ABSTRACT
Capsule: Barn swallows Hirundo rustica using artificial nest cups had greater reproductive success than those building and using natural nests.
Aims: To quantify reproductive success of Barn Swallows breeding in artificial man-made nests and compare with those using natural nests.
Methods: In 2012–16, the breeding attempts of 231 pairs of Barn Swallows were monitored in artificial and natural nests in an urban habitat in Denmark.
Results: Pairs breeding in old natural nests and artificial nests bred earlier, laid more eggs, and produced more nestlings and fledglings than pairs breeding in new natural nests. Inter-clutch intervals were shorter in Barn Swallow pairs using artificial nests and old natural nests. Nest cup volume and nest volume of newly built nests were significantly larger and more variable than artificial nests excluding an effect of nest size as an explanation for the larger clutches laid in artificial nests. Increased energy and time costs of nest construction may have reduced reproductive success for pairs building natural nests.
Conclusion: Artificial nests were readily accepted by Barn Swallows, they had a similar low predation rate as natural nests, and they proved to be a possible conservation option for this declining passerine breeding in urban habitats as evidenced by their 75% higher reproductive success in artificial nests.
Many farmland birds including the Barn Swallow Hirundo rustica have suffered marked declines in population size due to changes in agricultural practices in Europe (Ambrosini et al. Citation2011, Musitelli et al. Citation2016). This trend may also be linked to a more than 75% decline in insect biomass during the past three decades as reported in protected areas of Germany (Hallmann et al. Citation2017). In Denmark, the Barn Swallow population decline amounts to approximately 30% since 1976 (DOF Citation2018). The size of Barn Swallow breeding colonies is correlated positively to the presence of livestock, especially cattle, and studies suggest that the spatial distribution and number of breeding pairs are directly linked to the distribution of cattle farms (Ambrosini et al. Citation2011). In Denmark, the number of cattle farms were reduced by 61.2% from 1990–2006, and the numbers of cattle were reduced by 27.7% in the same period (Danmarks Statistik Citation2017). This non-negligible decline in the number of cattle farms have limited access by Barn Swallows to appropriate nesting sites in rural areas and a plausible dispersal of swallows to more urbanized habitats such as towns and suburban areas may be a consequence (Teglhøj Citation2017).
Nest boxes, plastic tubes and other man-made artificial nesting options may offer new possibilities for reproductive success in bird species where more traditional nest sites are limiting resources (Newton Citation1994, Corrigan et al. Citation2011, Libois et al. Citation2012, Lezekiel et al. Citation2017), and hence they may be important tools from a conservation perspective. Passerine use of nest boxes and the effects on important reproductive parameters such as laying date, clutch size, brood size and overall reproductive success have been studied in several passerine species (Major & Kendal Citation1996, Purcell et al. Citation1997, Ramstack et al. Citation1998, Lorenzón & Quiroga Citation2012, Rhim et al. Citation2013). No studies have so far focused on Barn Swallow acceptance of artificial nests as a breeding option and the effects of such artificial nests on nest type selection and breeding performance. Only one study has examined artificial wooden nests and the settlement patterns of Barn Swallows breeding in colonies (Mercadante & Stanback Citation2011). Barn swallows consider both proximity and visibility of established pairs when choosing nest sites within a colony (Mercadante & Stanback Citation2011).
Re-use of old nests is a common nesting strategy among Barn Swallows with up to 82% of pairs re-using old nests for their first breeding attempt (Safran Citation2006). The fitness-related benefits of re-using old nests include earlier laying dates of first clutches (Barclay Citation1988, Shields et al. Citation1988, Safran Citation2006), old nests may be important settlement cues for first-year breeders (Safran Citation2004), and the energetic costs of building a new nest may be reduced. In European and American Barn Swallows inter-specific nest re-use for both first and second broods is rare and opportunistic, and possibly functions as a time saving mechanism or as a result of limited nest sites (Hebda & Broughton Citation2017).
The costs of nest building and the relationship between nest building and clutch size have been the focus of some studies (Conrad & Robertson Citation1993, Gauthier & Thomas Citation1993, Mainwaring & Hartley Citation2013). They provide observational, comparative and experimental evidence that avian nest building is an energetically and temporally expensive activity, and it is important to incorporate such costs into avian life-history research (Moreno et al. Citation2010, Mainwaring & Hartley Citation2013). If activities during nest building are energetically costly, adult birds should benefit by using nesting strategies that reduce energy and time during nest construction. Re-use of old nests or artificial nest boxes may save time and energy and will enable an earlier initiation of egg laying. However, the use of old nests that may be infested by ectoparasites may negatively affect nestlings as well as adult swallows (Barclay Citation1988, Møller Citation1994).
The Barn Swallow is an aerial insectivorous passerine, with a body mass of approximately 19 g, predominately breeding in rural areas, but it is also found in European towns (Teglhøj Citation2017) and in larger cities in Asia (Turner Citation2006, Osawa Citation2015). Both sexes contribute to nest building, with the nest being cup shaped and composed of mud pellets, straw, feathers and hair (Møller Citation1994). Nest building in Barn Swallows is a sexually selected behaviour and temporal changes in nest size can be accounted for by indirect selection on tail length of males (Soler et al. Citation1998, Møller Citation2006). Short-tailed males contribute more to nest building than long-tailed males, and females paired with males that contribute a lot to nest building have earlier laying dates and lay more eggs than females with males that contribute little to nest building (Soler et al. Citation1998). Nest size and male tail length are positively related to clutch size (Møller Citation1982, Citation1994, Soler et al. Citation1998).
