ABSTRACT
Capsule: The diet of Black Guillemot Cepphus grylle grylle chicks in the Baltic Sea region was dominated by Viviparous Eelpout Zoarces viviparus. Risk of nest predation by avian and mammalian predators was perceived to be low, and hatching and fledging success were high.
Aims: To gain insight into the ecology of nestling Black Guillemots in the Baltic Sea region to fill knowledge gaps and benefit its conservation.
Methods: Two island groups in the Baltic Sea were visited several times during the breeding season of 2014 and 2015 to monitor nestling survival and fledging. In addition, camera traps were used in 2014 to monitor prey brought to chicks by adults and record possible nest predation events.
Results: Hatching success was 0.89 and 0.73 in 2014 and 2015, respectively, and fledging success was very high (0.95 and 0.97). No incidences of avian or mammalian predation were observed. Chicks fledged at night between 32 and 38 days after hatching. Viviparous Eelpout made up 95% of the prey items brought to the chicks by adults.
Conclusions: The hatching rate and fledging rate of the Black Guillemot was high in our study region. Juveniles seemed highly dependent on the availability of eelpout. Changes in the abundance of this species may therefore have negative effects on chick survival.
Currently, seabirds are the most threatened marine taxonomic group. Approximately 25% of seabirds are of special concern or are already threatened (Croxall et al. Citation2012). The Black Guillemot Cepphus grylle, a seabird belonging to the family Alcidae with a circumpolar distribution, is currently listed as ‘least concern’ on the red list of threatened species of the International Union for Conservation of Nature and Natural Resources (IUCN; BirdLife International Citation2016). However, not all subspecies and populations hold this favourable status. The subspecies Cepphus grylle grylle occurs in the Baltic Sea, together with the more widespread C. g. arcticus (HBW Citation2018). Whilst the breeding population of C. g. arcticus is assessed as least concern, its wintering population is listed as vulnerable on the Helsinki Commission red list, the red list of Baltic Sea species in danger of becoming extinct (Helsinki Commission Citation2013). On this same list, both the breeding and wintering populations of C. g. grylle are listed as near threatened. Almost all C. g. grylle breed off the coasts of Sweden and Finland where they have declined by 15–30% over the last 30 years (Helsinki Commission Citation2013).
A number of threats have been described that include predation by native species such as the Brown Rat Rattus norvegicus, Stoat Mustela erminea and Eurasian Otter Lutra lutra (Petersen Citation1981, Ewins & Tasker Citation1985). Predation by alien species, specifically by the American Mink Neovison vison, is thought to be an especially large threat (Nordström & Korpimäki Citation2004, Helsinki Commission Citation2013). This is illustrated by the fact that the American Mink wiped out a large colony (approximately 2600 individuals) breeding on an island in the Baltic Sea over a decade ago (Anonymous Citation2005, Jakt & Jägare Citation2005). The American Mink is predicted to be able to enlarge its geographic range in the region and is, therefore, a threat of particular concern (Hof et al. Citation2012). Fisheries bycatch, hunting and pollution are currently also listed as threats to the Black Guillemot (Koistinen et al. Citation1995, Žydelis et al. Citation2009, Helsinki Commission Citation2013). Other threats may include factors related to climate change, such as loss of potential breeding sites (Buchadas & Hof Citation2017), sea level rise and extreme weather events, which both increase the risk of nests being flooded (Hario Citation2001, Schreiber Citation2001, Crane & Auman Citation2008, Fort et al. Citation2009). Indeed, ground breeding species, such as the Black Guillemot, which tends to nest on rocky shorelines or at the base of cliffs, are thought to be more vulnerable to climate change than species that do not breed on the ground due to increased risk of nest predation by mammalian predators and the already mentioned risk of nest flooding (Hof et al. Citation2017). An additional risk of climate change is that it may affect the fish stock available to seabirds that are associated with sea ice via changes in the primary productivity of marine waters (Beaugrand et al. Citation2002, Hays et al. Citation2005, Österblom et al. Citation2007, Hoegh-Guldberg & Bruno Citation2010). Indeed, lack of sufficient food during the breeding season has already been mentioned as a potential threat to seabirds like the Black Guillemot in Alaska (Cepphus grylle mandti; Divoky Citation2011).
