ABSTRACT
Capsule: The use of woody material in the outer nest is significantly associated with heavier passerine species, whereas the use of grass, moss or leaves is unrelated to body mass.
Bird nests exhibit tremendous variation in their size and composition (Deeming & Mainwaring Citation2015). While the nests can be seen to have a variety of roles (Hansell Citation2000, Moreno Citation2012, Mainwaring et al. Citation2014), there is increasing evidence that the various materials within a nest also play different roles. For instance, animal-derived materials are often seen as being important insulators of the cup (Hansell Citation2000, McGowan et al. Citation2004, Hilton et al. Citation2004) while strong outer materials are associated with maintaining the structural integrity of the nest. Heenan & Seymour (Citation2011) suggested that the nest cup design more likely reflected structural properties than thermal characteristics although the premise of this conclusion has been challenged (Deeming & Mainwaring Citation2015). However, recent studies on finches (Eurasian Bullfinch Pyrrhula pyrrhula and Hawfinch Coccothraustes coccothraustes) and thrushes (Common Blackbird Turdus merula, Mistle Thrush Turdus viscivorous, Ring Ouzel Turdus torquatus, and Song Thrush Turdus philomelos) have shown that the mechanical properties of materials vary between parts of nests. Cup linings of grass and rootlets are thinner and less rigid than the woody stems found in the base and side of the nest (Biddle et al. Citation2015, Citation2017, Citation2018a, Citation2018b). Qualitative accounts of bird nests suggest that larger passerine species, e.g. the large Pine Grosbeak Pinicola enucleator and the Corvidae, also rely heavily on woody stems to construct nests (Pulliainen Citation1979, Ferguson-Lees et al. Citation2011). Horváth et al. (Citation2015) showed that nests constructed high in trees by Rooks Corvus frugilegus contain woody stems that average around 5 mm in diameter (range 2–15 mm), which are much thicker than used by smaller finches and thrushes and this may reflect the species’ large body mass (420–490 g; Dunning Citation2008). The nests of House Builders Pseudoseiura spp. are also made of woody twigs and are supposedly strong enough for a person to stand on (Collias & Collias Citation1984).
It could be argued that woody materials may provide a form of crypsis for species nesting in bushes or trees, but it is interesting to consider whether there is a relationship between the type and physical characteristics of the materials used in different parts of the nest, nest location and the mass of the birds involved. For instance, the Bullfinch nest is constructed at the end of branches and the woody stems offer better support from below than at the sides; by contrast, the heavier Hawfinch nests on thicker branches nearer the trunk and the woody stems at the sides of the nest are thicker, more rigid and stronger (Biddle et al. Citation2018a). Columbiformes and Accipitriformes are larger than most passerines and rely heavily on woody materials for nest building (Ferguson-Lees et al. Citation2011). It was hypothesized that nest materials would be related to the mass of the birds constructing the nest over a wide range of bird sizes.
Unfortunately, quantitative data for nest materials are very limited (Deeming & Mainwaring Citation2015, Biddle et al. Citation2018b, Wesołowski & Wierzcholska Citation2018). Rather than await the extensive research required to generate further data, this study used categories based on a qualitative assessment of the materials observed to be present in the outer nest wall. This criterion was based on the assumption that the materials in the nest wall would be providing support for the nest cup (Biddle et al. Citation2015, Citation2017). Passerine nests were broadly split into groups having outer nests dominated by either woody materials or other non-woody materials. The Passeriformes were chosen because of the wide divergence in adult size and for the diversity in nest structures and composition. It was appreciated that there could be possible selection biases in the recording of data but these were traded-off against having a large sample of species collected from several geographical regions. Recording of data was, however, conservative in its approach the, such that any species without a clear photograph or an ambiguous description was not included in the dataset.