The objective of this study was to examine Barn Swallow acceptance of artificial woodcrete nests as breeding options in an urban town habitat and how the choice of different nest types during double-brooded breeding cycles influenced important reproductive variables such as laying date, clutch size, fledgling success and inter-clutch interval. Specifically, the following predictions were made: (1) if nest building is an energetically costly activity, Barn Swallow pairs choosing to build new nests will have less energy to invest in eggs compared to swallows that re-use old nests or artificial nests, and consequently they will have an overall lower total number of eggs, nestlings and fledglings; (2) if nest building is a non-negligible activity in terms of time investment during the reproductive cycle of double-brooded Barn Swallow pairs, investment in a new nest in the first breeding attempt should result in later laying dates, and construction of a new nest for the second brood should increase the inter-clutch interval compared to swallows choosing to breed in combinations of artificial and old natural nests; (3) if Barn Swallows accept artificial nests, these nests may represent a possibility to lower costs of nest building, reduce reproductive failure linked to detachment of natural nests from walls and beams, and reduce the time used for nest construction and repair, which may result in earlier laying dates, larger clutch sizes and a higher fledgling output, and (4) if nest volumes and cup volumes of artificial nests are larger than those of new natural nests a size-effect is expected, predicting larger clutches in artificial nests.
Methods
Study area
Svendborg is an old Danish market town situated by the Sound of Svendborg (55°3′35.038 N, 10°36′25.909 E) in the southern part of Fyn, Denmark. The centre of the town is dominated by old buildings some of which have gates where swallows build their nests. The town periphery is predominately composed of open pastures, sports fields and scattered buildings. For further details on study areas, see Teglhøj (Citation2017).
Reproductive variables
Reproductive variables were recorded by regular visits to individual nests during laying, incubation and nestling periods 2012–16. A total of 231 pairs were followed of which 179 were double-brooded pairs initiating two successive broods within the same breeding season. Clutch size was defined as the maximum number of eggs in active nests, and the size of the brood at hatching and fledging as the number of nestlings present after hatching and the last day before fledging. The total number of eggs, nestlings and fledglings per pair was calculated as the sum of eggs, nestlings and fledglings from the first and the second broods, respectively. Inter-clutch interval was defined as the period between laying of the first egg in the first clutch and laying of the first egg in the second clutch. The laying dates of the first and the second clutches were calculated from the fledging date by using May 1st as day 1, and by assuming that one egg was laid per day, an average incubation period of 15 days and an average nestling period of 21 days (Møller Citation1994, Engstrand & Bryant Citation2002, Teglhøj Citation2017). Since Svendborg is a relatively densely populated town where capture of adult swallows with mist nests or trapping by other methods is considered unfeasible, ethical considerations overrode the advantages of obtaining individual identification of swallows in this study. However, the lack of individual identification was considered to be of minor importance, since the majority of swallow pairs were breeding as single pairs (51.1%), as 2 pairs (24.2%) or in very small colonies of 3–5 pairs (15.2%). Only 9.5% of the pairs bred in colonies of 6–9 pairs. These colonies were observed more intensively during the breeding seasons to register potential immigration of new individuals. However, no changes in breeding pair composition were observed. Due to the lack of ringing possibilities effects of age of breeding swallows were excluded from this study.
Artificial nests
A total of 164 artificial nests were placed at 77 different breeding sites within a radius of 5.5 km from the town centre of Svendborg. Artificial nests were all situated on walls or beams with a distance of 8 cm from the top of the nest to the ceiling, 4–5 cm from the closest wall when situated in corners, and 2–5 m above ground level. Most artificial nests were placed inside buildings, a few were placed on the outside of buildings under the eaves. This corresponded to the placement of natural nests in the study area (pers. obs.). Artificial nests are open cup nest boxes of woodcrete (Vivara®) resembling natural nests ((A)). Their tight attachment with screws onto walls or beams makes these nests robust to destruction and demolition by avian predators and cats.
Figure 1. (A) An artificial woodcrete nest with five chicks, (B) a female Barn Swallow building a new natural nest, (C) and an old natural nest with four chicks

Potential drawbacks and experimental artefacts of passerine nest box studies include differences in predation rates of birds breeding in nest boxes compared to birds breeding in natural nest cavities. Consequently the utility of artificial nests as a tool for assessing factors influencing the success of natural nests may be questioned (Møller Citation1989, Major & Kendal Citation1996). When placing artificial nest boxes in my study area special attention was paid to minimize such artefacts by placing the artificial nests in accordance with the placement of natural nests with the aim of avoiding different predation rates on such nests. The total predation rate of 1.2% for my urban study population of Barn Swallows () was similar to predation rates of 1.0–1.2% reported for Barn Swallows in rural areas (Møller Citation1987, Citation2010). Only five nests were depredated during the five breeding seasons: three natural nests (1.2%) and two artificial nests (1.3%). The similar predation rates of natural and artificial nests thus exclude differential attraction of predators to the different nest types, which makes comparison of breeding variables of different nest types a reliable tool for evaluating factors influencing the timing and the breeding success of Barn Swallow pairs choosing different nesting options.
Removal of old nesting material after each breeding season may introduce an artefact in nest box studies since parasite loads of old nests may influence nestling growth and overall breeding success (Møller Citation1989). Removal of nesting material may also well affect nest site choice and nest building behaviour as demonstrated in Tree Swallows Tachycineta bicolor (Rendell & Verbeek Citation1996). However, Koenig et al. (Citation1992) argued that combined results from studies of effects of parasites and old nest material on breeding success of hole-nesting birds offer no opportunity to generalize from one study to the other and emphasized that nest boxes offer important possibilities and allow study and manipulation of phenomena that usually are difficult to address in ecological studies. In order to compare Barn Swallow nest type selection and breeding performance in different nest types, old nesting material was removed from artificial nests between breeding seasons, and after the fledging of the first broods from such nests, allowing for comparison of the breeding performance in clean non-infested artificial nests and natural nests that potentially could be infested by ectoparasites during the breeding cycle.