A sound knowledge of the ecology of a species is the foundation of its successful conservation. Although the breeding ecology of the Black Guillemot has been well documented for Northern America (Divoky et al. Citation1974, Preston Citation1974, Cairns Citation1980, Citation1987a, Citation1987b, Divoky Citation1998), surprisingly little is known about the Palearctic populations (Ewins Citation1992), especially the Baltic Sea population. Currently, basic knowledge on the survival and diet of Black Guillemot chicks in the Baltic Sea is limited (but see Hario Citation2001), which hampers accurate predictions of the magnitude of potential threats to this population (Buchadas & Hof Citation2017) and hinders effective conservation planning. The aim of this project was, therefore, to gain insight into the survival and diet of Black Guillemot chicks in the Baltic Sea region, more specifically of the subspecies Cepphus grylle grylle. Several field site visits were made during the breeding seasons of 2014 and 2015 to estimate the survival rate of chicks and the timing of fledging. For the breeding season of 2014, we were able to use remotely triggered camera-traps stationed near the entrance of Black Guillemot nests to record images of prey brought to chicks by adults and the occurrence of nest predation.
Methods
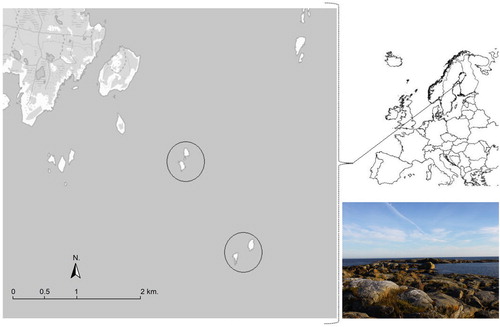
The study was conducted in two Black Guillemot colonies located on two island groups (). Each island group consisted of two small islands separated no more than 200 m from each other. The island groups were situated in the Baltic Sea, off the coast of Obbola in northern Sweden. The island group closest to the coast, Obbola-Lillbådan (63.647507N 20.342045E), was located 2.0 km from the mainland. The other island group, Obbola-Storbådan (63.635008N 20.360584E), was located 3.7 km from the mainland. The habitat on both island groups consisted of rocky outcrops with sparse shrub vegetation (). Both island groups were estimated to have approximately 30–50 breeding pairs of Black Guillemots.
Both island groups were visited 11 times in 2014 and 6 times in 2015. Every first visit of the year, the islands were searched for Black Guillemot nests. When a nest was found, a rock near the nest was marked with a unique number in red paint so the nest could be found during subsequent visits. The whole island was searched for nests in 2014 as well as in 2015, but in 2015 the nests found in 2014 were also re-visited. Chicks were marked with a metal ring and a colour-ring once they were of an appropriate size, which was approximately 21 days after hatching. An attempt to weigh them was also made each visit. When adults were captured, they were also ringed with a metal ring, colour-ringed, and their weight and wing-lengths were measured.
Camera trap data were available from six camera traps (HCO ScoutGuard SG560C with full colour LEDs), which were set up near Black Guillemot nests from 21 May 2014, before the eggs hatched, until the 17 August 2014, when no chicks were left in the nest. Camera trap data for 2015 were not available. The cameras produce very clear, high resolution (180 dpi) colour pictures or videos with a date and time-stamp (). The cameras were mounted so that they had a clear view of what we deemed to be the main entrance to the nest, and that they would not block the entrance to the nest or in another way disturb the wildlife present. Once a motion was detected, the camera trap was triggered, and a colour picture (one image every 5–6 seconds if motion continued) was taken or a colour video was recorded (15 seconds per video), depending on the setting used. The camera stored pictures and videos on an SD-card. Initially, the picture setting was used, but from 17 July 2014 onwards, the video setting was used since we wanted to avoid the approximate 5 second time lag between each picture. From these 6 camera traps, we collected a total of 34 298 images and 11 045 videos. We analysed the camera trap data to identify nest predation events, performed diet analyses and determined fledging behaviour. Only cases in which a prey item was clearly visible were considered for the diet analysis. Multiple images taken within a short time span, and clearly showing the same adult with the same prey, were only counted once. The moment of hatching was assumed to be on the same day that we observed the first prey being brought to the chicks on the images/videos. The moment of fledging was assumed to be on the same day that we observed the chicks outside the nest for the last time on images/videos. Their disappearance from the nest was then confirmed at the first subsequent visit to the field site.