Photographs of nests were obtained from a series of field guides of nests (Harrison Citation1979a, Citation1979b, Tarboton Citation2001, Beruldsen Citation2003, Ferguson-Lees et al. Citation2011) supported by online resources, particularly Birds of North America online (birdsna.org). Species were from a wide range of passerine families from Europe, North America, Southern Africa, and Australia. Materials predominately used on the outside of the nests were assessed from a photographic image of the nest in conjunction with written descriptions. Outer nest materials were classified as being dominated by either materials resembling woody stems (hereafter ‘woody materials’), i.e. whole twigs or branches (sample size = 120 species) or by non-woody materials (sample size = 471 species), which included, in order of highest to lowest frequency, grass (300 species), moss (41 species), leaves (38 species), bark, plant fibres, mud, rootlets, animal-derived materials, or a variety of different plant-derived materials. The nest site was also categorized as being above ground, on the ground, or in a cavity/crevice. Once the nest had been categorized, mean female body mass (g) was recorded from Dunning (Citation2008) and this ranged from 5.1 to 1147.1 g across the data set.
A phylogenetic tree of the 591 species in the dataset was produced based on a Hackett backbone using birdtree.org. Using this tree, phylogenetically controlled general linear modelling (pglm) was performed in R (ver. 3.2.1; Paradis et al. Citation2004) using the packages ape, mvtnorm and MASS (Deeming, Citation2018; code provided by Carl Soulsbury, pers. comm.), to test for the effect of nest materials (i.e. woody versus non-woody) on Log10-transformed values of female body mass.
The use of woody materials was not exclusively limited to larger birds but generally species weighing less than 30 g relied on non-woody materials on the outside of their nests, whereas for birds weighing above 30 g woody materials dominated (). Eighty-five per cent of the species with woody materials in the outer nest built nests above ground compared with only 58% of species not using woody stem materials. 26.5% of species not using woody materials nested on the ground.
Figure 1. A frequency distribution plot for female body mass (g) in categories based on Log10 values rounded to 0.1 for species with woody materials (dashed line) and non-woody materials (solid line) in the outer nest.
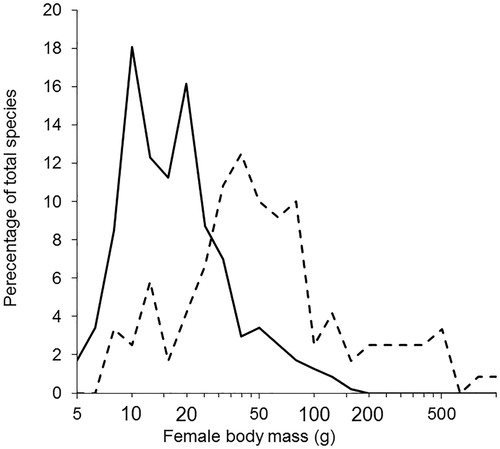
Controlling for phylogenetic relationships, female body size (analysed as LogFBM) was significantly 4–5 times greater for those species predominately using woody materials in their outer nest compared with species using non-woody materials (; F1,586 = 21.00, P < 0.00001). There was a very strong phylogenetic signal (λ = 0.9954) but the model explained very little of the variation in the data (R² = 3.5%). A second analysis found no significant effect of the three commonest non-woody materials, i.e. grass, moss and leaves, on LogFBM (F2,370 = 0.54, P = 0.583, λ = 0.9952, R² = 0.3%).
Figure 2. Mean (±se) values for female body mass (g) for species with woody materials and non-woody materials in the outer nest.
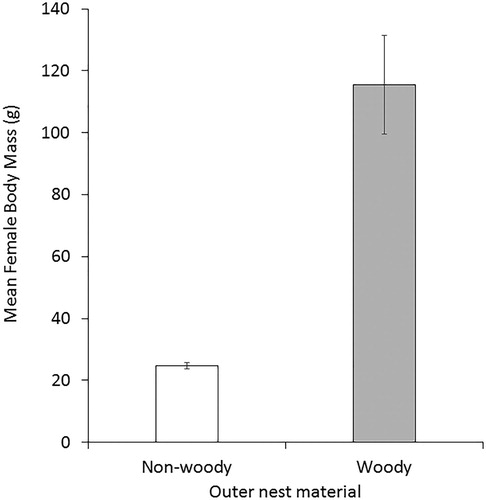
This analysis confirmed that heavier bird species are associated with the use of woody materials in the outer nest but no effect on mean bird mass was found for the commonest non-woody nest materials. This would seem reasonable because the nest constructed by larger birds will have to support not only the incubating adult but also the proportionately larger clutch of eggs and nestlings, often at sites that are above the ground.