Calculation of energy and time investment in nest building
To estimate the energy costs and the time spent on nest building in urban and rural habitats the model developed by Withers (Citation1977) and modified by Gauthier & Thomas (Citation1993) was used: Ct = ((tm × 0.83) + Cf) × number of trips, where Ct is the total cost of nest construction in kJ, tm is the time required to collect a mud pellet (10.5 s) and insert it into the nest wall (10.6 s), 0.83 W is the estimated metabolic rate (2 × basal metabolic rate) during mud collection (Gauthier & Thomas Citation1993), and Cf is the cost of flight of 2.10 W (Schmidt-Wallenburg et al. Citation2007). The average distance to the nearest mud source in the urban habitat and in rural habitats was set to 300 m (pers. obs.) and 100 m, respectively (Samuel Citation1971, Møller Citation1994). The flight speeds of nest building Barn Swallows were set to 11.9 m/s (urban habitat) and 7.9 m/s (rural habitat) using the relation between the distance to the mud source (x) and the flight speed (y): y = 5.89 + 0.02x, r2 = 0.76, P < 0.01, reported for the similar sized Cliff Swallow Hirundo pyrrhonota (Gauthier & Thomas Citation1993). The calculated flight speeds are in accordance with specific flight speeds obtained in studies on Barn Swallows (Blake et al. Citation1990, Bird Life international Citation2015a). For calculating the energy and time used on new nest construction in urban and rural habitats, each nest was assumed to contain an average of 1400 mud pellets, needing 1400 return trips to the mud source, with an average nest building activity of 3 h per day, which is within the range of values reported for other Hirundo species (2.3–3.7 h per day; Withers Citation1977, Earlé Citation1986, Gauthier & Thomas Citation1993).
Comparison of cup and nest volume of natural and artificial nests
To assess potential differences in nest cup and nest volume size related to nest type, the inner and the external diameters, and the depth of 50 new natural nests and 50 artificial nests were measured to the nearest millimetre using a ruler. The nest volume and the cup volumes were calculated as , where a is the smallest radius of an ellipsoid, b is the largest radius of an ellipsoid, and x is the fraction of an ellipsoid equal to 0.25 (Soler et al. Citation1998, Møller Citation2006).
Number of broods initiated in artificial nests and the re-use of nests
The percentage of broods initiated in artificial nests was calculated as the total number of broods from first and second broods raised in artificial nests divided by the total number of broods, i.e. the sum of broods raised in artificial and natural nests, multiplied by 100. The percentage of pairs re-using first brood nests for their second broods was calculated as the number of pairs using the same nest for both broods divided by the total sum of pairs using either the same nest or different nests for their first and second broods.
Statistical analyses
JMP version 10 (SAS Citation2012) and SOCS (Citation2017) were used for all statistical analyses. Best-fit generalized linear models were employed to test for year and nest type effects on laying date, total number of eggs, total number of nestlings, total number of fledglings, and inter-clutch interval. Nest type choices during the reproductive cycle of double-brooded Barn Swallow pairs were analysed using the following nest combinations for the first and second broods: 1: ‘new-new’, 2: ‘new-other’, 3: ‘other-new’ and 4: ‘other-other’, where ‘new’ refers to a new natural nest and ‘other’ refers to either an old natural nest or an artificial nest. Nest cup volumes and nest volumes were compared using log transformed data and by using a Welsh one-way analysis of variance (anova) test allowing for unequal variances. A one-way anova was used to test for differences in mean number of eggs between different nest types in both first and second clutches.
Results
Phenology
Barn swallows choosing artificial or old natural nests for their first brood laid eggs significantly earlier than swallows building new nests (χ2 = 22.46, df = 3, P < 0.001). Laying date varied significantly among years (χ2 = 15.16, df = 4, P = 0.0044; ). Mean laying date of first clutch was 29 May for new nests and 20 May for other nests (old natural and artificial nests), whereas mean laying date of the second clutch was 18 July for new nests and 11 July for old natural and artificial nests.
Table 1. Predictors of the laying date, the total number of eggs, the total number of nestlings, the total number of fledglings and the inter-clutch interval of double brood Barn Swallow pairs. Sample size was 179 pairs. Years: 2012–16. Nest combinations: 1: New-new, 2: New-other, 3: Other-new, 4: other-other, where “new” refers to a new natural nest, and “other” refers to an old natural nest or an artificial nest. Mean (SE) for variables were laying date 22.99 (10.42), N = 186, total number of eggs 7.96 (0.16), N = 226, total number of nestlings 7.22 (2.68), N = 232, total number of fledglings 5.61 (0.19), N = 232, inter-clutch interval 53.23 (0.47), N = 123. AICc is Akaike’s information criteria corrected for small sample sizes.
Mean clutch size and total number of eggs, nestlings and fledglings
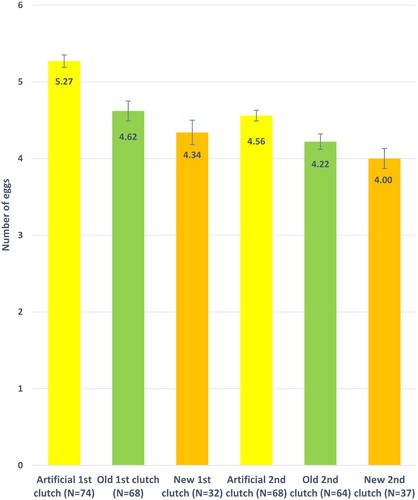
Mean clutch size of artificial nests was larger than mean clutch sizes of natural nests in both first clutch (F2,171 = 16.67, P < 0.001) and second clutch (F2, 166 = 8.23, P < 0.001; , ).
Figure 2. Mean number of eggs (±se) of first and second clutches in different nest types. Artificial = artificial woodcrete nest, new = new build natural nest, old = old natural nest. First clutch: F2,171 = 16.67, P < 0.001, Second clutch: F2, 166 = 8.23, P < 0.001.