Results
We found in total 36 nests on the islands in 2014 and 31 in 2015 (see for a summary of the data). Of the 36 nests found in 2014, 26 contained 2 eggs and 10 contained 1 egg, the total number of eggs found in 2014 was therefore 62 (1.7 eggs per nest). Of the 31 nests found in 2015, 14 contained 2 eggs and 17 contained 1 egg, which equates to a total of 45 eggs in 2015 (1.5 eggs per nest). We weighed 16 eggs and the average weight of these eggs was 51 g (SD = 5 g). In 2014, 55 of the 62 eggs hatched and in 2015, 33 of the 45 eggs hatched. The hatching success was therefore higher in 2014 (0.89) than in 2015 (0.73). We were unable to ring all chicks due to access problems (chicks being too far below the rock or in crevices where we could not reach them), but we were able to ring 72 chicks (48 in 2014 and 24 in 2015). Of the 55 chicks in 2014, three juveniles were not found again at subsequent field visits hence 52 likely fledged and the perceived fledging success was therefore 0.95. Of the 33 chicks in 2015, one chick died in the nest and 32 likely fledged, providing a perceived fledging success of 0.97 ().
Table 1. Summary of breeding parameters of Black Guillemots.
All encountered chicks were weighed on one or more occasions. The lightest chick (found in 2015) was 33.9 g, but it was unknown how old this individual was. In 2014, the lightest chick, which was estimated to be no more than one day old, weighed 48 g. The change in weight over time of several chicks for which we were able to estimate hatching date based on camera trap data and for which we had more than two measurements is shown in . Based on these data, chicks exhibited a logarithmic growth pattern with higher growth rates when they were younger which slowed down one to two weeks before fledging. Fledging occurred between 32 and 38 days after hatching (). Around an age of 31–33 days, three chicks weighed well over 300 g, but one weighed only 230 g. We also ringed six adults in 2014 and five adults in 2015. The average weight of the adults in 2014 was 357 g (SD = 102 g) and their average wing length was 176 mm (SD = 3 mm). The average weight of the adults ringed in 2015 was 424 g (SD = 42 g). Their average wing length was 173 mm (SD = 3 mm). Differences in weight and wing length between 2014 and 2015 were not significant (Wilcoxon rank sum test weight: W = 9.5, P = .360; wing length: W = 25, P = .079).
Figure 3. Weight change of Black Guillemot nestlings against age (days after hatching) for five nestlings from three nests (1, 2 and 3).
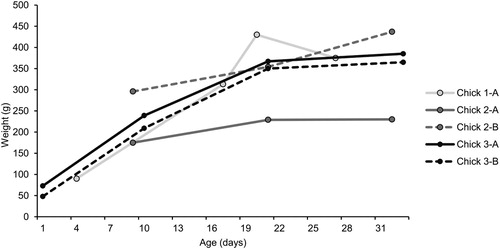
Table 2. Estimated hatching and fledging dates for Black Guillemots at each nest and for each nestling.
Among the 6 camera traps, we recorded in total 393 feeding events at the nest. The main source of prey brought to the juveniles was Viviparous Eelpout Z. viviparus constituting 95% of the diet. The remaining 5% consisted of 3% herring Clupeidae sp. and of 2% other (a species of snail and other unknown species). We could distinguish the parents on some nests because of the colour-rings so it was clear that both parents provided food to the chicks.
Several species that could predate on Black Guillemot eggs, chicks or adults were identified on the camera trap images including Herring Gull Larus argentatus, Common Gull Larus canus, Great Black-backed Gull Larus marinus, Lesser Black-backed Gull Larus fuscus and Hooded Crow Corvus cornix. The cameras recorded several incidences of gulls or crows near a nest and even peering into a nest, but we did not observe any cases of chick predation or kleptoparasitism.
