ABSTRACT
Capsule: Studies of nest predation using artificial nests need to consider the effect of egg size on the types of predator that are detected.
Aims: To estimate the nest predation rate in the Patagonian temperate forest and evaluate the influence of egg size on predator guild.
Methods: On different plant species, we placed 108 nests each containing eggs of either Atlantic Canary Serinus canaria or Common Quail Coturnix coturnix, and a model clay egg of equal size to the real egg. Nest predators were identified from the marks left on the clay eggs or by videos recorded using camera traps.
Results: 86% of the nests were predated. Birds, mainly Chimango Caracara Milvago chimango, were the main nest predators. A marsupial, the Monito del Monte Dromiciops gliroides, and rodents also contributed to nest predation. Nest predation rates were similar for both egg sizes but the nest predator guild was different. Birds and rodents preyed on both eggs but the Monito del Monte consumed mainly small eggs.
Conclusion: Egg size did not influence the rate of nest predation but, instead, affected the nest predator guild. Consequently, in order to avoid underestimating the impacts of small predators, egg size should be considered in studies of nest predation.
Nest predation has been described as a leading cause of nesting failure among a diverse range of bird species (Ricklefs Citation1969, Martin Citation1993b, Arcese et al. Citation1996), and it is an important factor in their ecology, evolution and behaviour (Martin Citation1995, Martin & Clobert Citation1996, Martin et al. Citation2011). Nest predation can be highly variable in space and time due to predator diversity and habitat features (Martin Citation1993a, Weidinger & Kočvara Citation2010). Because predation is the main cause of mortality during bird nesting (Eggers et al. Citation2005, Brawn et al. Citation2011, Ricklefs Citation1969), some researchers have focused on nest predation as a process that determines the structure of bird communities (e.g. Marini Citation1997, Martin Citation1988).
Studying nest predation using natural nests is complex because of the difficulty of finding nests and following their development without the observer interfering directly with nesting success (Major Citation1990). Furthermore, the direct observation of a natural nest predation event is rare and the presence of the observer can dissuade predators. Instead, nest predation rates can be estimated using artificial nests containing natural and/or artificial eggs of different types (Oliveira et al. Citation2013, Montevecchi Citation1976, Lindell Citation2000). However, the size and shell thickness of eggs used in nest predation studies can affect predation frequency as well as the predator species detected (Maier & DeGraaf Citation2000, Roper Citation1992, Haskell Citation1995, Oliveira et al. Citation2013). For example, studies using artificial nests attempting to simulate predation in passerine nests used quail Coturnix sp. eggs (Keyser et al. Citation1998, Picman et al. Citation1993, Wilcove Citation1985, Thompson & Burhans Citation2004) that are thicker and 30–100% wider than the eggs of most passerine species (Haskell Citation1995). Consequently, some predators may not be able to effectively handle these relatively large eggs and the results of these studies could underestimate predation rates by, for example, small mammals (Roper Citation1992, Haskell Citation1995, DeGraaf & Maier Citation1996, Maier & DeGraaf Citation2000). Several studies have been published on nest predation in the tropics (Skutch Citation1985, Gibbs Citation1991, Sieving Citation1992) and forests from the northern hemisphere (Wilcove Citation1985, Martin Citation1995, Martin et al. Citation2000b, Robinson et al. Citation1995) but there are few studies of nest predation for the South America temperate forests (Willson et al. Citation2001, Vergara & Simonetti Citation2003).