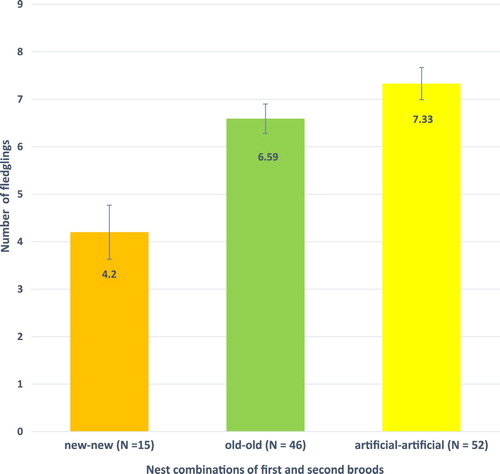
Double-brooded Barn Swallow pairs breeding in combinations of artificial and old natural nests had more nestlings (χ2 = 8.92, df = 3, P = 0.0303) and more fledglings (χ2 = 21.06, df = 3, P < 0.001) compared with pairs including new nests in the breeding cycle (). In nest combinations involving the same nest type for both first and second broods mean number of fledglings was 75% and 11% higher for artificial nests compared to new and old natural nest, respectively (χ2 = 15.87, df = 2, P < 0.001) ().
Inter-clutch interval
The inter-clutch interval was shorter (χ2 = 35.88, df = 3, P < 0.001) for Barn Swallow pairs choosing artificial nests and old nest combinations (52 days) compared with pairs involving new nests in their second breeding attempts (58 days) ().
Size differences between artificial nests and new natural nests
Nest cup volume and nest volume of natural new nests were significantly larger (cup: F1, 54.481 = 33.70, P < 0.001, nest volume: F1, 53.49 = 13.56, P < 0.001) and more variable than for artificial nests ().
Table 2. Nest cup volume and nest volume of natural and artificial nests. Sample size was 50 new natural nests and 50 artificial nests. Mean (se) of nest cup volume was 766 cm3 (13), N = 100 and of nest volume was 1478 cm3 (26), N = 100.
Number of broods initiated in different nest types and re-use of nests
The percentage of broods initiated in artificial nests, new and old natural nests was 37.1%, 24.4% and 38.5%, respectively, over the 5 breeding seasons (). In total 33.5% of double-brooded pairs used the same nest for both first and second clutches, whereas 66.5% used different nests for their two clutches. A total of 31.7% of pairs that re-used nests were breeding in artificial nests whereas 68.3% of nest re-users bred in old natural nests (). A contingency table analysis based on nests that were re-used or not and natural or artificial nests revealed a likelihood ratio χ2 = 3.08, df = 1, P = 0.079 showing no significant difference in the proportion of pairs re-using artificial or natural nests.
Table 3. Total number of broods raised in different nest types and the predation rates of different nest types. Total broods N = 410. Years 2012–16.
Table 4. Re-use of natural and artificial nests within a double-brooded breeding cycle. Sample size 179 pairs. Years 2012–16.
Discussion
The major findings of this study were that: (1) Barn Swallows invested more in egg laying when using artificial nests, whereas new nest building was associated with a reduction in the number of eggs laid; (2) the fledgling output from Barn Swallow pairs using artificial nests twice during a double-brooded breeding cycle was 75% greater than pairs using a new natural nest for both broods; (3) double-brooded Barn Swallow pairs breeding in combinations of artificial and old natural nests laid earlier, and had more nestlings and fledglings compared with birds breeding in nest combinations involving new natural nests; (4) inter-clutch intervals were shorter for double-brooded pairs choosing artificial nests and old natural nest combinations compared with pairs that included new nests once or twice in the same breeding season; and (5) more eggs in artificial nests could not be linked to a nest size-effect, since nest cup volumes and nest volumes of newly built nests were significantly larger and more variable than for artificial nests.
Nest type selection, nest re-use and reproduction
In my study area 77.5% of Barn Swallow pairs were double-brooded, a higher proportion than reported for two rural populations in Kraghede and Hjortkær, Denmark, where 60.3% and 68.0% of breeding pairs laid a second clutch (Møller Citation1994, Thellesen Citation2000), but within the range 65–83% reported for a central Polish population (Banbura & Zielinski Citation1998). A total of 33.5% double-brooded pairs re-used their nest for both first and second broods, whereas 66.5% used another nest for their second brood. Shields (Citation1984) found a high re-use frequency of old nest across breeding seasons, and that secondary nests were used for second broods. Hebda & Broughton (Citation2017) reviewed inter-specific nest re-use of Barn Swallows in Europe and North America and reported that nest re-use is rare and opportunistic, and may function as a time saving mechanism or as a result of limited nest sites. In my study population nest sites were limited, which may be the primary cause of the relatively high degree of nest re-use (Teglhøj Citation2017). A total of 68.3% of re-used nests were natural nests, whereas 31.7% were artificial. The latter nest type may contribute to a higher nest re-use rate, since such nests were cleaned after the first brood had fledged and may have encouraged pairs to re-use such nests for their second breeding attempt.
Mean clutch size of both first and second clutches was larger in artificial nests compared to natural new and old nests (). Mean clutch size in all natural nests in first and second clutches were 4.53 eggs and 4.17 eggs, respectively, somewhat lower than reported for rural populations in northern Denmark (first clutch: 4.94; second clutch: 4.41, Møller Citation1994), central Poland (first clutch: 4.86; second clutch 4.47, Banbura & Zielinski Citation1998) and western Poland (first clutch: 4.98; second clutch: 4.31, Zduniak et al. Citation2011). Double-brooded Barn Swallows breeding in combinations of artificial and old natural nests laid more eggs and had more nestlings and more fledglings compared with Barn Swallows using nest combinations involving new natural nests. Pairs using artificial nests for both first and second broods produced in total 75% and 11% more fledglings than pairs using either new nests or old nests twice during the breeding season (). In an earlier study, Safran (Citation2006) found that on average Barn Swallow pairs had greater seasonal reproductive success in old nests compared to new nests regardless of individual age, supporting the findings of the present study. However, Barclay (Citation1988) found no differences in mean reproductive success per pair using either old or new nests. Purcell et al. (Citation1997) found benefits from nestbox breeding of Western Bluebirds Sialia mexicana, Plain Titmice Parus inornatus and House Wrens Troglodytes aedon compared to natural nesting sites in tree cavities. All three species had higher nesting success and fledged more young when using nest boxes, and House Wrens laid larger clutches in nest boxes. In a study of Eastern Kingbirds Tyrannus tyrannus, however, no differences in breeding success were found between birds using natural or artificial nests (Cancellieri & Murphy Citation2013).
