Citation1992, Cramp & Perrins Citation1993; Tryjanowski & Goławski Citation2004; Goławski & Mitrus Citation2008). Close breeding of the two species is possible through nesting niche partitioning, that is, a different preference for the height of nest location (Gotzman Citation1965; Kuźniak et al. Citation2001) and different foraging strategies (Gotzman Citation1965). The co-occurrence of Red-backed Shrikes and Barred Warblers has been well documented (Dresscher Citation1910; Schmidt Citation1981; Neuschulz Citation1988; Cramp Citation1992; Schönfeld Citation1998), however, habitat preferences of the two species from the areas of their sympatric occurrence are still poorly understood (Polak & Filipiuk Citation2014).
Both species prefer nesting in extensive agriculture and set-aside habitats, such as bushes or fallow fields (Goławski Citation2006b; Polak Citation2012; Polak & Filipiuk Citation2014). After a considerable decline in the European breeding populations of both species in the 1960s (Bibby Citation1973; Tucker & Heath Citation1994; Lefranc & Worfolk Citation1997; Tryjanowski et al. Citation2006), recent population trends are stable or uncertain (European Bird Census Council Citation2015; BirdLife International Citation2018), although Polish populations seem to have increased since 2005 (Kuczyński & Chylarecki Citation2012; Chodkiewicz et al. Citation2016). Nevertheless, since areas of suitable habitat are currently disappearing across the whole of Europe (BirdLife International Citation2013; European Environment Agency Citation2015), both the Red-backed Shrike and the Barred Warbler are still threatened by population declines (e.g. Brambilla et al. Citation2007). As migrants, both species are also influenced by factors operating on their wintering grounds which, in turn, may affect breeding populations (Krebs Citation1997; Lefranc & Worfolk Citation1997). In this context, detailed knowledge of habitat preferences of both species is important for their conservation.
Here, we examined distribution patterns and habitat preferences of the Red-backed Shrike and the Barred Warbler, breeding sympatrically in a semi-natural mosaic of wetland and agricultural habitats in north Poland. Habitat characteristics of the study area offered optimal foraging and breeding conditions, so we expected high breeding densities of both species. Considering the high habitat diversity of the study area, we expected that territories of both species would be non-randomly distributed, situated mainly in set-aside habitats (e.g. bushes, woodlots, fallow fields). Given similar habitat preferences of both species (Kuźniak et al. Citation2001; Polak & Filipiuk Citation2014), we also expected territory overlap of Red-backed Shrikes with Barred Warblers. However, considering the higher densities of Red-backed Shrike comparing to Barred Warbler reported from similar habitats in Poland (Kuźniak et al. Citation2001; Goławski Citation2007; Polak Citation2012; Polak & Filipiuk Citation2014), we expected a higher density of Red-backed Shrike territories, including those occupied exclusively by this species. We also expected, that the spatial structure of the landscape (landscape configuration and composition) might be an important factor in territory choice for both Red-backed Shrike and Barred Warbler.
Methods
Study area and field methods
We conducted the study in the Druzno Lake Natura 2000 area in Żuławy Elbląskie, the alluvial plain in the Vistula River delta, Poland. The semi-natural landscape was formed of a mosaic of habitats between agriculture landscape and wetlands around Druzno Lake, a shallow and overgrowing eutrophic water body. Vegetation around the lake was dominated by reed-beds, willow shrubs and alder forests. The whole lake and wetland areas were separated from surrounding farmland by levees. Farmland areas consisted mainly of arable fields and extensively used meadows and pastures, with a large area of fallow, shrubs (mainly willows Salix sp. and Black Elder Sambucus nigra) and small woodlots (Buliński et al. Citation2013).
Numbers of breeding territories of Red-backed Shrikes and Barred Warblers were estimated along eleven linear transects (total length 32.1 km, range 1.6–5.1 km) around the lake in the 100 m buffer zone on each side of the transect: total width 200 m. The central axes of transects were canals or levees. The study area covered in total 644 ha ().
Figure 1. Location of the study area: (a) Druzno Lake in Europe, (b) Druzno Lake with the transect line, a 200 m wide buffer zone (100 m each side) and territories of Red-backed Shrikes (RBS, black dots) and Barred Warblers (BW, white dots) and (c) zoomed transect line with the buffer zone.
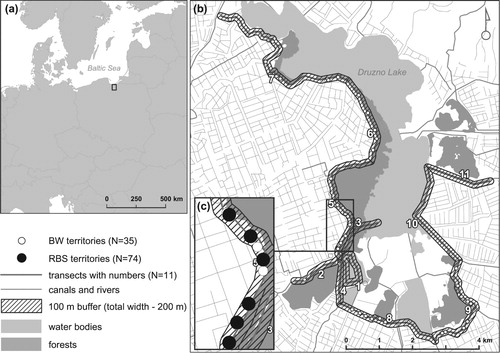
Each transect was monitored five times during the breeding seasons of both species, that is from 17 May to 3 July 2014, every 6–11 days. Monitoring was carried out on foot or using a boat (in canal transects) during the whole day from 06:00 to 19:00 hours. Each location of Red-backed Shrikes and Barred Warblers was recorded with a global positioning system (GPS) handset receiver (Garmin GPSmap 62). For each bird we also recorded sex (if possible) and type of behaviour (see below).
Breeding status
We classified all observations of the studied species as: (a) ‘probable breeding’ when we observed a male and female together, an individual at least twice at the same place, and/or an individual with nesting material and/or alarm calling and (b) ‘certain breeding’ when we observed parent bird/s carrying food or faecal sacs, and/or fledglings being fed outside the nest (Sikora et al. Citation2007). In cases when individuals were observed only once and did not meet requirements of probable or certain breeding, the observations were excluded from the analysis.