Five camera traps recorded a clear moment of fledging on video. All juvenile Black Guillemots fledged during the night. However, siblings did not always fledge on the same night. At four nests, siblings fledged at the same time, but at one nest they fledged on separate nights. Juveniles would often emerge from the nest a few days before the moment of fledging. They would move around in the vicinity of the nest entrance and also flap or stretch their wings. These behaviours predominately occurred at night but were also observed during the day. At the moment of fledging, there were no adult Black Guillemots in view at any of the nests studied.
Discussion
Our study identified a high hatching rate (0.73–0.89) and fledging rate (0.95–0.97) for the Black Guillemot, especially when compared to other seabird species (Whittow et al. Citation2002; Mavor et al. Citation2006). A high fledging rate (0.97) has also been found for a colony of Black Guillemots in the Gulf of St. Lawrence, Canada, which was only lightly disturbed by humans (Cairns Citation1980). Substantially lower fledging rates were observed for colonies that were more disturbed by humans (0.38–0.64; Cairns Citation1980). Cairns (Citation1980), however, reported substantially lower hatching rates for all studied colonies (0.32–0.66). Available shelter from avian predators, and apparent lack of mammalian predators, yielded fledging success in excess of 90%. Although fledging success was high during both our study years, we identified large variation in hatching rates with a much lower hatching rate in 2015. Interestingly, reproductive output was much lower in 2015 compared to 2014. Not only did we find fewer nests, even though we knew the nest locations of the previous year, but the number of eggs per nest were also lower. The camera traps yielded novel insights into the feeding and reproductive ecology of the Black Guillemot. Diet of chicks was dominated by the Viviparous Eelpout and highlights the potential importance of this prey item in the region.
The probability of breeding appeared to decrease from 2014 to 2015. Although we knew the exact location of nests in 2014, several of these were not used in 2015 and overall fewer nesting attempts were made. Alcidae, and Charadriiformes in general, have been described as annual breeders as opposed to biennial breeders like the albatrosses which normally skip a year of breeding (Hamer et al. Citation2001). However, annually breeding seabirds may commonly skip a year of reproduction, especially if environmental conditions are unfavourable for reproduction (Cubaynes et al. Citation2011 , Reed et al. Citation2015). Skipped breeding has recently been described for the Common Guillemot Uria aalge and both individual-level constraints and environmental factors explained the frequency of skipped breeding (Reed et al. Citation2015). We had an incidental observation of an adult Black Guillemot colour-ringed in 2014 returning to the exact same nest in 2015, indicating annual breeding and high site fidelity. Similar to Reed et al. (Citation2015), we also observed much higher than average sea surface temperatures during the winters of 2013/14 and 2014/15 (Siegel & Gerth Citation2015, Citation2016), which may impact negatively on reproduction in subsequent years. As mentioned, human disturbance may have a significant negative impact on breeding colonies (Cairns Citation1980), we thus cannot rule out that the presence of researchers on the islands may have had a contributing effect to the lower breeding rates in 2015. However, a significant negative effect was not observed by Cairns (Citation1980) for colonies that were only lightly disturbed by human presence. We, therefore, do not think that our limited presence on the islands was a significant contributing factor to the reduced reproductive performance in 2015. The low probability of breeding in 2015 was also coupled with a smaller clutch size. In many species, the clutch size is an indication of individual body condition and hence the large proportion of single-egg nests may indicate that breeding conditions were worse in 2015, although there was no significant difference in weight of adults between 2014 and 2015. Hence, further research is needed regarding how environmental and individual-level factors influence the probability of breeding and clutch size. Currently, it is not known how other seabird species in the Baltic Sea region were affected during the breeding season of 2015 and future studies comparing reproductive success amongst seabirds would help identify whether they may be similarly affected.