Phenology
Laying dates of first and second clutches were in the range 20–29 May and 11–18 July, respectively. These dates were in accordance with the range 18–30 May (first clutch) but later than second clutches (5–9 July) reported by Banbura & Zielinski (Citation1998) for a population of Barn Swallows in central Poland. Laying dates for a northern Danish population (Kraghede) were somewhat later (5 June for first clutch, 20 July for second clutch; Møller Citation1994). Laying dates of first and second clutches in Svendborg were significantly earlier for old and artificial nests compared with new natural nests, the differences being 9 days for first clutches and 7 days for second clutches, respectively, corresponding well with the estimated time to construct new nests in the urban habitat (see below). Similar observations were reported by Safran (Citation2006), who found 12 days earlier laying of Barn Swallows using old natural nests compared with swallows using new natural nests for their first brood. Purcell et al. (Citation1997) studied breeding biology of passerine birds in nest boxes and natural tree cavities and found earlier laying dates in Western Bluebirds breeding in nest boxes. These findings suggest that re-use of old nests or the use of artificial nests may save time for female Barn Swallows preparing for egg laying, enabling an earlier initiation of the clutch.
Time investment in nest building
Time used for nest building may directly influence phenology of both first and second clutches, and since early laying dates are associated with larger clutches and more fledglings, this may influence the overall reproductive success and the fitness of individuals (Dunn Citation2004, Safran Citation2006, Møller Citation2008). Time investment in nest building was studied in Cliff Swallows by Gautheir & Thomas (Citation1993) who found, that birds minimized time allocated to nest construction while increasing the net energy cost of nest building. Conrad & Robertson (Citation1993) studied Eastern Phoebes Sayornis phoebe, and their data suggested that building of new nests may be more time consuming and possibly more energetically costly than repairing old nests. Barn Swallow nest building requires around 750–2000 trips to a mud source and takes from 3 to more than 21 days depending on the availability of nesting material and distances to mud sources (Møller Citation1994, Shields et al. Citation1988, pers. obs.). Mud pellets in drought periods may be transported more than 1500 m (Møller Citation1994) and long commuting distances may be the rule rather than the exception in urban habitats with extensive paved areas and reduced access to mud sources (Teglhøj Citation2017). The time used to build a nest in rural areas with short distances to a mud source (100 m) was estimated as 6 days. This is in accordance with the average time for nest construction of 6.4-7.5 days reported in earlier studies (Samuel Citation1971, Møller Citation1994). Barn Swallows in the urban habitat required 9 days for nest completion based on average commuting distances of 300 m. The calculated longer nest building period in urban habitat was supported by later laying dates in newly built nests (7–9 days) and longer inter-clutch intervals (5–6 days) for pairs breeding in newly built nests in the second breeding attempt. Barn swallow pairs choosing artificial nests and old nest combinations reduced inter-clutch intervals significantly compared with pairs involving new nests once or twice in a double-brooded breeding cycle (). Clean artificial nests with a low ectoparasite infestation may be linked to the reduced inter-clutch intervals, since it has previously been observed, that Barn Swallows breeding in natural nests with low ectoparasite infestation reduce inter-clutch intervals due to the re-use of such nests for second clutches (Møller Citation1990).
Energy investment in nest building
Energy invested in nest building and egg production can be a non-negligible part of the total breeding costs of birds (Conrad & Robertson Citation1993, Gauthier & Thomas Citation1993, Williams Citation2005, Mainwaring & Hartley Citation2013). By comparison of the energy budgets in Cliff Swallows Hirundo pyrrhonota building new nests with those re-using old nests Gauthier & Thomas (Citation1993) found that nest building affects the energy balance of adults by depleting parental fat reserves. Parents that build new nests had lower fat reserves when provisioning young and supplied less food to their young than did parents that re-used old nests (Gauthier & Thomas Citation1993). Cliff Swallows acted to minimize time investment, but the effect of the higher energy expenditure on new nest building resulted in earlier depletion of fat resources compared to swallows re-using old nests. Female Pied Flycatchers Ficedula hypoleuca that had been saved nest construction efforts before egg laying expressed higher provisioning at early nestling periods and thus improved the growth of nestlings (Moreno et al. Citation2010).
The calculated total energy cost of Barn Swallows building new nests in the urban habitat was 86 kJ. Assuming that the female contributes on average 72.3% of nest building (Møller Citation1994), she invests in total 62 kJ in nest construction. A similar calculation for Barn Swallows building nests in rural habitats with easy access to mud sources the net cost of nest construction for a female would be 38 kJ. In Barn Swallows the energy cost of egg synthesis is small in relation to routine energy requirements during reproduction and swallows form their eggs from current food intake, but it cannot be excluded that micronutrients are used from stored reserves (Ward Citation1996, Ward & Bryant Citation2006). Since insect densities in Svendborg town were significantly lower compared to insect densities in the town periphery (Teglhøj Citation2017) during the whole breeding season, Barn Swallows building new nests in the town center could be faced with lower food availability and longer commuting distances between the nest and the mud source during the nest building period (Teglhøj Citation2017). The energy content of a Barn Swallow egg is 9.84 kJ/egg (Ward & Bryant Citation2006) and the energy content of fat is 38 kJ/g (Briggs & Calloway Citation1984). Energy invested in new nest construction equals the energy content of 1.6 g fat or 6.3 eggs for females in the town habitat compared to 1.0 g fat or 3.9 eggs for females in rural areas. Thus, females in the urban habitat use a theoretical non-negligible 8.4% of their body weight on new nest construction compared to 5.3% for females in rural areas. In this study area, female Barn Swallows building new nests laid fewer first and second clutch eggs, and the seasonal total egg output from nest combinations involving new nests was lower compared to nest combinations involving old and artificial nests. This indicates that the amount of energy allocated to egg production and egg laying was reduced and could be linked to the higher energy cost of females investing in new nests compared to swallows that chose to breed in old natural nests or artificial nests where energy requirement for nest repair and nest lining is considered minimal, the latter only taking a few days (Møller Citation1994).