Breeding territories
Boundaries of the territories were plotted based on records fulfilling the criteria of probable or certain breeding described above. First, minimal convex polygons were plotted, based on positions (3–5) where individuals of a given territory were recorded. We considered the geometrical centre of the polygon as the centre of the territory (Polak & Filipiuk Citation2014). In cases where only two positions were recorded, the point located at the middle distance between those positions was considered as a territory centre. Then, we plotted the territories as circles around the centre of territory with 70 m radius for Red-backed Shrike (Goławski & Meissner Citation2008) and 50 m radius for Barred Warbler (Waldenström et al. Citation2004) resulting in areas of 1.539 ha for Red-backed Shrike and 0.785 ha for Barred Warbler. In total, we denoted territories of 74 pairs of Red-backed Shrikes and 35 pairs of Barred Warblers.
Landscape structure
To characterize habitat structure of the study area and territories of Red-backed Shrike and Barred Warbler, we used various landscape metrics. We have chosen arbitrary metrics describing landscape features that are possibly important for habitat choice in both species, for example, size and shape of patches, mutual location and diversity (all habitat variables are described in ). We calculated landscape metrics in FRAGSTATS 4.2 software (McGarigal et al. Citation2012) based on data about habitat types, transformed into a raster dataset, that is, a matrix consisting of pixels (cells of the same size), where each pixel has a value. Here each pixel had a value identifying it as a habitat type. Adjacent pixels of the same value create ‘patches’ which are areas covered by one habitat type. In this study, the ‘landscape’ is considered as a mosaic of different patches of habitat types within the boundary of the study area or breeding territory. ‘Landscape structure’ should be interpret as a spatial structure of the landscape, for example, size of patches, their shape and mutual location as well as diversity of habitat types.
Table 1. The description of the landscape metrics used in the analyses to characterize landscape structure of the study area and breeding territories of the Red-Backed Shrike and the Barred Warbler. Pixels – cells of the same size; adjacent pixels with the same habitat type create a ‘patch’ of one habitat type; ‘class’ – is a specific habitat type (all patches of one habitat type); ‘landscape’ – a mosaic of different patches of various habitat types within a defined boundary (here, a boundary of the study area or territory); ‘landscape structure’ – a spatial structure of the landscape, e.g. diversity of habitat types, size of patches, their shape and mutual location; ‘edge segments’ – pixels located at the edge of the patch; ‘area of interest’ (AOI) – here transect buffer (the study area, 200 m wide) or breeding territories of the birds.
Habitat characteristics
Habitat types were characterized within and outside birds’ territories, within the 100 m buffer zones around transects (the study area, total width 200 m). We distinguished 18 habitat types () based mainly on field observations supported by up-to-date colour orthophotomaps from 2014 (provided by Google Earth Pro). We calculated the proportion of each habitat type within the territories of both species using Quantum GIS software (Valmiera 2.2.0; QGIS Development Team Citation2014).
Table 2. Characteristics and relative area (%) of habitat types in all studied buffers combined, ordered descendingly by relative area of habitat types.
Statistical analyses
To compare (1) the distance between territories, (2) relative area of habitat types and (3) values of the various landscape metrics (listed in ) between Red-backed Shrikes and Barred Warblers, we used the non-parametric Mann–Whitney U tests due to non-normal distribution of this data.
Spearman’s rank correlation tests were used to examine relationships between species densities on transects and: (1) proportion of habitat types within the same transect buffers and (2) values of landscape metrics of transect buffers (within the 200 m buffer zone). Separate correlation analyses were performed for the two metrics due to the relatively small number of transects (N = 11). To eliminate the problem of multiple correlations, we applied the Bonferroni correction (Simes Citation1986).
Multivariate ordination analyses were used to compare: (1) habitat composition variability of Red-backed Shrike and Barred Warbler territories, (2) values of Jacobs preference index (D; see below for description) and (3) habitat composition variability between Red-backed Shrike territories overlapping and not overlapping Barred Warbler territories. Unimodal methods were used in both indirect [the Detrending Correspondence Analysis (DCA)] and direct [the Canonical Correspondence Analysis (CCA)] ordination, with bird species as predictors and habitat types within territories or Jacobs preference index values as response variables. Firstly, to visualize the variability of species territories and recognize the length of the gradient of predictors (i.e. bird species), we performed the DCA. The length of the gradient is the measure of variability expressed in standard deviation (sd) units and describes the type of data distribution (<3 sd for linear and >4 sd for unimodal data distribution). To test the relationship between response variables and predictors, we performed the direct ordination. In our data all gradient values were >3 sd which justifies use of the unimodal method, the CCA (Braak & Šmilauer Citation2002; Šmilauer & Lepš Citation2014). Due to non-normal distribution, all data were transformed using a log(x + 1) transformation. To check statistical significance of canonical axis, we performed Monte Carlo permutation test. This test, except of the pseudo-F and the P value, calculates the percentage of possible explained variability of tested parameters (here: variability of habitat composition). In the case of non-significant differences, values of explained variability are low, due to lack of variability. We visualized results on graphs with two ordination axes, where Axis 1 (horizontal) represents the main axis of predictor variability (explains the greatest part of data variability) and Axis 2 (vertical) is the second most important axis explaining a lower part of overall variability. Each axis is characterized by the eigenvalue, which measures the amount of variability explained by this axis (the explanatory power of the axis). Each axis explains as much variability as possible and eigenvalues decrease with the order of the axis (Braak & Šmilauer Citation2002; Šmilauer & Lepš Citation2014). In our study, we have two predictors and due to that only one axis in the CCA is canonical. The other axes are not canonical, since only one independent constraint can be formed from the predictors. The distance between points representing particular territories or habitat types represents the similarity between them – the shorter the distance, the higher the similarity (Braak & Šmilauer Citation2002; Šmilauer & Lepš Citation2014).