Hatching success was lower in 2015 than in 2014, which was likely due to adverse weather at the start of the breeding season in 2015. Lower lying nests that were first found with eggs, had disappeared after heavy rainfall events and several days with high water levels in the Baltic Sea and hence a high water table (personal observations by the authors). Indeed, historic water level data obtained by the Swedish Meteorological and Hydrological Institute (SMHI, http://opendata-download-ocobs.smhi.se/explore/?parameter=4#) at the nearest measuring station (Ratan) shows that the water level was on average 33 cm higher in May and June 2015 than in May and June 2014. We did not find any evidence that these failed nests were substituted with second broods. This may indicate that extreme weather events and a rise of the water table, likely consequences of ongoing climate change in the region, may indeed have a negative effect on ground nesting seabirds like the Black Guillemot (Schreiber Citation2001, Crane & Auman Citation2008, Fort et al. Citation2009, Hof et al. Citation2017).
Fledging success appeared to be very high both in 2014 (0.95) and in 2015 (0.97). Since these estimates were based on visual inspections of the nests, incidences of mammalian or avian predation in between the penultimate and final visits may have been overlooked. Three chicks did indeed ‘disappear’ in 2014 and one nestling died in 2015 due to an unknown cause. Unfortunately, there were no cameras positioned at these nests, we, therefore, cannot rule out predation as a cause. Given that the disappearances occurred during the early phases of chick growth, we are confident that the high fledgling rates accurately describe the situation on the island groups.
The fate of the fledglings could also be confirmed by the camera traps that were placed on the islands. The use of camera traps has been increasing in avian ecology studies, especially studies that investigate nest predation and behavioural aspects of nesting ecology (Bolton et al. Citation2007, O’Brien & Kinnaird Citation2008). Analyses of our camera trap data support the high fledging rates since we did not observe any incidences of nest predation and it is therefore thought that the predation pressure by avian predators is low on these islands. It should be noted that cameras were not positioned at every nest and that cameras may have missed incidences of predation by mammals or birds, especially since other researchers have found proof of gull predation on both chicks and adult auk species (Birkhead Citation1977, Nettleship Citation1996, Finney Citation2002). We found no evidence of the presence of potential mammalian predators (personal observations by the authors). Analyses of the camera trap data can in many cases confirm the successful fledging of chicks. However, there is a risk that a fledging event is not captured due to various reasons such as malfunctioning of the camera, and the fledgling leaving via another entrance than the one covered by the camera. We can therefore not solely rely on camera trap data to accurately estimate survival rates of chicks. Placement of miniature cameras inside the nest may be a better solution, provided they do not disturb the chicks or adults.
Based on the camera trap data, chicks fledged between 32 and 38 days after hatching, which is comparable to findings in other regions that range from 30 to 40 days (Nettleship Citation1996). We further observed that prior to the moment of fledging, chicks could be seen near the entrance of the nest for several days in a row, mostly at night but also occasionally during daytime, during which they seemed to explore or train their wings. During the moment of fledging, which in all observed incidences occurred at night, the chicks did not appear to be accompanied by their parents. We did not observe any signs of adults forcing or luring juveniles out of the nest as seen by Winn (Citation1950). Nettleship (Citation1996) also stated that juveniles fledge at night, likely because of the risk of gull predation during the day. Although we did not observe any evidence of gull predation, a number of gull species were present and hence gull predation may be a real threat meaning that fear of gull predation may also drive night-time fledging in our study population. However, we would also encourage further research to consider the probability of detecting predation events, which may have been missed in our study, and in the absence of avian predation events, to investigate whether other factors may drive night-time fledging.
The camera traps revealed that the diet of juveniles was dominated by the Viviparous Eelpout, which confirms earlier findings by Hario (Citation2001). Other species have been reported to be important in other regions. For instance, in Shetland, the Rock Gunnel Pholis gunnellus and the Sandeel Ammodytes marinus were found to be important items in the diet of Black Guillemot chicks. In total, 70–80% of their diet consisted of the sandeel and the rock gunnel (Ewins Citation1990). It is currently not known how likely it is that adult Black Guillemots would provide a larger variety of prey items to chicks if one source of prey becomes rare, and how well chicks would cope with different types of prey. The diet composition of chicks, however, did display variation with the decrease in the potentially preferred sandeel in the study by Ewins (Citation1990). Although fish are integral for adult Black Guillemots, there is an indication that their diet is of greater variety – containing both fish and invertebrates – than that of chicks, which contained mostly fish (Bradstreet & Brown Citation1985, Barrett et al. Citation2016). Reasons for this difference are currently not known but may be linked to nutritional value and energy expenditure. Since there may be a link between the catchability of eelpout and sea temperature (Hario Citation2001), changes in sea temperature may strongly affect food availability for Black Guillemot chicks (Hario Citation2001) and may thus require monitoring to ensure the species wellbeing.