Clutch size – nest size, age or parasite effect?
The relationship between clutch size and size of natural nests or nest boxes have been studied in different passerine species. Slagvold (Citation1989) found a positive correlation between the clutch size and the nest cup size in passerines. Furthermore, positive correlations have been found between nest box size and clutch size in Great Tits Parus major, Pied Flycatchers and Tree Swallows (Purcell et al. Citation1997). Møller (Citation1982) found a similar positive correlation between the size of natural nests and clutch size of Barn Swallows. In this study, a higher number of eggs was laid in artificial nests in both first and second clutches compared with natural old and new nests, an effect that could not be explained by a nest size-effect since natural new nests were significantly larger compared to artificial nests (, ).
Age-related effects of breeding females could contribute to the observed lower number of eggs in newly built nests, since it has earlier been documented that young female Barn Swallows lay smaller clutches than older females (Banbura & Zielinski Citation1998). The age of breeding individuals could not be assessed in this study area but age-related effects are considered to be insignificant since all analyses were carried out on data from double-brooded pairs, where older females are predominant. It has previously been estimated that only 50% of first-year breeding females have a second clutch compared with older females, which all produce a second clutch (Banbura & Zielinski Citation1998), and individual age does not affect the observed greater seasonal reproductive success in old nests compared to new nests (Safran Citation2006).
Barn Swallows are able to assess the degree of parasite nest infestation and heavily infested nests are typically avoided for second clutches (Møller Citation1990, Citation1994). An increased number of mites in Barn Swallow nests decreases reproductive success in terms of the number of fledglings of first clutches and in terms of clutch size, brood size, and number of fledglings of second clutches (Møller Citation1990). Non-infested artificial nests, where old nesting material had been removed between and within seasons, may have acted as an important nest site and nest selection cue, where minimal parasite loads could be interpreted by females as optimal conditions for increasing investment in egg laying.
Conclusion
Barn Swallow nest type selection during a double-brooded breeding cycle influenced laying dates, inter-clutch interval, investment in eggs and overall reproductive output. A smaller number of eggs in newly built nests and longer inter-clutch intervals of pairs that used new nests for their second broods were linked to non-negligible energy and time expenditure by females building new nests in the urban habitat. Female Barn Swallows may save time and energy by choosing males that can offer an old natural nest or an artificial nest, allowing her to lay eggs earlier and invest more in egg production, reduce the inter-clutch interval, and achieve a total higher number of fledglings, contributing to an overall fitness increase. Investment in a new natural nest may reduce the individual fitness in the breeding season of nest construction, but may on the other hand be regarded as possible long-term fitness investment, if the female is able to survive to forthcoming breeding seasons and is able to occupy the same old nest for consecutive years.
This study documents that artificial man-made nests may be a suitable breeding option for Barn Swallows occupying urban habitats, since Barn Swallows gradually adapted to these nests, the number of broods raised in artificial nests increased from 16% in 2012 to 52% in 2016. Reproductive output from artificial nests was higher than natural nests, and predation rates were low and of a similar magnitude to that of natural nests. From a conservation perspective, artificial nests may constitute a possible measure that could be used to halt the decline of Barn Swallows, which has been observed in many European countries (Ambrosini et al. Citation2006, Sicurella et al. Citation2014, Bird Life International Citation2015b, DOF Citation2018).
Acknowledgments
I am grateful to the Danish Ornithological Association for financing part of the artificial nests used in the study. I am also very thankful to the private citizens of Svendborg who allowed me to place artificial nest at their properties and enabling consultation of these nests for all five breeding seasons. Many thanks to A. P. Møller for his help with the design of appropriate statistical models and tests. Finally, I would like to thank two anonymous reviewers for their constructive reviews of the manuscript.
ORCID
Peter Györkös Teglhøj http://orcid.org/0000-0003-1230-1508
References
- Ambrosini, R., Ferrari, R.P., Martinelli, R. & Romano, M. 2006. Seasonal, meterological and microhabitat effects on breeding success and offspring phenotype in the Barn Swallow, Hirundo rustica. Ecoscience 13: 298–307. doi: 10.2980/i1195-6860-13-3-298.1
- Ambrosini, R., Bani, L., Massimino, D., Fornasari, L. & Saino, N. 2011. Large-scale spatial distribution of breeding Barn swallows Hirundo rustica in relation to cattle farming. Bird Study 58: 495–505. doi: 10.1080/00063657.2011.609883
- Barclay, R.M.R. 1988. Variation in the costs, benefits, and frequency of nest reuse by Barn Swallows (Hirundo rustica). Auk 105: 53–60.
- Banbura, J. & Zielinski, P. 1998. Timing of breeding, clutch-size and double-broodedness in Barn Swallows Hirundo rustica. Ornis Fenn. 75: 177–183.
- Bird Life International. 2015a. http://www.rspb.org.uk/discoverandenjoynature/discoverandlearn/birdguide/name/s/swallow/migration.aspx.
- Bird Life International. 2015b. http://www.birdlife.org/datazone/speciesfactsheet.php?id=7116.