To examine habitat preferences in the territories of the two species, and compare habitat types used versus those available within the transect buffer area, we performed compositional analysis. Additionally, to examine the strength and direction (preference or avoidance) of habitat selectivity towards habitat types, we calculated the Jacobs preference index (D) (Jacobs Citation1974) separately for Red-backed Shrikes and Barred Warblers:where r is the proportion of the habitat type in the occupied territories (used) and p is the proportion of the same habitat type in transect buffer (available). This index ranges from −1.00 (total avoidance) to 1.00 (strong preference). Values of zero indicates that the type of habitat represented in a territory was used in the same proportion that was represented in the buffer. First, we calculated D values for each habitat type in each territory and then, due to non-normal distribution, we calculated the median D value for the habitat type for the bird species.
To investigate overall spatial patterns of territories, we compared the distribution of distances between territories of the two nearest neighbours of each species separately, using the Shapiro–Wilk test. To compare proportion of overlapping and exclusive Red-backed Shrike territories, we used the χ2 test.
We performed ordination analyses (DCA, CCA) in Canoco version 4.5 (Braak & Šmilauer Citation2002) and compositional analysis applying compana function from adehabitatHS package (Calenge Citation2006) in R 3.3.2. software (R Core Team Citation2016). All other analyses were carried out in STATISTICA 10 (Statsoft Incorporated Citation2011). We considered results significant at the level of P < 0.05.
Results
Landscape structure
Territories of Red-backed Shrikes and Barred Warblers differed significantly in landscape structure. Territories of Red-backed Shrike were characterized by significantly higher landscape shape index, range of patch area and range of radius of gyration. Territories of Barred Warblers had significantly higher patch density, patch richness density and Simpson’s evenness index than those of Red-backed Shrikes (). Other landscape metrics were similar for both species (all P > 0.05). There was no significant relationship between densities of both species and landscape metric values in the transect buffers (Spearman’s rank correlation with the Bonferroni correction, all P > 0.05, ).
Habitat characteristics
The territories of Red-backed Shrike consisted mainly of levees, fallow fields, meadows and arable land. Levees, fallow fields, bushes and meadows served as the main components of Barred Warbler territories (). Relative area of levees in Red-backed Shrike territories was significantly lower compared to those occupied by Barred Warblers (Mann–Whitney U test, Z35,74 = −4.67, P < 0.001; ). Proportions of other habitat types were similar in territories of both species (all P > 0.05; ).
Figure 2. The proportion of habitat types in the territories of Red-backed Shrikes (RBS) and Barred Warblers (BW). The star denotes a statistically significant difference (Mann–Whitney U test, Z35,74 = −4.67, P < 0.001). The codes of habitat types are described in .
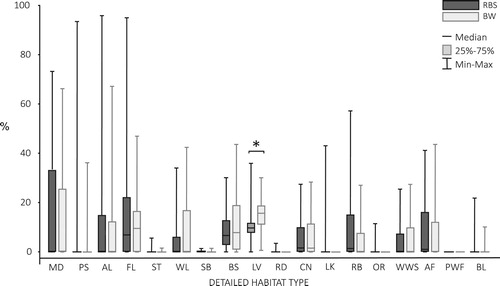
Table 3. Proportions of four most frequently used habitat types within the territories of Red-backed Shrikes and Barred Warblers. Q1 and Q3 are 25% and 75% percentiles. Habitat types ordered alphabetically.
Table 4. Comparison of the landscape metrics for the territories of Red-backed Shrikes and Barred Warblers. Q1 and Q3 are 25% and 75% percentiles. For description of landscape metrics, see . Only metrics differing significantly (P < 0.05) between the two bird species are presented. Landscape ordered ascendingly by P value.
In contrast to landscape structure, habitat composition of the territories did not differ significantly between the two species, which is confirmed by low values of explained variability (Monte Carlo permutation test, first canonical axis explained 1.0% of variability possible to explain, pseudo-F = 1.06, P = 0.36, ). However, habitat variability in Barred Warbler territories tended to be smaller compared to territories of Red-backed Shrikes ((a)). Red-backed Shrikes showed slightly higher preference towards meadows, single trees, arable lands and fallow fields than did Barred Warblers. Barred Warblers chose levees, wet willow shrubs, bushes and woodlots slightly more frequently than did Red-backed Shrikes. In addition, the gradient of data was >4 sd which means that some Red-backed Shrike territories included habitats not found in the Barred Warbler territories (e.g. roads, other rushes and lake; (b)). According to the Jacobs preference index (D), both species clearly preferred levees (Red-backed Shrike: D = 0.24; Barred Warbler: D = 0.43), fallow fields (D = 0.15; D = 0.28) and bushes (D = 0.15; D = 0.19), and avoided other habitat types. The values of the Jacobs preference index were not related to the species (Monte Carlo permutation test, first canonical axis explained 0.94% of variability possible to explain, pseudo-F = 1.00, P = 0.42).