The temperate forests of South America constitute a unique floristic type and are biogeographically isolated of other forest formations (Armesto et al. Citation1998). These forests have a high degree of endemism of plant and animal species, and a remarkable proportion of their flora depends on mutualistic interactions with animals for their pollination and seed dispersal (Armesto et al. Citation1996, Aizen & Ezcurra Citation1998). The northern portion of these temperate forests is the one with the greatest diversity, the highest concentration of endemism, and it is also the one with the lowest proportion of surface within protected areas (Armesto et al. Citation1998). Although the ecological interactions that occur in this environment are very varied and diverse, nest predation has not yet been examined. The aim of the study was to determine the guild of nest predators in the northern part of Patagonia temperate forest and compare the nest predation rates for eggs of different sizes. The egg sizes chosen were those commonly used in most artificial nest predation studies (quail egg) and those that simulate the egg size of most of the passerines from the forest (canary egg). Specifically, we asked: (1) which are the main predators of bird nests in the northern part of Patagonia temperate forest? (2) Are there differences in nest predation according to egg size? And (3) is predator guild determined by the size of the egg? If egg size affects the nest predator guild, we expect that small eggs (canary eggs) would be consumed by all predators, and that large eggs (quail eggs) would be taken only by large predators with mouths or beaks capable of manipulating the egg or breaking the shells.
Methods
Study site
The study was carried out in the Llao-Llao Municipal Reserve, an area of 1226 ha of continuous forest located 25 km west of San Carlos de Bariloche, Argentina (41°08”S, 71°19” W). The vegetation of the area belongs to the sub-Antarctic biogeographic region (Morrone Citation2015). The dominant trees were an evergreen southern beech Nothofagus dombeyi and the conifer Austrocedrus chilensis. The understory was dominated by the bamboo Chusquea culeou and the shrubs Aristotelia chilensis and Azara microphylla. Mean annual precipitation in the study area is approximately 1000 mm. Mean austral summer temperature (January) is 15°C, and mean austral winter temperature (July) is 3°C (Mermoz & Martin Citation1987). Regarding the avifauna, 44 bird species have been described inhabiting these temperate forests, with a high degree of endemism (66%) (Rozzi et al. Citation1996). Twenty-three bird species belonging to 16 families have been identified in the study area where the open-cup nester White-crested Elaenia Elaenia albiceps and the cavity nester Thorn-tailed Rayadito Aphrastura spinicauda were the most abundant bird species (Amico & Aizen Citation2005).
Sampling design
We used 108 artificial nests simulating White-crested Elaenia open-cup nests, constructed with natural forest materials (grasses and mosses), and placed them in 12 plots. To assess whether the egg size determined rate of predation and the type of predators, we baited 60 nests with the egg of Atlantic Canary Serinus canaria and a non-toxic model clay egg of equal size. The artificial eggs were used so that nest predators could be identified by the beak (birds) and/or teeth (mammals) marks left in the clay. The remaining 48 nests were baited with eggs of Common Quail Coturnix coturnix and their equivalent sized clay egg. Common Quail eggs were approximately twice the size of Atlantic Canary eggs (mean ± se, quail eggs diameter = 26.8 ± 0.55 mm, length = 33.8 ± 1.48 mm, n = 20; canary eggs diameter = 14.0 ± 0.65 mm, length = 18.1 ± 1.27 mm, n = 20) and nine times heavier (mean ± se, quail eggs weight: 12.5 ± 0.73 g, n = 20; canary eggs weight: 1.4 ± 0.39 g, n = 20). In each of the 12 plots, we placed nine nests, five contained canary eggs and four contained quail eggs, on different plant species (between 0.5 and 3 m above the ground) that are commonly used by White-crested Elaenia. We separated each of these nests at least by 15 m and each plot by at least by 150 m from other plots, to minimize the chances of a single animal visiting more than one plot. All nests were placed simultaneously and for a period of 15 days, which is the incubation period for most of the Passeriformes (Mezquida & Marone Citation2001). After the period, we visited each nest and recorded the number of predated eggs. To identify the predator species that were attacking the eggs, we examined the model clay eggs remaining in the nests for imprints of bills and teeth. Additionally, we recorded diurnal and nocturnal visits using camera traps (Bushnell® Trophy Cam Infra Red) facing two nests containing quail eggs and sixteen nests containing canary eggs.