Our study provides several insights into the reproductive ecology of the Black Guillemot, and of the subspecies Cepphus grylle grylle in particular, and how these may impact upon the conservation status of the species. In the perceived absence of mammalian predators and lack of clear evidence of avian predation, fledging rates were extremely high which underlines the importance of excluding mammalian predators from islands. We observed relatively large inter-annual variation in reproductive output due to a lower number of breeding pairs, smaller clutch sizes and lower hatching rates in one of the years. The cause of the lower hatching rates was largely due to adverse weather conditions, but further research is needed to understand causes of variation in breeding probability and clutch size. The strong preference of feeding Viviparous Eelpout to chicks is an important finding and future conservation efforts may need to consider potential threats not only to the Black Guillemot but also to its preferred food source. Further research is also needed about how adaptable Black Guillemots are and whether alternative prey items can be relied upon. A comparison with the reproductive ecology between Cepphus grylle grylle and the more widespread C. g. arcticus may further identify bottlenecks. Our study improves our understanding of the ecology of the Black Guillemot that can aid its conservation. However, we have also highlighted a number of factors that need further research if we are to effectively understand potential causes for the ongoing decline of the species in this region.
Acknowledgements
We are grateful to Erik Andersson and several field assistants for help during the fieldwork. We thank Tomas Brodin for suggestions during the fieldwork set-up phase and identification of the eelpout. We further thank Göran Spong for allowing us to use the camera traps. Lastly, we thank an anonymous reviewer for useful comments that helped improve this article.
ORCID
Anouschka R. Hof http://orcid.org/0000-0001-6743-0089
Andrew M. Allen http://orcid.org/0000-0002-0119-2425
Additional information
Funding
References
- Anonymous. 2005. Black Guillemots near Holmön wiped out (In Swedish: Tobisgrisslor vid Holmön utplånade). SVT Nyheter 22 Juli 2005. https://www.svt.se/nyheter/lokalt/vasterbotten/tobisgrisslor-vid-holmon-utplanade.
- Barrett, R.T., Christensen-Dalsgaard, S., Anker-Nilssen, T., Langset, M. & Fangel, K. 2016. Diet of adult and immature North Norwegian Black Guillemots Cepphus grille. Seabird 29: 1–14.
- Beaugrand, G., Reid, P.C., Ibañez, F., Lindley, J.A. & Edwards, M. 2002. Reorganization of North Atlantic Marine Copepod biodiversity and climate. Science 296: 1692–1694. doi: 10.1126/science.1071329
- Birdlife International. 2016. Cepphus grylle. http://www.iucnredlist.org.
- Birkhead, T. 1977. The effect of habitat and density on breeding success in the common guillemot (Uria aalge). J. Anim. Ecol. 46: 751–764 doi: 10.2307/3638
- Bolton, M., Butcher, N., Sharpe, F., Stevens, D. & Fisher, G. 2007. Remote monitoring of nests using digital camera technology. J. Field Ornithol. 78: 213–220. doi: 10.1111/j.1557-9263.2007.00104.x
- Bradstreet, M.S. & Brown, R.G. 1985. Feeding ecology of the Atlantic Alcidae. In Nettleship, D.N. & Birkhead, T.R. (eds) The Atlantic Alcidae, 263–318. Academic Press, London.
- Buchadas, A.R. & Hof, A.R. 2017. Future breeding and foraging sites of a southern edge population of the locally endangered Black Guillemot Cepphus grylle. Bird Study 64: 306–316. doi: 10.1080/00063657.2017.1358251
- Cairns, D. 1980. Nesting density, habitat structure and human disturbance as factors in Black Guillemot reproduction. Wilson Bullet 92: 352–361.