Figure 3. Characteristics of Red-backed Shrike (RBS) and Barred Warbler (BW) breeding territories: (a) ordination diagram from the DCA analysis displaying first two axes, explaining, respectively, 18% and 12% of the total variability in habitat composition of breeding territories of: Red-backed Shrike (black diamonds) and Barred Warbler (white diamonds) and (b) predictors (species) – response variables (habitat types) biplot from the CCA analysis, visualizing similar habitat preferences of the two species. The average proportion of specific habitat type, calculated in relation to other habitat types (black triangles) and species (grey triangles). Ordination Axis 1 (horizontal) – the one canonical axis, characterizes the most important variability in habitat composition; represents the length of the gradient in proportion of habitat types in the species’ territories (calculated in number of standard deviations of data). Axis 2 (vertical) – additional axis; explains second most important range of variability. The closest distance between symbols the higher similarity. The codes of habitat types are given in .
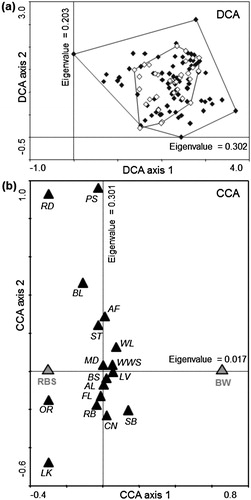
Habitat types within the territories of both Red-backed Shrike and Barred Warbler differed significantly from the overall study area (Red-backed Shrike: Δ = 0.091, P = 0.002 and Barred Warbler: Δ = 0.017, P = 0.002 by randomization). The simplified matrix ranked habitat types (by rows) in order to their preference for the Red-backed Shrike and Barred Warbler (for the ranking order see online Table S1 for Red-backed Shrike and online Table S2 for Barred Warbler). The top-ranking habitat type in Red-backed Shrike territories, i.e. levees, showed significantly greater use than remaining habitat types (compared to their availability at the study area; Table S1). In Barred Warblers, levees were also used significantly more frequently compared to its availability (Table S2).
Inter-species overlap
Significantly more Barred Warbler territories (91%) than territories of Red-backed Shrike (44%) were located close (territories overlapped) to the co-occurring species (
= 21.65, P < 0.001). The majority of Barred Warbler territories (71%) were located within 50 m of the centre of a Red-backed Shrike territory, and 40% were entirely within a Red-backed Shrike territory. In contrast, only 34% of Red-backed Shrike territories were located within 50 m of the centre of a Barred Warbler territory.
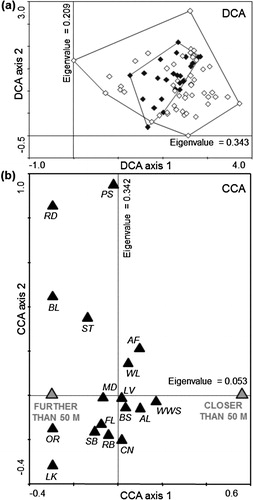
Habitat composition in Red-backed Shrike territories located within 50 m of Barred Warbler territories differed significantly from those further away (Monte Carlo permutation test, first canonical axis explained 2.5% variability possible to explain, pseudo-F = 2.4, P = 0.006). Red-backed Shrike territories overlapping significantly with those of the Barred Warbler which consisted mainly of levees, wet willow shrubs, bushes, arable lands and woodlots. Territories occupied exclusively by Red-backed Shrikes were characterized by more meadows, fallow land, single bushes, reed-beds and single trees. Moreover, such habitat types as roads, buildings, other rushes and lake surface were represented only in Red-backed Shrike territories distant from Barred Warbler territories ().
Figure 4. Characteristics of Red-backed Shrike territories located within 50 m of Barred Warbler territories in comparison with those located over 50 m away: (a) ordination diagram from the DCA analysis displaying first two axes, explaining, respectively, 20% and 12% of the total variability in habitat composition of Red-backed Shrike territories located up to 50 m (black diamonds) and those located over 50 m (white diamonds) and (b) predictors (two groups of Red-backed Shrike territories) – response variables (habitat types) biplot from the CCA analysis, visualizing significantly different habitat preferences of these two groups of territories (grey triangles) towards the habitat composition, displayed as the average proportion of habitat type in relation to other habitat types (black triangles). The codes of habitat types are given in .
Breeding densities
Mean breeding density was estimated at 2.3 territories per km of linear transect (range 0.6–4.8 territories per km) for the Red-backed Shrike and 1.1 territories per km (range 0.0–2.5) for the Barred Warbler. Analysis of distance between territories of the two nearest neighbours of given species indicated a non-random distribution (Shapiro–Wilk test, Red-backed Shrike: W = 0.838, P < 0.001, Barred Warbler: W = 0.792, P < 0.001). The territories of the Red-backed Shrike tended to be situated closer to each other (median = 274 m, percentiles 25–75% = 193–544 m) compared to those of the Barred Warbler (median = 360 m, 25–75% = 239–822 m; Mann–Whitney U test, Z35,74 = 1.83, P = 0.067). Densities of the two species were not related to the area of habitat types along the transects (Spearman’s rank correlation with Bonferroni correction, all P > 0.05).
Discussion
We examined habitat characteristics and territory distribution for Red-backed Shrikes and Barred Warblers breeding sympatrically in a mosaic of agricultural and wetland habitats. To our knowledge, this is the first study to explore landscape structure in the territories of both species.
Despite similarity in territory composition, both species showed significant differences in landscape structure selectivity. Red-backed Shrike territories had a simpler landscape structure with fewer habitat types. However, this species seemed to be more flexible in habitat choice, using a significantly wider range of patch sizes and shapes than the Barred Warbler. In contrast to the Red-backed Shrike, Barred Warblers tended to select more complex and diverse landscapes, with a higher density and evenness (more equal distribution of area among patches) of habitat patches and higher diversity of habitat types. Barred Warblers were also more specific in choice of size and shape of habitat patches. All these results suggest a stronger preference of the Barred Warbler for particular habitat structure, a lower tolerance to suboptimal habitat and, therefore, a lower plasticity in nesting habitat choice, compared to the Red-backed Shrike. The close vicinity of the lake area, other rushes and roads were used only by Red-backed Shrikes. This additionally suggests, that this species is more flexible in habitat choice and able to breed even in suboptimal conditions, e.g. in close proximity to anthropogenic areas and wetlands. Density in transects of both species was related to neither the particular habitat type, nor to the richness and diversity of the transect buffer mosaic.