Statistical analysis
We calculated the nest predation rate as the number of attacked nests divided by the number of total nests for all plots. We also calculated that rate, in the same way, for each predator species. We compared nest predation according to the egg size using generalized linear mixed models (glmer – R package ‘lme4’) with a binomial distribution because we were measuring the proportion of successful/failed nests, which is a binary variable. In the model, we included nest fate (success or failure) as the response variable, the egg size (canary = small egg and quail = big egg) as the explanatory variable and plot as a random factor. Additionally, to evaluate if the size of the egg affected the predatory species we used generalized linear mixed models (glmer – R package ‘lme4’) with a binomial distribution. In this case, we made a subset with only predated nests and now the egg size became a binary variable (large predated egg vs. small predated egg). So, we considered the preyed egg size as a response variable, the nest predator species as an explanatory variable and plot as a random factor. We performed all analyses using the open source software R, version 3.3.2 (R Core Team Citation2017).
Results
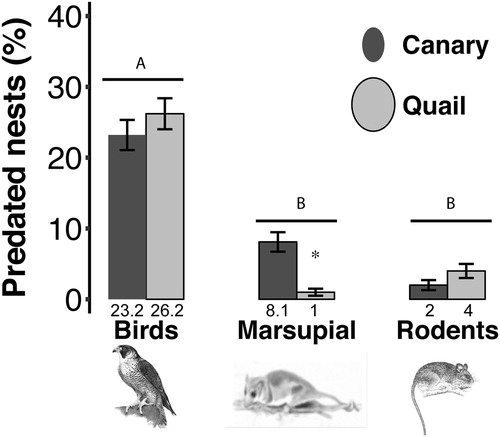
We found that 86% of the nests showed signs of being attacked by nest predators. Birds were the main nest predators (49%). The Chimango Caracara was the species detected most frequently by the cameras, then the Monito del Monte (9%) and rodents (6%) (Χ2 = 23.7, df = 2, P < 0.001). The other category, ‘unidentified’, corresponded to nests containing eggs without recognizable marks (12%). The remaining nests (24%) represented unavailable data, either because the clay eggs were not in the nest or because the predators ate the natural egg only and left no marks on the clay egg. In total, the camera traps recorded 18 visits by Chimango Caraca during the day, 13 visits by the Monito del Monte during the night, and one visit by a rodent during the night.
We found no significant differences in nest predation rate between the two sizes of egg (X2 = 0.15, df = 1, P = 0.69; ). Instead, we found that egg size determined which predator species preyed upon each egg type (quail vs. canary). Birds consumed large eggs five times more than did Monito del Monte and rodents (). Additionally, the Monito del Monte consumed mostly small eggs (estimate ± se = −2.16 ± 1.09, z = −1.97, P = 0.04, ).
Figure 1. Egg size and nest predation. Canary and quail eggs were preyed upon in similar proportions (X2 = 0.15, df = 1, P = 0.69).
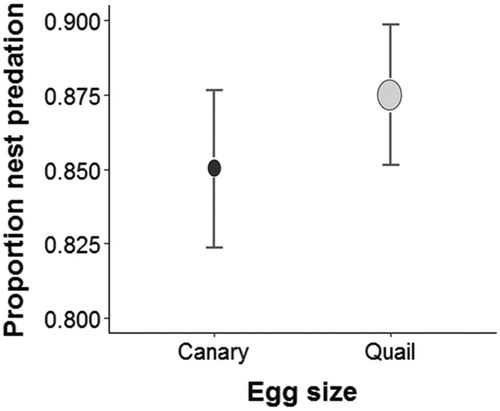
Figure 2. Percentage of nests predated by each predator group. Birds predated both sizes of eggs in a greater proportion than mammals (X2 = 23.7, df = 2, P < 0.0001). Marsupials preferred to prey upon small eggs (canary) (estimate ± se = −2.16 ± 1.09, z = −1.97, P = 0.04). Rodents did not show a preference for egg size. The numbers below the bars show the mean values. Different letters show significant differences in nest predation between predator groups (P < 0.05) and the asterisk shows the significant difference for egg sizes taken by the Monito del Monte.