- Blake, R.W., Kolotylo, R. & de la Cueva, H. 1990. Flight speeds of the Barn swallow, Hirundo rustica. Can. J. Zool. 68: 1–5. doi: 10.1139/z90-001
- Briggs, G.M. & Calloway, D.H. 1984. Nutrition and Physical Fitness, 11th edn. New York: CBS College Publishing.
- Cancellieri, S. & Murphy, M.T. 2013. Experimental examination of nest reuse by an open-cup-nesting passerine: time/energy savings or nest site shortage? Animal Behav. 85: 1287–1294.
- Conrad, K.F. & Robertson, R.J. 1993. Clutch size in eastern phoebes (Sayornis phoebe). I. The cost of nest building. Can. J. Zool. 71: 1003–1007. doi: 10.1139/z93-133
- Corrigan, R.M., Scrimgeour, G.J. & Paszkowski, C. 2011. Nest boxes facilitate local-scale conservation of common Goldeneye (Bucephala clangula) and Bufflehead (Bucephala albeola) in Alberta, Canada. Avian Conserv. Ecol. 6: 1.
- Danmarks Statistik. 2017. http://www.statistikbanken.dk/BRUG6.
- DOF. 2018. https://dofbasen.dk/ART/art.php.
- Dunn, P. 2004. Breeding dates and reproductive performance. Adv. Ecol. Res. 35: 69–87. doi: 10.1016/S0065-2504(04)35004-X
- Engstrand, S.M. & Bryant, D.M. 2002. A trade-off between clutch size and incubation efficiency in the Barn Swallow Hirundo rustica. Funct. Ecol. 16: 782–791. doi: 10.1046/j.1365-2435.2002.00681.x
- Earlé, R.A. 1986. Time budget of South African Cliff Swallows during breeding. S-Afr. Tydskr. Dierk. 21: 57–59.
- Gauthier, M. & Thomas, D.W. 1993. Nest site selection and cost of nest building by Cliff Swallows (Hirundo pyrrhonota). Can. J. Zool. 71: 1120–1123. doi: 10.1139/z93-152
- Hallmann, C.A., Sorg, M., Jongejans, E., Siepel, H., Hofland, N., Schwan, H., Stenmans, W., Müller, A., Sumser, H., Hörren, T., Goulson, D. & de Kroon, H. 2017. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12: e0185809. DOI: 10.1371/journal.pone.0185809.
- Hebda, G. & Broughton, R.K. 2017. Inter-specific nest re-use by Barn swallows Hirundo rustica. Bird Study 64: 112–115. doi: 10.1080/00063657.2016.1274288
- Koenig, W.D., Gowaty, P.A. & Dickinson, J.A. 1992. Boxes, barns and bridges: confounding factors or exceptional opportunities in ecological studies? Oikos 63: 305–308. doi: 10.2307/3545392
- Lezekiel, S., Yosef, R., Bakaloudis, D.E., Papakosta, M.A., Vlachos, C.G., Antoniou, A. & Zdnuiak, P. 2017. The endemic Cyprus Wheatear (Oenanthe cypriaca) adapts readily to artificial nest sites. Biol. Conserv. 213: 1–4. doi: 10.1016/j.biocon.2017.06.040
- Libois, E., Gimenez, O., Oro, D., Minguez, E., Pradel, R. & Sanz-Aguilar, A. 2012. Nest boxes: A successful management tool for the conservation of an endangered seabird. Biol. Conserv. 155: 39–43. doi: 10.1016/j.biocon.2012.05.020
- Lorenzón, R.E. & Quiroga, M.A. 2012. Breeding biology of the White-Rumped Swallow (Tachycineta leucorrhoa; Hirundinidae in a wetland: a comparative approach. Avian. Biol. Res. 5: 47–53. doi: 10.3184/175815512X13267955937297
- Mainwaring, M.C. & Hartley, I.R. 2013. The energetic costs of nest building birds. Avian. Biol. Res. 6: 12–17. doi: 10.3184/175815512X13528994072997
- Major, R.E. & Kendal, C.E. 1996. The contribution of artificial nest experiments to understanding avian reproductive success: a review of methods and conclusions. Ibis 138: 298–307. doi: 10.1111/j.1474-919X.1996.tb04342.x
- Mercadante, A.N. & Stanback, M.T. 2011. Out of sight, out of mind? Visual obstructions affect settlement patterns in Barn swallows (Hirundo rustica). Auk 128: 230–236. doi: 10.1525/auk.2011.10162
- Moreno, J., Lobato, E., González-Braojos, S. & Ruiz-De Castaneda, R. 2010. Nest construction costs affect nestling growth: a field experiment in a cavity-nesting passerine. Acta Ornithol. 45: 139–145. doi: 10.3161/000164510X551291
- Musitelli, F., Romano, A., Møller, A.P. & Ambrosini, R. 2016. Effects of livestock farming on birds of rural areas in Europe. Biodivers. Conserv. DOI:10.1007/s10531-016-1087-9.
- Møller, A.P. 1982. Clutch size in relation to nest size in the swallow Hirundo rustica. Ibis 124: 339–343. doi: 10.1111/j.1474-919X.1982.tb03780.x
- Møller, A.P. 1987. Advantages and disadvantages of coloniality in the swallow, Hirundo rustica. Animal Behav. 35: 819–832. doi: 10.1016/S0003-3472(87)80118-5
- Møller, A.P. 1989. Parasite, predators and nest boxes: facts and artefacts in nest box studies of birds? Oikos 56: 421–423. doi: 10.2307/3565628
- Møller, A.P. 1990. Effects of parasitism by the haematophagous mite Ornithonyssus bursa on reproduction in the Barn swallow Hirundo rustica. Ecology 71: 2345–2357. doi: 10.2307/1938645
- Møller, A.P. 1994. Sexual Selection and the Barn Swallow. Oxford: Oxford University Press.