The breeding territories of both Red-backed Shrike and Barred Warbler were non-randomly distributed and the habitat within territories differed significantly from a random selection of the available habitats. As in other areas (Kuper et al. Citation2000; Kuźniak et al. Citation2001; Waldenström et al. Citation2004; Goławski Citation2007; Polak Citation2012; Polak & Filipiuk Citation2014), the Red-backed Shrike used levees, bushes, single bushes, fallow areas and single tress more frequently than expected, given availability of such habitats. Fallow areas with high shrubs and bushes are often used by this species as nest sites (Horvath et al. Citation2000), and bushes and single trees serve as perches for hunting and vigilance (Cramp Citation1992). Preference of levees and fallow areas is likely to be associated with the Red-backed Shrike’s main feeding strategy, which is hunting of invertebrates moving on the ground in open areas, often with low vegetation (mown or grazed). The highest biomass of Red-backed Shrike’s main prey items, that is invertebrates larger than 10 mm (Cramp Citation1992; Tryjanowski et al. Citation2003; Goławski Citation2006a) has been reported from such habitats (Goławski & Goławska Citation2008). Also dirt roads with power lines running along levees provided a surface without vegetation and many vantage points for detecting and hunting large invertebrates (Morelli et al. Citation2016). As in other studies (Polak Citation2012; Polak & Filipiuk Citation2014), Red-backed Shrikes avoided anthropogenic areas, water and wetland habitats such as reed-beds, alder forest and wet willow shrubs. Similarly, the territories of Barred Warblers were non-randomly distributed, being located within habitat types characterized by levees, bushes, single bushes, single trees and fallow areas. Such habitat types serve as potential nest sites for Barred Warblers as well as perches for singing males (Cramp Citation1992; Payevsky Citation1999). Preference of bushes and trees, observed also in East Poland (Polak Citation2012; Polak & Filipiuk Citation2014), could also be a consequence of the feeding strategy (collecting insects from tree branches) and the rather secretive behaviour of this species (Schmidt Citation1981; Cramp Citation1992). Barred Warbler preference towards riverbank habitats have been reported by many authors (Dyrcz Citation1991; Czechowski et al. Citation2002; Wesołowski et al. Citation2003; Wilniewczyc Citation2005; Brauze Citation2007). Our study area resembles a riverbank environment with a linearly distributed mosaic of wet willow shrubs, bushes, levees and agricultural areas (arable, meadows and pastures). This similarity could explain the high density of Barred Warblers in the present study.
As we prediccted, the majority of Barred Warbler territories (71%) in our study were located within 50 m of the centre of Red-backed Shrike territories and a considerable proportion (40%) of Barred Warbler territories were located entirely inside Red-backed Shrike territories. Also, in West Poland, 82% and 100% of Barred Warbler territories overlapped with those of Red-backed Shrike, in a degraded pine forest and intensively used farmland, respectively (Kuźniak et al. Citation2001). In East Poland, 92% of Barred Warbler and 21–42% of Red-backed Shrike territories were located close to each other (Goławski Citation2007; Polak & Filipiuk Citation2014). Kuźniak et al. (Citation2001) suggested, that in optimal habitats offering plenty of randomly distributed nesting places, Barred Warblers will nest further from Red-backed Shrikes more frequently. Due to this conclusion we suggest, that our study area offers good breeding conditions with plenty of nest sites for the Barred Warbler, as a great proportion of Barred Warbler territories (29%) were distant (>50 m) from Red-backed Shrike territories.
The density of Red-backed Shrikes in our study was higher than mean values reported from most regions in Poland and some other European countries (e.g. Kuźniak Citation1991; Pugacewicz Citation1997; Winiecki et al. Citation1997; Dombrowski et al. Citation2000; Sikora Citation2007; Brambilla et al. Citation2009; online Table S3). Higher densities have been noted in some sites in Poland, Italy and Hungary, mainly in traditionally used agricultural areas or abandoned open areas with vegetation succession. Similarly, the density of Barred Warbler territories was higher compared to most regions of Poland, with the exception of a few sites (e.g. Pugacewicz Citation1997; Sikora Citation2007; Polakowski Citation2013; online Table S4). Observed relatively high average densities of Red-backed Shrike and Barred Warbler (very high in case of some transects) may have resulted from nesting in an optimal habitat mosaic. High habitat heterogeneity and high contributions of semi-natural habitats around Druzno Lake, affect positively the diversity of invertebrates (main prey of both species; Goławski Citation2006a, Goławski & Goławska Citation2008) and constitute a suitable nesting habitat. On the other hand, comparisons with literature should be treated with some reservation as data were collected by various authors in different habitat mosaics and using different methods (i.e. study plots and linear transects) which could affect density values.
Due to the methods applied, some limitations are inherently associated with our study. We conducted the surveys in transects along the levees, representative mainly for a narrow zone along the reserve border, characterized by a high diversity habitat mosaic. Thus, some habitat types, for example, levees and bushes, might be overrepresented compared to areas situated further from the reserve border. All this might affect density and distribution of the birds’ territories as the study area does not include potentially more unsuitable habitat further from the lake edge. Also the territory size might affect probability of presence of particular habitat types in breeding territories. Moreover, we have not included in our study micro-habitat features (e.g. vertical structure of vegetation or plant community composition), which might affect habitat selection of both species (Polak Citation2012).