It is known that as female body mass increases then the dimensions of nests also increases (Deeming Citation2013), but our current understanding of the mechanical properties of materials used in nest construction is relatively poor. Placement of materials with varying characteristics into different parts of a nest has been shown in various finches and thrushes to be non-random (Biddle et al. Citation2015, Citation2017, Citation2018a). Hawfinches Coccothraustes coccothraustes have been observed snipping off twigs from living trees (Mountford Citation1957) but whether these would have different mechanical properties is unknown. In addition, captive Zebra Finches Taeniopygia guttata are able to recognize the mechanical properties of different types of string that they are offered for nest building (Bailey et al. Citation2014). Male Cape Weavers Ploceus capensis choose longer and stronger grasses to build the outer nest of their nests before adding increasingly shorter and weaker materials as nest building progresses (Bailey et al. Citation2016). In these cases, individual birds must have some means of discerning the physical characteristics of the materials used in nests. It is suggested that finches and thrushes are able to recognize the mechanical properties of the materials with the bills, possibly assessing material diameter that correlates with strength and rigidity (Biddle et al. Citation2018a).
‘Softer’ plant-derived materials, e.g. grass, moss and leaves, presumably offer less support than woody material but it will be more flexible. Slagsvold (Citation1989) suggested that nest walls should offer a degree of flexibility to allow expansion as the clutch of nestlings grew and softer materials would presumably accommodate this. However, the value of these materials may vary depending on the mode of nest construction. Weaverbirds (Ploceidae) rely on grass to weave their suspended nests (Crook Citation1963) and can select materials preferentially according to the stage of construction (Bailey et al. Citation2016). By contrast, the grass that forms 60% of a Common Linnet Linaria cannabina nest (Biddle et al. Citation2018b) appears to be randomly arranged in the outer cup walls (Lucia Biddle pers. comm.). Presumably, this reflects the fact that these nests are supported from below and so have little direct role in load-bearing.
Although the effect was significant, the outer nest material category explained very little of the variation in body mass and the very high phylogenetic signal suggests that their species relatedness was crucial in this distribution. Presumably, nests construction is more similar within a family than between families but the factors driving the evolution of nest-building behaviours are poorly understood (Winkler & Sheldon Citation1993, Price & Griffith Citation2017). In addition, selection of materials by some species may be influenced by their availability in the habitat (Britt & Deeming Citation2011, Surgey et al. Citation2012, Álvarez et al. Citation2013, Briggs & Deeming Citation2016, Lambrechts et al. Citation2017, Wesołowski & Wierzcholska Citation2018). Nest site may also be crucial. Although not part of the analysis described here, around 80 species in this study nested in a cavity but less than 10 of these used woody stems in the outer parts of their nests. The walls and floor of the cavity may well provide the appropriate support for these nests. However, it is difficult to tease apart the cause and effect here – does ready availability of materials override mechanical considerations? More research is needed to better understand the physical characteristics of the materials used in nests (Biddle et al. Citation2018a, Citation2018b) that possibly underlie the behavioural choices made by birds during nest construction.
This study focussed on passerine species but non-passerines build a variety of nest types. Field guides suggest that Columbiformes all construct nests using woody materials and, given that the smallest species weigh approximately 30 g (Dunning Citation2008), it would be interesting to assess the relationship between body mass and the use of woody materials in this order. The impression given by many field guides (Harrison Citation1979a, Citation1979b, Tarboton Citation2001, Beruldsen Citation2003, Ferguson-Lees et al. Citation2011) is that large non-passerines that nest in trees (e.g. Accipitriformes, Ciconiiformes) rely on woody materials in their nests. By contrast, species nesting on the ground (e.g. Anseriformes, Galliformes) appear to not to rely on woody materials. It would be interesting to assess the relationships between nest site location, body mass and nest materials in a wider range of avian taxa.