Discussion
This study provides novel insights on nest predation in the temperate forest of Patagonia and their predator guild. The nest predation rate that we found was high, close to 90%. It is necessary to be cautious about the interpretation of the result because the artificial nests have been criticized as not reliably reflecting predators and predation rates of natural nests (Faaborg Citation2004). This is because artificial nests differ from real nests in a number of important ways such as nest type, egg type, concealment, nest spacing, odour, missing adults, etc. (Major & Kendal Citation1996, King et al. Citation1999). However, the use of artificial nests is a good approximation to cover the lack of information on nest predation that exists in the forests of Patagonia. In turn, the percentage of nest predation found in this study was similar to that reported in other areas of the temperate forest of southern South America (Willson et al. Citation2001). This value, also, coincides with that reported for other different environments in Argentina (Mezquida & Marone Citation2003). The predators detected in our study were birds, a marsupial, the Monito del Monte, and rodents. Our results are similar to those of other studies, where birds appear among the main nest predators (Paton Citation1994, Willson et al. Citation2001, Martin et al. Citation2000a, Mezquida & Marone Citation2002). The Chimango Caracara was also the main nest predator reported in the temperate forest of Chile (Willson et al. Citation2001). A novel finding in our study was the influence of the Monito del Monte on bird nest predation. This marsupial is one of the most common arboreal mammals in these temperate forests (Fontúrbel et al. Citation2012) and plays a key ecological role as seed disperser (Amico et al. Citation2009). Lastly, similar to studies in other forests of South America (Willson et al. Citation2001, Vergara & Simonetti Citation2003), we found that rodents were also acting as nest predators. Moreover, it has been shown that the presence of rodents can cause changes in the nesting behaviour of birds as a consequence of their attempts to find safer breeding places (Wesołowski et al. Citation2009).
We found no significant differences in predation rates in relation to egg size. This finding differs from most studies that have used small eggs, which generally showed that small eggs were predated more than large ones (Oliveira et al. Citation2013, Coppedge et al. Citation2007, Davison & Bollinger Citation2000), possibly due to the presence of small predators. In this study, the nest predation rate was the same for both egg sizes, which could be due to a greater presence of birds capable of eating quail eggs.
Although egg size did not affect the overall predation rate, the nest predator species detected were different for each size of egg. Our results support previous studies that have also shown how the predator guild is affected by the egg type (Maier & DeGraaf Citation2000, DeGraaf & Maier Citation1996, Roper Citation1992). We observed that the predation rate of small and large eggs was similar for birds and rodents but the Monito del Monte preferred to prey upon small eggs. In other areas of the temperate forest, some authors (Willson et al. Citation2001, Vergara & Simonetti Citation2003) found that birds and rodents were the main nest predators but they did not report Monito del Monte as a nest predator. This may be because in their study the authors only used quail eggs which we found that the Monito del Monte barely consumed. For this reason, the egg size should be considered in studies of nest predation in order to avoid underestimating the impact of small predators on bird communities. However, to date, the studies that have used artificial nests to evaluate the impacts of nest predators have frequently used eggs that are too large or thick-shelled to reveal potential small nest predators (Keyser et al. Citation1998, Picman et al. Citation1993, Wilcove Citation1985, Thompson & Burhans Citation2004). Most of these studies use commercially obtained quail eggs as bait, which have thick shells due to diet supplements (DeGraaf & Maier Citation1996). Studies in captivity, where small marsupials were offered quail eggs, showed that these animals failed to break the shell or refused to eat them (Fulton & Ford Citation2003, Roper Citation1992). On the other hand, Haskell (Citation1995) measured the jaw gape of Eastern Chipmunks Tamias striatus and found that the mean jaw gape was smaller than average width of quail eggs, so that it would have been very difficult for the chipmunks to break or carry a quail egg with their teeth. The same could be happening with the Monito del Monte, which eats small eggs because they are within the maximum gape size of this species.
Thus, taking into account these limitations, egg size must be considered in studies of nest predation. Most studies have used quail eggs as bait but these are typically larger than eggs of the native species. Some small-mouthed mammals appear to be unable to eat the relatively large eggs. Therefore, quail egg experiments may not accurately reflect predation by small-mouthed mammals in populations of birds with small eggs. In conclusion, the egg size of native species should be used as models for artificial nest predation experiments. Based on our findings, we recommend small and thin-shelled eggs as an alternative to quail eggs when simulating passerine nests.