In conclusion, our study demonstrated that both the Red-backed Shrike and the Barred Warbler shared similar habitat preferences in terms of habitat types but differed significantly in selectivity towards the spatial structure of territories. Overall, the Red-backed Shrike appeared to be a more flexible and tolerant species compared with the Barred Warbler, which was more selective in habitat choice. Our results suggest that spatially diverse and mosaic habitats in semi-natural landscapes of the alluvial delta area of the Vistula River, with prevalence of traditional agricultural use (i.e. set-aside habitats such as bushes and fallow fields), provides good breeding conditions for the Red-backed Shrike and the Barred Warbler. Nevertheless, expected intensification in agriculture and a move towards rape and maize monocultures, may negatively affect breeding and foraging conditions for both the Red-backed Shrike and the Barred Warbler.
Supplemental Material
Download MS Word (38.5 KB)Acknowledgements
We thank Adam Bernatowicz for help in the fieldwork and spatial analyses. We are also very grateful to Adrian Zwolicki for valuable statistical advice and to Piotr Dzieszko for great help in performing landscape metrics analyses. We also thank anonymous reviewers for helpful comments on earlier version of the manuscript and George Day for linguistic editing. The study was performed under permission of the Provincial Nature Protection Bureau. Source of hydrographic data used to create the map of the study area is the Map of Hydrographic Division of Poland made by Institute of Meteorology and Water Management on request of the Minister of the Environment and financed by the National Fund of Environmental Protection and Water Management.
ORCID
Zuzanna Pestka http://orcid.org/0000-0001-5126-4346
Additional information
Funding
References
- Bang, J., Jensen, B. & Sunde, P. 2005. Wood pigeons Columba palumbus breeding in open land associate with Kestrel Falco tinnunculus nests. Bird Study 52: 93–95. doi: 10.1080/00063650509461378
- Bibby, C. 1973. The Red-backed Shrike: a vanishing British species. Bird Study 20: 103–10. doi: 10.1080/00063657309476365
- BirdLife International. 2013. State of the world’s birds: indicators for our changing world. Cambridge: BirdLife International.
- BirdLife International. 2018. IUCN Red List for birds. http://www.birdlife.org.
- Braak, C.J.F. & Šmilauer P. 2002. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: software for canonical community ordination (version 4.5). https://www.canoco.com.
- Brambilla, M., Rubolini, D. & Guidali, F. 2007. Between land abandonment and agricultural intensification: habitat preferences of Red-backed Shrikes Lanius collurio in low-intensity farming conditions. Bird Study 54: 160–167. doi: 10.1080/00063650709461471
- Brambilla, M., Casale, F., Bergero, V., Crovetto, G.M., Falco R., Negri I., Siccardi P. & Bogliani, G. 2009. GIS-models work well, but are not enough: Habitat preferences of Lanius collurio at multiple levels and conservation implications. Biol. Conserv. 142: 2033–2042. doi: 10.1016/j.biocon.2009.03.033
- Brauze, T. 2007. Population size and habitat preferences of the Barred Warbler Sylvia nisoria in floodplain terrace of lower Vistula River. Notatki Ornitologiczne 48: 1–10 (in Polish).
- Buliński, M., Markowski, R. & Sągin, P. 2013. Szata roślinna rezerwatu ‘Jezioro Drużno’. In Nitecki, C. (ed) Jezioro Druzno – Monografia Przyrodnicza, 33–82. Wyd. Mantis, Olsztyn (in Polish).
- Calenge, C. 2006. The package ‘adehabitat’ for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 197: 516–519. doi: 10.1016/j.ecolmodel.2006.03.017
- Chodkiewicz, T., Meissner, W., Chylarecki, P., Neubauer, G., Sikora, A., Pietrasz, K., Cenian, Z., Betleja, J., Kajtoch, Ł., Lenkiewicz, W., Ławicki, Ł., Rohde, Z., Rubacha, S., Smyk, B., Wieloch, M., Wylegała, P., Zielińska, M. & Zieliński, P. 2016. Monitoring Ptaków Polski w latach 2015–2016. Biuletyn Monitoringu Przyrody 15: 65–77 (in Polish).
- Cody, M.L. 1985. An introduction to habitat selection in birds. In Cody, M.L. (ed) Habitat Selection in Birds, 3–56. Academic Press, New York.
- Cramp, S. 1992. Handbook of the Birds of Europe, the Middle East and North Africa. The Birds of the Western Palearctic, Vol. VI: Warblers. Oxford University Press, Oxford.
- Cramp, S. & Perrins, C.M. 1993. Handbook of the Birds of Europe, the Middle East and North Africa. The Birds of the Western Palearctic, Vol. VII: flycatchers to shrikes. Oxford University Press, Oxford.
- Czechowski, P., Rubacha, S., Wąsicki, A., Bocheński, M., Jędro, G., Kajzer, Z. & Sidelnik, M. 2002. Awifauna lęgowa środkowego odcinka doliny Odry. Notatki Ornitologiczne 43: 163–176 (in Polish).
- Dombrowski, A., Goławski, A., Kuźniak, S. & Tryjanowski, P. 2000. Stan i zagrożenia populacji gąsiorka Lanius collurio w Polsce. Notatki Ornitologiczne 42: 139–148 (in Polish).
- Dresscher, E. 1910. Bestehen Beziehungen zwischen Sperbergersmücke und Würger. Ber Ver Schles Ornithol. 3: 68 (in German).