- Møller, A.P. 2001. The effect of dairy farming on Barn swallow Hirundo rustica abundance, distribution and reproduction. J. Appl. Ecol. 38: 378–389. doi: 10.1046/j.1365-2664.2001.00593.x
- Møller, A.P. 2006. Rapid change in nest size of a bird related to change in a secondary sexual character. Behav. Ecol. 17: 108–116. doi: 10.1093/beheco/arj003
- Møller, A.P. 2008. Climate change and micro-geographic variation in laying date. Oecologia 155: 845–857. doi: 10.1007/s00442-007-0944-3
- Møller, A.P. 2010. The fitness benefit of association with humans: elevated success of birds breeding indoors. Behav. Ecol. 21: 913–918. doi: 10.1093/beheco/arq079
- Newton, I. 1994. The role of nest sites in limiting the numbers of hole-nesting birds: A review. Biol. Conserv. 70: 265–276. doi: 10.1016/0006-3207(94)90172-4
- Osawa, T. 2015. Importance of farmland in urbanized areas as a landscape component for Barn swallows (Hirundo rustica) nesting on concrete buildings. Environ. Manag. 55:1160–1167. doi: 10.1007/s00267-015-0457-5
- Purcell, K.L., Verner, J. & Oring, L.W. 1997. A comparison of the breeding ecology of birds nesting in boxes and tree cavities. Auk 114: 646–656. doi: 10.2307/4089284
- Ramstack, J.M., Murphey, M.T. & Palmer, M.R. 1998. Comparative reproductive biology of three species of swallows in a common environment. Wilson Bull. 110: 233–243.
- Rendell, W.B. & Verbeek, N.A.M. 1996. Old nest material in nest boxes of tree swallows: effects on reproductive success. Condor 98: 142–152. doi: 10.2307/1369517
- Rhim, S.J., Son, S.H., Kim, K.J. & Hwang, H.S. 2013. Breeding ecology of tits using artificial nest boxes in coniferous and deciduous forests in Mt. Namsan, Seoul Metropolitan, Korea. For. Sci. Technol. 9: 147–150.
- Safran, R.J. 2004. Adaptive site selection rules and variation in group size of Barn swallows: individual decisions predict population patterns. Am. Nat. 164: 121–131. doi: 10.1086/422198
- Safran, R.J. 2006. Nest-site selection in the Barn swallow Hirundo rustica: what predicts seasonal reproductive success? Can. J. Zool. 84: 1533–1539. doi: 10.1139/z06-176
- Samuel, D.E. 1971. The breeding biology of Barn swallows and Cliff Swallow in West Virginia. Wilson Bull. 83: 284–301.
- SAS. 2012. JMP version 10.0. Cary, NC: SAS Institute Inc.
- Schmidt-Wellenburg, C.A., Biebach, H., Daan, S. & Visser, G.H. 2007. Energy expenditure and wing beat frequency in relation to body mass in free flying Barn swallows (Hirundo rustica). J. Comparat. Physiol. B 177: 327–337. doi: 10.1007/s00360-006-0132-5
- Shields, W.M. 1984. Factors affecting nest site fidelity in Adirondack Barn swallows (Hirundo rustica). Auk 101: 780–789. doi: 10.2307/4086904
- Shields, W.M., Crook, J.R., Hebblethwaite, M.L. & Wiles-Ehmann, S.S. 1988. Ideal free colonility in the swallows. In C.N. Slobodchifoff (ed) The Ecology of Social Behavior, 189–228. Academic Press, San Diego, CA.
- Sicurella, B., Caprioli, M., Romano, A., Romano, M., Rubolini, D., Saino, N. & Ambrosini, R. 2014. Hayfield enhanced colony size of the Barn Swallow Hirundo rustica in northern Italy. Bird Conserv. Int. 24: 17–31. doi: 10.1017/S095927091300021X
- Slagvold. 1989. On the evolution of clutch size and nest size in passerine birds. Oecologia 79: 300–305. doi: 10.1007/BF00384308
- SOCS. 2017. http://www.socscistatistics.com/.
- Soler, J.J., Cuervo, J.J., Møller, A.P. & de Lope, F. 1998. Nest building is a sexually selected behavior in the Barn swallow. Anim. Behav. 56:1435–1442. doi: 10.1006/anbe.1998.0938
- Teglhøj, P.G. 2017. A comparative study of insect abundance and reproductive success of Barn swallows Hirundo rustica in two urban habitats. J. Avian Biol. 48: 846–853. doi: 10.1111/jav.01086
- Thellesen, P.V. 2000. Bestanden af Landsvale Hirundo rustica på en gård i Hjortkær i Sydvestjylland, 1971–1998. Dan. Ornitol. Foren. Tidsskr. 94: 5–11.
- Turner, A. 2006. The Barn Swallow. London: T & AD Poyser.
- Ward, S. 1996. Energy expenditure of female Barn swallows Hirundo rustica during egg formation Physiol. Zool. 69: 930–951. doi: 10.1086/physzool.69.4.30164236
- Ward, S. & Bryant, D.M. 2006. Barn swallows Hirundo rustica form eggs mainly from current food intake J. Avian Biol. 37: 179–189. doi: 10.1111/j.0908-8857.2006.03262.x
- Williams, T.D. 2005. Mechanisms underlying the costs of egg production. BioScience 55: 39–48. doi: 10.1641/0006-3568(2005)055[0039:MUTCOE]2.0.CO;2
- Withers, P.C. 1977. Energetic aspects of reproduction by the Cliff Swallow. Auk 94: 718–725. doi: 10.2307/4085268
- Zduniak, P., Czechowski, P. & Jedro, G. 2011. The effect of nesting habitats on reproductive output in the Barn swallow Hirundo rustica. A comparative study of populations from atypical and typical habitats in western Poland. Belg. J. Zool. 141: 38–43.