Acknowledgements
We thank Hector Loretti for supplying the canary eggs, Yamila Sasal and Facundo Oddi for their assistance with data analysis and Silvia Quintas for manually making the artificial nests and clay eggs. We thank Ricardo Sage, Luis Marone and Chris Greyson-Gaito for providing comments that highly improved the quality of the manuscript. We also thank Secretary of the Environment of Río Negro for granting permit to work in the area. SV, MRC and GCA were supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Additional information
Funding
References
- Aizen, M.A. & Ezcurra, C. 1998. High incidence of plant-animal mutualisms in the woody flora of the temperate forest of southern South America: biogeographical origin and present ecological significance. Ecología Austral 8: 217–236.
- Amico, G.C. & Aizen, M.A. 2005. Dispersión de semillas por aves en un bosque templado de Sudamérica austral:¿ quién dispersa a quién?. Ecología Austral 15: 89–100.
- Amico, G. C., Rodríguez-Cabal, M.A. & Aizen, M.A. 2009. The potential key seed-dispersing role of the arboreal marsupial Dromiciops gliroides. Acta Oecol. 35: 8–13. doi: 10.1016/j.actao.2008.07.003
- Arcese, P., Smith, J. & Hatch, M.I. 1996. Nest predation by cowbirds and its consequences for passerine demography. Proc. Natl. Acad. Sci. U. S. A. 93: 4608–4611. doi: 10.1073/pnas.93.10.4608
- Armesto, J.J., Rozzi, R., Smith-Ramírez, C. & Arroyo, M.T.K. 1998. Conservation targets in South America temperate forest. Science 282: 1271–1272.
- Armesto, J.J., Smith-Ramírez, C. & Sabag, C. 1996. The importance of plant-bird mutualisms in the temperate rainforest of Southern South America. In Lawford, R.G., Alaback, P.B. & Fuentes, E.R. (eds) High-latitude Rainforests and Associated Ecosystems of the West Coast of the Americas, 248–265. Springer, New York.
- Brawn, J.D., Angehr, G., Davros, N., Robinson, W.D., Styrsky, J.N. & Tarwater, C.E. 2011. Sources of variation in the nesting success of understory tropical birds. J. Avian Biol. 42: 61–68. doi: 10.1111/j.1600-048X.2010.04897.x
- Coppedge, B.R., Talent, L.G. & Engle, D.M. 2007. Effects of olfactory attributes and size of egg on rates of predation of artificial ground nests in tallgrass prairie. The Southwest Nat 52: 453–460. doi: 10.1894/0038-4909(2007)52[453:EOOAAS]2.0.CO;2
- Davison, W.B. & Bollinger, E. 2000. Predation rates on real and artificial nests of grassland birds. Auk 117: 147–153. doi: 10.1642/0004-8038(2000)117[0147:PRORAA]2.0.CO;2
- DeGraaf, R. & Maier, T. 1996. Effect of egg size on predation by white-footed mice. Wilson Bull. 108: 535–539.
- Eggers, S., Griesser, M., Andersson, T. & Ekman, J. 2005. Nest predation and habitat change interact to influence Siberian jay numbers. Oikos 111: 150–158. doi: 10.1111/j.0030-1299.2005.13802.x
- Faaborg, J. 2004. Truly artificial nest studies. Conserv. Biol. 18: 369–370. doi: 10.1111/j.1523-1739.2004.00486.x
- Fontúrbel, F.E., Franco, M., Rodríguez-Cabal, M.A., Rivarola, M.D. & Amico, G.C. 2012. Ecological consistency across space: a synthesis of the ecological aspects of Dromiciops gliroides in Argentina and Chile. Naturwiss 99: 873–881. doi: 10.1007/s00114-012-0969-2
- Fulton, G.R. & Ford, H.A. 2003. Quail eggs, modelling clay eggs, imprints and small mammals in an Australian woodland. Emu 103: 255–258. doi: 10.1071/MU02007
- Gibbs, J.P. 1991. Avian nest predation in tropical wet forest: an experimental study. Oikos 60: 155–161. doi: 10.2307/3544861
- Haskell, D.G. 1995. Forest fragmentation and nest predation: Are experiments with Japanese quail eggs misleading? Auk 112: 767–770.