- Dyrcz, A. 1991. Jarzębatka – Sylvia nisoria (Bechst., 1758). In Dyrcz, A., Grabiński, W., Stawarczyk, T. & Witkowski, J. (eds) Ptaki Śląska. Monografia faunistyczna, 374. Wrocław University, Wrocław (in Polish).
- European Bird Census Council 2015. Trends of common birds in Europe, 2015 update. http://www.ebcc.info/index.php?ID = 587.
- European Environment Agency. 2015. State of Nature in the EU. Reporting under the nature directives 2007-2012. EEA Technical Report. Publications Office of the European Union, 65–95. doi: 10.2800/603862
- Fretwell, S.D. 1972. Populations in a Seasonal Environment. Princeton University Press, Princeton, NJ.
- Goławski, A. 2006a. Comparison of methods for diet analysis and prey preference: A case study on the Red-backed Shrike Lanius collurio. Ornis Fenn. 83: 108–116.
- Goławski, A. 2006b. Biologia lęgowa gąsiorka Lanius collurio w ekstensywnym krajobrazie rolniczym wschodniej Polski. Notatki Ornitologiczne 47: 1–10 (in Polish).
- Goławski, A. 2007. Does the Red-backed Shrike (Lanius collurio L.) benefit from nesting in the association with the Barred Warbler (Sylvia nisoria Bechst.)? Pol. J. Ecol. 55: 601–604.
- Goławski, A. & Goławska, S. 2008. Habitat preference in territories of Red-Backed Shrike Lanius collurio and their food richness in an extensive agriculture landscape. Acta Zool Hungar 54: 89–97.
- Goławski, A. & Kuźniak, S. 2015a. Jarzębatka Sylvia nisoria. In Chylarecki, P., Sikora, A., Cenian, Z. & Chodkiewicz, T. (eds) Monitoring ptaków lęgowych. Poradnik metodyczny, 2nd edn, 597–601. GIOŚ, Warszawa (in Polish).
- Goławski A. & Kuźniak S. 2015b. Gąsiorek Lanius collurio. In Chylarecki P., Sikora A., Cenian Z., Chodkiewicz T (eds) Monitoring ptaków lęgowych. Poradnik metodyczny, 2nd edn, 549–553. GIOŚ, Warszawa (in Polish).
- Goławski, A. & Meissner, W. 2008. The influence of territory characteristics and food supply on the breeding performance of the Red-backed Shrike (Lanius collurio) in an extensively farmed region of eastern Poland. Ecol. Res. 23: 347–353. doi: 10.1007/s11284-007-0383-y
- Goławski, A. & Mitrus, C. 2008. What is more important: nest site concealment or aggressive behaviour? A case study of the Red-backed Shrike Lanius collurio. Ann. Zool. Fenn. 57: 403–410.
- Gotzman, J. 1965. Die transspecifischen raumlichen beziehungen zwischen dem Neuntöter (Lanius collurio L.) und Sprbergersmücke (Sylvia nisoria Bechst.)] in der Brutzeit. Ekol. Pol. A 13: 1–22 (in German).
- Horvath, R., Farkas, R. & Yosef, R. 2000. Nesting ecology of the Red-backed Shrike (Lanius collurio) in northeastern Hungary. Ring 22: 127–132.
- Isenmann, P. & Fradet, G. 1995. Is the nesting association between the Orphean Warbler (Sylvia hortensis) and the Woodchat Shrike (Lanius senator) an anti-predator oriented mutualism? J. Ornihol. 136: 288–291. doi: 10.1007/BF01651297
- Jacobs, J. 1974. Quantitative measurement of food selection. Oecologia 14: 413–417. doi: 10.1007/BF00384581
- Krebs, C.J. 1997. Ekologia. Analiza eksperymentalna występowania i rozmieszczenia, 4th edn. Polskie Wydawnictwo Naukowe, Warszawa (in Polish).
- Kuczyński, L. & Chylarecki, P. 2012. Atlas pospolitych ptaków lęgowych Polski: rozmieszczenie, wybiórczość siedliskowa, trendy, 148. GIOŚ, Warszawa (in Polish).
- Kuper, J., van Duinen, G., Nijssen, M., Geertsman, M. & Esselink, H. 2000. Is the decline of the Red-backed Shrike (Lanius collurio) in the Dutch costal dune area caused by the decrease in insect diversity? Ring 22: 11–25.
- Kuźniak, S. 1991. Breeding ecology of the Red-backed Shrike Lanius collurio in the Wielkopolska region (Western Poland). Acta Ornithol. 26: 67–84.
- Kuźniak, S. & Tryjanowski, P. 2003. Gąsiorek. Wydawnictwo Klubu Przyrodników, Świebodzin (in Polish).
- Kuźniak, S., Bednorz, J. & Tryjanowski, P. 2001. Spatial and temporal relations between the Barred Warbler Sylvia nisoria and the Red-backed Shrike Lanius collurio in the Wielkopolska region (W Poland). Acta Ornithol. 36: 129–133. doi: 10.3161/068.036.0205
- Lefranc, N. & Worfolk, T. 1997. Shrikes. A Guide to the Shrikes of the World, 87–93. Pica Press, Sussex.
- McGarigal, K., Cushman, S.A. & Ene, E. 2012. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps. Computer software program produced by the authors at the University of Massachusetts, Amherst. http://www.unmass.edu./landeco/research/fragstats/fragstats.html.
- Morelli, F., Mróz, E., Pruscini, F., Santolini, R., Goławski, A. & Tryjanowski, P. 2016. Habitat structure, breeding stage and sex affect hunting success of breeding Red-backed Shrike (Lanius collurio). Ethol. Ecol. Evol. 28: 136–147.