- Cairns, D. 1987a. Diet and foraging ecology of Black Guillemots in northeastern Hudson Bay. Can. J. Zool. 65: 1257–1263. doi: 10.1139/z87-196
- Cairns, D. 1987b. The ecology and energetics of chick provisioning by Black Guillemots. Condor 89: 627–635. doi: 10.2307/1368652
- Crane, M. & Auman, H., 2008. The ecological effects of climate change on seabirds. Agreement on the conservation of Albatrosses and Petrels, Fourth Meeting of Advisory Committee, Cape Town.
- Croxall, J.P., Butchart, S.H., Lascelles, B., Stattersfield, A.J., Sullivan, B., Symes, A. & Taylor, P. 2012. Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv. Int. 22: 1–34. doi: 10.1017/S0959270912000020
- Cubaynes, S., Doherty, P.F. Jr., Schreiber, E.A. & Gimenez, O. 2011. To breed or not to breed: a seabird’s response to extreme climatic events. Biol. Lett. 7: 303–306 doi: 10.1098/rsbl.2010.0778
- Divoky, G. J., Watson, G. E. & Bartonek, J. C. 1974. Breeding of the Black Guillemot in northern Alaska. Condor 76: 339–343. doi: 10.2307/1366350
- Divoky, G. J. 1998. Factors affecting the growth of a Black Guillemot Colony in Northern Alaska. Phd thesis, University of Alaska.
- Divoky, G. 2011. Black Guillemots in a melting Arctic: responding to shifts in prey, competitors, and predators. In Watson, R., Cade, T., Fuller, M., Hunt, G. & Potapov, E. (eds) Gyrfalcons and Ptarmigan in a Changing World, 125–130. The Peregrine Fund, Boise, ID.
- Ewins, P. J. 1990. The diet of Black Guillemots Cepphus grylle in Shetland. Holarct. Ecol. 13: 90–97.
- Ewins, P. J. 1992. Growth of Black Guillemot Cepphus grylle chicks in Shetland in 1983-84. Seabird 14: 3–14.
- Ewins, P. J. & Tasker, M. L. 1985. The breeding distribution of Black Guillemots Cepphus grylle in Orkney and Shetland, 1982–84. Bird Study 32: 186–193. doi: 10.1080/00063658509476877
- Finney, S. K. 2002. The dynamics of gull-puffin interactions: implications for management. PhD thesis, University of Glasgow.
- Fort, J., Porter, W.P. & Grémillet, D. 2009. Thermodynamic modelling predicts energetic bottleneck for seabirds wintering in the Northwest Atlantic. J. Exp. Biol. 212: 2483–2490. doi: 10.1242/jeb.032300
- Hamer, K.C., Schreiber, E.A & Burger, J. (2001) Breeding biology, life histories and life history-environment interactions in seabirds. In Schreiber, E. & Burger, J. (eds) Biology of Marine Birds, 217–261. CRC Press, Boca Raton, FL.
- Hario, M. (2001). Chick growth and nest departure in Baltic Black Guillemots Cepphus grylle. Ornis Fenn. 78:97–108.
- Hays, G.C., Richardson, A.J. & Robinson, C. 2005. Climate change and marine plankton. Trends Ecol. Evol. 20: 337–344. doi: 10.1016/j.tree.2005.03.004
- HBW. 2018. Handbook of the birds of the world. http://www.hbw.com/species/black guillemot-cepphus-grylle. [Accessed 25 April 2017].
- Helsinki Commission. (2013). HELCOM red list of Baltic Sea species in danger of becoming extinct (Baltic Sea Environment Proceedings No. 140). http://www.helcom.fi/lists/publications/bsep140.pdf [Accessed 25 April 2017].
- Hoegh-Guldberg, O. & Bruno, J.F. 2010. The impact of climate change on the world’s marine ecosystems. Science 328: 1523–1528. doi: 10.1126/science.1189930
- Hof, A.R., Jansson, R. & Nilsson, C. 2012. Future climate change will favour non-specialist mammals in the (sub)arctics. Plos One 7: E52574. doi: 10.1371/journal.pone.0052574
- Hof, A.R., Rodríguez-Castañeda, G., Allen, A.M., Jansson, R. & Nilsson, C., 2017. Vulnerability of subarctic and Arctic breeding birds. Ecol Appl. 27: 219–234. doi: 10.1002/eap.1434
- Jakt & Jägare. 2005. Mink Tog Hela Fågelkolonin. Jakt & Jägare https://archive.is/20120525102939/http://www.jaktojagare.se/en/article_print.php?id=164433 [Accessed July 2018].