- Keyser, A.J., Hill, G.E. & Soehren, E.C. 1998. Effects of forest fragment size, nest density, and proximity to edge on the risk of predation to ground-nesting passerine birds. Conserv. Biol. 12: 986–994. doi: 10.1046/j.1523-1739.1998.97177.x
- King, D.I., DeGraaf, R.M., Griffin, C.R. & Maier, T.J. 1999. Do predation rates on artificial nests accurately reflect predation rates on natural bird nests?(Es Acaso la Tasa de Depredación en Nidos Artificiales un Reflejo de la Tasa de Depredación en Nidos Naturales?). J Field Ornithol 70: 257–262.
- Lindell, C. 2000. Egg type influences predation rates in artificial nest experiment. J. Field Ornithol. 71, 16–21. doi: 10.1648/0273-8570-71.1.16
- Maier, T.J. & DeGraaf, R.M. 2000. Predation on Japanese quail vs. House Sparrow eggs in artificial nests: small eggs reveal small predators. Condor 102, 325–332. doi: 10.1650/0010-5422(2000)102[0325:POJQVH]2.0.CO;2
- Major, R.E. 1990. The effect of human observers on the intensity of nest predation. Ibis 132: 608–612. doi: 10.1111/j.1474-919X.1990.tb00285.x
- Major, R.E. & Kendal, C.E. 1996. The contribution of artificial nest experiments to understanding avian reproductive success: a review of methods and conclusions. Ibis 138: 298–307. doi: 10.1111/j.1474-919X.1996.tb04342.x
- Marini, M.Â. 1997. Predation-mediated bird nest diversity: an experimental test. Can J Zoolog 75, 317–323. doi: 10.1139/z97-040
- Martin, T.E. 1988. Processes organizing open-nesting bird assemblages: competition or nest predation? Evol. Ecol. 2, 37–50. doi: 10.1007/BF02071587
- Martin, T.E. 1993a. Nest predation among vegetation layers and habitat types: revising the dogmas. Am. Nat. 141, 897–913. doi: 10.1086/285515
- Martin, T.E. 1993b. Nest predation and nest sites. BioScience 43, 523–532. doi: 10.2307/1311947
- Martin, T.E. 1995. Avian life history evolution in relation to nest sites, nest predation, and food. Ecol. Monogr. 65: 101–127. doi: 10.2307/2937160
- Martin, T.E. & Clobert, J. 1996. Nest predation and avian life-history evolution in Europe versus North America: a possible role of humans? Am. Nat. 147: 1028–1046. doi: 10.1086/285891
- Martin, T.E., Lloyd, P., Bosque, C., Barton, D.C., Biancucci, A.L., Cheng, Y.R. & Ton, R. 2011. Growth rate variation among passerine species in tropical and temperate sites: an antagonistic interaction between parental food provisioning and nest predation risk. Evolution 65: 1607–1622. doi: 10.1111/j.1558-5646.2011.01227.x
- Martin, T.E., Martin, P., Olson, C., Heidinger, B. & Fontaine, J. 2000a. Parental care and clutch sizes in North and South American birds. Science 287: 1482–1485. doi: 10.1126/science.287.5457.1482
- Martin, T.E., Scott, J. & Menge, C. 2000b. Nest predation increases with parental activity: separating nest site and parental activity effects. Proc Roy Soc London B: Sci-Biol 267: 2287–2293. doi: 10.1098/rspb.2000.1281
- Mermoz, M. & Martin, C. 1987. Mapa de vegetación del parque y la Reserva Nacional Nahuel Huapi. Administración de Parques Nacionales. Delegación Regional Patagonia. Secretaría de Ciencia y Técnica de la Nación. Buenos Aires.