Further research is required to assess the mechanical properties of nest materials. For instance, the thickness of twigs and branches used in bird nests should correlate with body mass. Data for Rooks suggest that heavier birds will use thicker twigs (Horváth et al. Citation2015), but more work is needed to assess the thickness of materials used in various parts of nests of much bigger species than those studied to date (27–130 g). In addition, it would be useful to better understand whether the direct correlation between woody stem thickness and its strength and rigidity observed in finch and thrush nests (Biddle et al. Citation2018a) extends to thicker twigs. If this were the case, it would be possible to measure twig diameter in different parts of nests to ascertain their mechanical properties without destroying the structure.
Acknowledgements
I thank Lucia Biddle for discussions and for comments on a previous draft of this manuscript.
ORCID
D. Charles Deeming http://orcid.org/0000-0002-9587-6149
References
- Álvarez, E., Belda, E.J., Verdejoc, J. & Emilio Barba, E. 2013. Variation in Great Tit nest mass and composition and its breeding consequences: a comparative study in four Mediterranean habitats. Avian Biol. Res. 6: 39-46. doi: 10.3184/175815513X13609517587237
- Bailey, I.E. Morgan, K.V., Bertin, M., Meddle, S.L. & Healy, S.D. 2014. Physical cognition: birds learn the structural efficacy of nest material. Proc. R. Soc. B 281: 20133225. doi: 10.1098/rspb.2013.3225
- Bailey, I.E. Morgan, K.V., Oschadleus, H.D., DeRuiter, S.L., Meddle, S.L. & Healy, S.D. 2016. Nest-building males trade off material collection costs with territory value. Emu 116: 1–8. doi: 10.1071/MU15022
- Beruldsen, G. 2003. Australian Birds Their Nests and Eggs. G & E Beruldsen, Brisbane.
- Biddle, L.E., Deeming, D.C. & Goodman, A.W. 2015. Morphology and biomechanics of the nests of the Common Blackbird Turdus merula. Bird Study 62: 87–95. doi: 10.1080/00063657.2014.988119
- Biddle, L.E., Goodman, A.M. & Deeming, D.C. 2017. Construction patterns of birds’ nests provide insight into nest-building behaviours. Peer J 5: e3010. doi: 10.7717/peerj.3010
- Biddle, L.E., Deeming, D.C. & Goodman, A.W. 2018a. Birds use structural properties when selecting materials for different parts of their nests. J. Orn. doi:10.1007/s10336-018-1571-y.
- Biddle, L.E., Broughton, R.E., Goodman, A.M. & Deeming, D.C. 2018b. Composition of bird nests is a species-specific characteristic. Avian Biol. Res. 22: 132–153. doi: 10.3184/175815618X15222318755467
- Briggs, K.B. & Deeming, D.C. 2016. Use of materials in nest construction by pied flycatchers Ficedula hypoleuca reflects localized habitat and geographical location. Bird Study 63: 516–524. doi: 10.1080/00063657.2016.1238867
- Britt, J. & Deeming, D.C. 2011. First egg date and air temperature affect nest construction in Blue Tits Cyanistes caeruleus but not in Great Tits Parus major. Bird Study 58: 78–89. doi: 10.1080/00063657.2010.524916
- Collias, N.E. & Collias, E.C. 1984. Nest Building and Bird Behaviour. Princeton University Press, Princeton.
- Crook, J.H. 1963. A comparative analysis of nest structure in the weaver birds (Ploceinae). Ibis 105: 238–262. doi: 10.1111/j.1474-919X.1963.tb02498.x
- Deeming, D.C. 2013. Effects of female body size and phylogeny in avian nest dimensions. Avian Biol. Res. 6: 1–11. doi: 10.3184/175815512X13528955707337
- Deeming, D.C. 2018. Effect of composition on shape of bird eggs. J. Avian Biol. 49: jav-01528. doi: 10.1111/jav.01528
- Deeming, D.C. & Mainwaring, M.C. 2015. Functional properties of nests. In Deeming, D.C. & Reynolds, S.J. (eds) Nest, Eggs, and Incubation: New Ideas Avian Reproduction, 29–49. Oxford University Press, Oxford.
- Dunning, J.B., Jr 2008. CRC Handbook of Avian Body Masses, 2nd edn. CRC, Baton Rouge.
- Ferguson-Lees, J., Castell, R. & Leech, D. 2011. A Field Guide to Monitoring Nests. British Trust for Ornithology, Thetford.