- Koistinen, J., Koivusaari, J., Nuuja, I. & Paasivirta, J. 1995. PCDES, PCBS, PCDDS and PCDFS in Black Guillemots and white-tailed Sea Eagles from the Baltic Sea. Chemosphere 30: 1671–1684. doi: 10.1016/0045-6535(95)00053-B
- Mavor, R.A., Parsons, M., Heubeck, M. & Schmitt, S. 2006. Seabird numbers and breeding success in Britain and Ireland, 2005. UK Nature Conservation, No. 30. Peterborough, Joint Nature Conservation Committee.
- Nettleship, D. 1996. Family Alcidae (Auks). In J. Del Hoyo, A. Elliott & J. Sargatal (eds) Handbook of the birds of the world. Volume 3. Hoatzin to Auks, 678–722. Lynx Edicions, Barcelona.
- Nordström, M. & Korpimäki, E. 2004. Effects of island isolation and feral mink removal on bird communities on small islands in the Baltic Sea. J. Anim. Ecol. 73: 424–433.
- O’Brien, T. G. & Kinnaird, M. F. 2008. A picture is worth a thousand words: the application of camera trapping to the study of birds. Bird Conserv. Internat. 18: S144–S162. doi: 10.1017/S0959270908000348
- Österblom, H., Hansson, S., Larsson, U., Hjerne, O., Wulff, F., Elmgren, R. & Folke, C. 2007. Human-induced trophic cascades and ecological regime shifts in the Baltic Sea. Ecosystems 10: 877–889. doi: 10.1007/s10021-007-9069-0
- Petersen, A. 1981. Breeding biology and feeding ecology of Black Guillemots. PhD thesis, University of Oxford.
- Preston, W. C. 1974. Breeding ecology and social behavior of the Black Guillemot, Cepphus grylle. PhD thesis, University of Michigan.
- Reed, T.E., Harris, M.P. & Wanless, S. 2015. Skipped breeding in common guillemots in a changing climate: restraint or constraint? Front. Ecol. Evol. 3: 1–13. doi: 10.3389/fevo.2015.00001
- Schreiber, E. 2001. Climate and weather effects on seabirds. In Schreiber, E. & Burger, J. (eds) Biology of Marine Birds, 179–215. CRC Press, Boca Raton, FL.
- Siegel, H. & Gerth, M. 2015. Development of Sea Surface Temperature (SST) in the Baltic Sea 2014. http://www.helcom.fi/Documents/Baltic%20sea%20trends/Environment%20fact%20sheets/Sea%20surface%20temperature_BSEFS%202015.pdf [Accessed 03 May 2018].
- Siegel, H. & Gerth, M. 2016. Development of Sea Surface Temperature (SST) in the Baltic Sea 2015. http://www.helcom.fi/Documents/Baltic%20sea%20trends/Environment%20fact%20sheets/SST%20development%20in%202015_BSEFS%202016.pdf [Accessed 03 May 2018].
- Whittow, G. C., Schreiber, E. A. & Burger, J. (2002). Seabird reproductive physiology and energetics. In Schreiber, E. & Burger, J. (eds) Biology of Marine Birds, 409–427. CRC Press, Boca Raton, FL.
- Winn, H. E. 1950. The Black Guillemots of Kent Island, Bay of Fundy. The Auk 67: 477–485. doi: 10.2307/4081088
- Žydelis, R., Bellebaum, J., Österblom, H., Vetemaa, M., Schirmeister, B., Stipniece, A., Dagys, M., Van Eerden, M. & Garthe, S. (2009). Bycatch in gillnet fisheries – an overlooked threat to waterbird populations. Biol. Conserv. 142: 1269–1281. doi: 10.1016/j.biocon.2009.02.025