- Mezquida, E.T. & Marone, L. 2001. Factors Affecting nesting success of a bird Assembly in the Central Monte Desert, Argentina. J. Avian Biol. 32: 287–296. doi: 10.1111/j.0908-8857.2001.320401.x
- Mezquida, E.T. & Marone, L. 2002. Microhabitat structure and avian nest predation risk in an open Argentinean woodland: an experimental study. Acta Oecol. 23: 313–320. doi: 10.1016/S1146-609X(02)01160-8
- Mezquida, E.T. & Marone, L. 2003. Are results of artificial nest experiments a valid indicator of success of natural nests? Wilson Bull. 115: 270–276. doi: 10.1676/02-117
- Montevecchi, W.A. 1976. Egg size and the egg predatory behaviour of crows. Behaviour 57: 307–320. doi: 10.1163/156853976X00587
- Morrone, J.J. 2015. Biogeographical regionalisation of the Andean region. Zootaxa 3936: 207–236. doi: 10.11646/zootaxa.3936.2.3
- Oliveira, C.W.d.S., Almeida, G.P., Paiva, L.V.d. & França, L.F. 2013. Predation on artificial nests in open habitats of central Brazil: effects of time and egg size. Biota Neotropica 13: 142–146. doi: 10.1590/S1676-06032013000100016
- Paton, P.W. 1994. The effect of edge on avian nest success: How strong is the evidence? Conserv. Biol. 8: 17–26. doi: 10.1046/j.1523-1739.1994.08010017.x
- Picman, J., Milks, M.L. & Leptich, M. 1993. Patterns of predation on passerine nests in marshes: effects of water depth and distance from edge. Auk 110: 89–94. doi: 10.2307/4088408
- R Core Team. 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Ricklefs, R. E. 1969. An Analysis of Nesting Mortality in Birds, Smithsonian Institution Press, Washington, DC.
- Robinson, S.K., Thompson III, F.R., Donovan, T.M., Whitehead, D.R. & Faaborg, J. 1995. Regional forest fragmentation and the nesting success of migratory birds. Science 267, 1987–1990. doi: 10.1126/science.267.5206.1987
- Roper, J. J. 1992. Nest predation experiments with quail eggs: Too much to swallow? Oikos 65, 528–530. doi: 10.2307/3545570
- Rozzi, R., Martinez, D.R., Willson, M.F. & Sabag C. 1996. Avifauna de los bosques templados de Sudamérica. Capítulo 7 pp. 135–152, en Armesto JJ, C Villagrán & M Kalin Arroyo (eds.) Ecología de los bosques nativos de Chile. Santiago, Editorial Universitaria.
- Sieving, K.E. 1992. Nest predation and differential insular extinction among selected forest birds of central Panama. Ecology 73: 2310–2328. doi: 10.2307/1941477
- Skutch, A.F. 1985. Clutch size, nesting success, and predation on nests of Neotropical birds, reviewed. Ornithol Monogr 36: 575–594. doi: 10.2307/40168306
- Thompson, F.R. & Burhans, D.E. 2004. Differences in predators of artificial and real songbird nests: evidence of bias in artificial nest studies. Conserv. Biol. 18: 373–380. doi: 10.1111/j.1523-1739.2004.00167.x
- Vergara, P.M. & Simonetti, J.A. 2003. Forest fragmentation and rhinocryptid nest predation in central Chile. Acta Oecol. 24: 285–288. doi: 10.1016/j.actao.2003.09.006
- Weidinger, K. & Kočvara, R. 2010. Repeatability of nest predation in passerines depends on predator species and time scale. Oikos 119: 138–146. doi: 10.1111/j.1600-0706.2009.17649.x
- Wesołowski, T., Rowiński, P. & Maziarz, M. 2009. Wood Warbler Phylloscopus sibilatrix: a nomadic insectivore in search of safe breeding grounds? Bird Study 56: 26–33. doi: 10.1080/00063650802681540
- Wilcove, D.S. 1985. Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66: 1211–1214. doi: 10.2307/1939174
- Willson, M.F., Morrison, J.L., Sieving, K.E., De Santo, T.L., Santisteban, L. & Díaz, I. 2001. Patterns of predation risk and survival of bird nests in a Chilean agricultural landscape. Conserv. Biol. 15, 447–456. doi: 10.1046/j.1523-1739.2001.015002447.x