- Hansell, M.H. 2000. Bird Nests and Construction Behaviour. Cambridge University Press, Cambridge.
- Harrison, H.H. 1979a. A Field Guide to the Birds’ Nests: United States East of the Mississippi River (Peterson Field Guides), Houghton Mifflin Company, Boston.
- Harrison, H.H. 1979b. A Field Guide to Western Birds’ Nests (Peterson Field Guides), Houghton Mifflin Company, Boston.
- Heenan, C.B. & Seymour, R.S. 2011. Structural support, not insulation, is the primary driver for avian cup-shaped nest design. Proc. R. Soc. B 278: 2924–2929. doi: 10.1098/rspb.2010.2798
- Hilton, G.M., Hansell, M.H., Ruxton, G.D., Reid, J.M. & Monaghan, P. 2004. Using artificial nests to test importance of nesting material and nest shelter for incubation energetics. Auk 121: 777–787. doi: 10.1642/0004-8038(2004)121[0777:UANTTI]2.0.CO;2
- Horváth, É., Solt, S., Kotymán, L., Palatitz, P., Sándor Piross, I. & Fehérvári, P. 2015. Provisioning nest materials for rooks; a potential tool for conservation management. Ornis Hunn. 23: 22–31. doi: 10.1515/orhu-2015-0002
- Lambrechts, M.M., Charmantier, A., Demeyrier, V., Lucas, A., Perret, S., Abouladzé, M., Bonnet, M., Canonne, C., Faucon, V., Grosset, S., le Prado, G., Lidon, F., Noell, T., Pagano, P., Perre, V., Pouplard, S., Spitaliéry, R., Bernard, C., Philippe Perret, P., Blondel, J. & Grégoire, A. 2017. Nest design in a changing world: Great tit Parus major nests from a Mediterranean city environment as a case study. Urban. Ecosyst.. 20: 1181–1190. doi: 10.1007/s11252-017-0670-5
- Mainwaring, M.C., Hartley, I.R., Lambrechts, M.M. & Deeming, D.C. 2014. The design and function of birds’ nests. Ecol. Evol. 4: 3909–3928. doi: 10.1002/ece3.1054
- McGowan, A., Sharp, S.P. & Hatchwell, B.J. 2004. The structure and function of nests of Long-tailed tits Aegithalos caudatus. Funct. Ecol. 18: 578–583. doi: 10.1111/j.0269-8463.2004.00883.x
- Mountford, G. 1957. The Hawfinch. Collins, London.
- Moreno, J. 2012. Avian nests and nest building as signals. Avian Biol. Res. 5: 238–251. doi: 10.3184/175815512X13534385822786
- Paradis, E., Claude, J. & Strimmer, K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. doi: 10.1093/bioinformatics/btg412
- Price, J.J. & Griffith, S.C. 2017. Open cup nests evolved from roofed nests in the early passerines. Proc. R. Soc. B 284: 20162708. doi: 10.1098/rspb.2016.2708
- Pulliainen, E. 1979. On the breeding of the pine grosbeak Pinicola enucleator in NE Finland. Ornis Fenn. 56: 156–162.
- Slagsvold, T. 1989. Experiments on clutch size and nest size in passerine birds. Oecologia 80: 297–302. doi: 10.1007/BF00379030
- Surgey, J., du Feu, C.R. & Deeming, D.C. 2012. Opportunistic use of a wool-like artificial material as lining of tit (Paridae) nests. Condor 114: 385–392. doi: 10.1525/cond.2012.110111
- Tarboton, W. 2001. A Guide to the Nests and Eggs of Southern African Birds. Struik Publishers (Pty) Ltd., Cape Town.
- Wesołowski T. & Wierzcholska S. 2018. Tits as bryologists: patterns of bryophyte use in nests of three species cohabiting a primeval forest. J. Orn. doi: 10.1007/s10336-018-1535-2
- Winkler, D.W. & Sheldon, F.H. 1993. Evolution of nest construction in Swallows (Hirundinidae): a molecular phylogenetic perspective. Proc. Nat. Acad. Sci. 90: 5705–5707. doi: 10.1073/pnas.90.12.5705