- Neuschulz, F. 1988. Zur Synökie von Sperbergersmücke Sylvia nisoria (Bechst. 1975) und Neuntöter Lanius collurio (L. 1958): Ergebnisse einer populationsbiologischen Studie. Luchow – Dannenberger Orn Jber 234 (in German).
- Newton, I. 1998. Population Limitation in Birds. Academic Press, London.
- Payevsky, V.A. 1999. Breeding biology, morphometrics and population dynamics of Sylvia warblers in the Eastern Baltic. Avian Ecol. Behav. 2: 19–50.
- Polak, M. 2012. Habitat preferences of Sympatric Barred Warbler (Sylvia nisoria) ant the Red-backed Shrike (Lanius collurio) breeding in central Poland. Ann. Zool. Fenn. 49: 355–363. doi: 10.5735/086.049.0509
- Polak, M. 2014. Protective nesting association between the Barred Warbler Sylvia nisoria and the Red-backed Shrike Lanius collurio: An experiment using artificial and natural nests. Ecol. Res. 29: 949–957. doi: 10.1007/s11284-014-1183-9
- Polak, M. & Filipiuk, M. 2014. Preferencje siedliskowe jarzębatki Sylvia nisoria i gąsiorka Lanius collurio na Środkowym Roztoczu. Ornis Polonica 55: 22–33 (in Polish).
- Polakowski, M. 2013. Wysokie zagęszczenie jarzębatki Sylvia nisoria w Kotlinie Biebrzańskiej w latach 2012 i 2013. Ornis Polonica 54: 205–211 (in Polish).
- Pugacewicz, E. 1997. Awifauna lęgowa Puszczy Białowieskiej, 108, 123, 196–197, 210–211. Północnopodlaskie Towarzystwo Ochrony Ptaków, Białowieża (in Polish).
- QGIS Development Team. 2014. QGIS Geographic Information System. Open Source Geospatial Foundation Project. https://www.qgis.org/.
- Quinn, J.L. & Ueta, M. 2008. Protective nesting associations in birds. Ibis 150: 146–167. doi: 10.1111/j.1474-919X.2008.00823.x
- R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/.
- Richardson, D.S. & Bolen, G.M. 1999. A nesting association between semi-colonial Bullock’s orioles and yellow-billed magpies: evidence for the predator protection hypothesis. Behav. Ecol. Sociobiol. 46: 373–380. doi: 10.1007/s002650050632
- Schmidt, E. 1981. Die Sperbergrasmucke. Die Neue Brehm – Bucherei, Wittenberg (in German).
- Schönfeld, M. 1998. Zum Vonkommen. Neststand und Nestern des Neuntöters Lanius collurio und zum syntopen Vorkommen des Sperbergersmücke Sylvia nisoria im Mittelbegebiet bei Wittenberg/Sachsen-Anhalt. Ornithol. Mitt. 50: 221–226 (in German).
- Sikora, A. 2007. Gniazdowanie cennych gatunków Ptaków na Wysoczyźnie Elbląskiej. Notatki Ornitologiczne 48: 246–258 (in Polish).
- Sikora, A., Rohde, Z., Gromadzki, M., Neubauer, G. & Chylarecki, P. (eds) 2007. Atlas rozmieszczenia Ptaków lęgowych Polski 1985–2004, 13–15. Bogucki Wydawnictwo Naukowe, Poznań (in Polish).
- Simes, R.J. 1986. An improved Bonferroni procedure for multiple tests of significance. Biometrika 73: 751–754 doi: 10.1093/biomet/73.3.751
- Šmilauer, P. & Lepš, J. 2014. Multivariate Analysis of Ecological Data Using Canoco 5. Cambridge University Press, Cambridge.
- StatSoft, Inc. 2011. STATISTICA (data analysis software system), version 10.
- Tryjanowski, P. & Goławski, A. 2004. Sex differences in nest defence by the Red-backed Shrike Lanius collurio: effects of offspring age, brood size and stage of breeding season. J. Ethol. 22: 13–16. doi: 10.1007/s10164-003-0096-9
- Tryjanowski, P., Karg, M.K. & Karg, J. 2003. Diet composition and prey choice by the Red-backed Shrike lanius collurio in western Poland. Belgian J. Zool. 133: 157–162.
- Tryjanowski, P., Sparks, T.H. & Crick, H.Q.P. 2006. Red-backed Shrike (Lanius collurio) nest performance in a declining British population: a comparison with a stable population in Poland. Ornis Fenn. 83: 181–186.
- Tucker, G.M. & Heath, M.F. 1994. Birds in Europe: their conservation status. BirdLife International, Cambridge.
- Waldenström, J., Rhönnstad, P. & Hasselquist, D. 2004. Habitat preferences and population trends in the Barred Warbler Sylvia nisoria in the Ottenby area, southern Sweden. Ornis Svecica 14: 107–116.
- Wesołowski, T., Czeszczewik, D., Mitrus, C. & Rowiński, P. 2003. Ptaki Białowieskiego Parku Narodowego. Notatki Ornitologiczne 44: 1–31 (in Polish).
- Wilniewczyc, P. 2005. Jarzębatka – Sylvia nisoria (Bechst., 1785). In Chmielewski, S. (ed) Ptaki Gór Świętokrzyskich. Monografia faunistyczna, 372–374. TBOP, Kielce (in Polish).
- Winiecki, A., Grzybek, J., Krupa, A. & Mielczarek, S. 1997. Awifauna lęgowa doliny środkowej Wartu – stan aktualny i kierunki zmian. Notatki Ornitologiczne 38: 87–120 (in Polish).
