ABSTRACT
Capsule: Plumage colour of Northern Saw-whet Owls Aegolius acadicus was strongly associated with body condition and may be used to distinguish the highest quality individuals. Relationships between eye colour and body condition were more complex and deserve further study.
Aims: We explored the association of colouration with body condition of Northern Saw-whet Owls during their autumnal migration across Pennsylvania, USA from 1999 to 2012.
Methods: We used fat and keel scores of female owls to index body condition. Since feathers are laid down during pre-migration moult, we hypothesized that facial white plumage would be more strongly associated with long-term condition (keel scores) whereas eye colour should indicate short-term condition (fat scores).
Results: Facial white plumage and eye colour were largely uncorrelated, but were strongly associated with both fat and keel scores. Contrary to our hypothesis, owls with more facial white plumage had both higher fat and keel scores, indicating that facial white was strongly associated with both short- and long-term condition. This appears to be because facial white was highest in individuals most capable of maintaining good condition in both scores (the highest quality owls). Relationships between condition and eye colour were more complex, since owls with highest fat scores but lowest keel scores had lightest eyes, possibly resulting from trade-offs with pigment function and immunocompetence. Our results also demonstrated environmental forcing (cyclic prey availability) of colouration and body condition, although not the relationship between them which remained consistent between years and for different ages.
Conclusion: Facial white, but not eye colour, was a robust predictor of short- and long-term body condition, permitting detection of individuals in the best and most consistent condition. Further study of colouration and condition are needed to elucidate the extent of genetic control and environmental factors in feather melanization.
The development of methods to quickly and simply assess individual health and condition in birds is of considerable importance. Measures of body condition, such as subcutaneous fat deposits and pectoral muscle scoring, have been widely used to assess the health of birds (Labocha & Hayes Citation2012, Tellería et al. Citation2013). Links have been found in some species between migratory body condition and survival (Burton et al. Citation2006, Cresswell Citation2009) so that, although the physiology of migration is not well documented for many taxa, body condition may offer a reliable way to assess the health of migratory bird populations. Similarly, avian colouration can convey information to conspecifics on individual quality (Andersson Citation1982) through plumages (Saino et al. Citation2015), pigmented skin (Velando et al. Citation2006, Avilés & Parejo Citation2013), eyes (Newton & Marquiss Citation1982), and keratinaceous structures (Préault et al. Citation2005). Two of the most common pigments in avian colouration are melanins and carotenoids (Fox Citation1976). Unlike melanins that can be synthesized from amino acids, carotenoids must be obtained through the diet (Møller et al. Citation2000, McGraw & Hill Citation2006, Benito et al. Citation2011). After their synthesis or assimilation, pigments can be metabolized into the follicle of a developing feather (or other integumentary tissues and epithelial cells of the iris) and will be deposited either as melanin granules or lipids (McGraw & Hill Citation2006), which can result in reliable patterns that convey information on nutritional condition and health (Svensson & Merilä Citation1996, Avilés & Parejo Citation2013).
Additionally, hormonal control can also drive pigmentation to convey sex (Rosenfield et al. Citation2003), age and sexual maturity (Newton & Marquiss Citation1982, Jouventin et al. Citation2005), and mate quality (Wiehn Citation1997). Past studies have demonstrated that elevated concentrations of stress hormone (e.g. corticosterone) are capable of reducing the extent of melanin- and carotenoid-based pigmentation (Roulin et al. Citation2008, Fairhurst et al. Citation2014). Given that white feathers, those lacking melanin, are more prone to degradation (Burtt Citation1986) and that carotenoids play an essential role in immune system function (McGraw & Ardia Citation2003), individuals that are able to produce and maintain costly colouration (e.g. from carotenoid pigmentation or absence of melanin) during stressful periods, such as breeding or over-wintering, have been interpreted as expressing high individual quality (Martínez-Padilla et al. Citation2013, Fairhurst et al. Citation2014, Saino et al. Citation2015).
For raptors, developmental changes in both plumage and eye colouration have been documented (Wiehn Citation1997, Bortolotti et al. Citation2003, Rosenfield et al. Citation2003), although the hormonal control of change in eye colour has been less well studied. Little is known about colouration during seasonal migration, but because feathers are influenced by nutritional conditions experienced at the time of their growth (Jaspers et al. Citation2006, Pap et al. Citation2008), for species that moult prior to migration, autumn plumage characteristics reflect post-breeding/pre-dispersal conditions. Conversely, non-feathered structures can change relatively quickly based on diet (Biard et al. Citation2009, Benito et al. Citation2011). Thus, colouration during autumn migration may provide crucial carry-over information on the stresses of breeding (Norris et al. Citation2004) and/or individual condition during or prior to the onset of migration (Heise & Moore Citation2003), and may act as an index of over-winter survival potential (Griffith et al. Citation2003). Here, we investigate connections between plumage and eye colourations and migratory body condition in a small raptor.
The Northern Saw-whet Owl Aegolius acadicus is a small, migratory owl (female autumn migratory mass average (± sd) = 95.3 ± 5.54 g; range = 74–120.1 g, n = 2195, Weidensaul unpublished data) with a broad breeding distribution across northern and mountainous North America. Northern Saw-whet Owls with definitive basic (adult winter) plumage are a dark brown (raw umber) with white spots and streaking and have a buff and white facial disk and throat (Rasmussen et al. Citation2008, Weidensaul et al. Citation2015). While plumage colouration in this species is not well studied, and to our knowledge structural colouration has not been investigated, melanin and porphyrin pigments are believed to be important components of plumage in these owls, resulting in the dark brown and buff feathers (Weidensaul et al. Citation2011, Citation2015). Three classes of pigments (carotenoids, pteridines, and purines) have been implicated in Northern Saw-whet Owl eye colour (Oliphant Citation1987) and, although carotenoid-based colour can change quickly with diet (Møller et al. Citation2000, McGraw & Hill Citation2006, Benito et al. Citation2011), much less is known about the other two pigments. Thus, eye colouration may reflect similar conditions as plumage colour, or may be a shorter-term measure of diet and body condition in Northern Saw-whet Owls.
Northern Saw-whet Owls undertake southerly migrations between September and November, with migratory populations largely consisting of females and juveniles (hatch-year) as most males are assumed to remain on the breeding range over winter (Brinker et al. Citation1997, Rasmussen et al. Citation2008, Brittain et al. Citation2009). The age structure of these migratory populations varies considerably (Brittain et al. Citation2009), comprising a large proportion of juvenile owls approximately every four years. This boom–bust population cycle appears to reflect availability of rodent prey during the breeding season and is thus reflected as higher juvenile recruitment (Brinker et al. Citation1997, Whalen & Watts Citation2002, Beckett & Proudfoot Citation2011, Confer et al. Citation2014). Links between body condition of owls and stage in this boom–bust cycle have also been reported (Whalen & Watts Citation2002, Stock et al. Citation2006). Thus, comparing colouration within a long-term study of this species may permit an understanding of the role of environmental forcing on pigmentation and how this relates to body condition, processes rarely reported for wild birds (McGraw & Hill Citation2006).
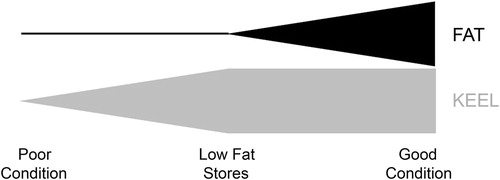
Here, we hypothesize that plumage and eye colourations are associated with body condition for Northern Saw-whet Owls during autumnal migrations. Subcutaneous fat deposits and pectoral muscle measures are widely used as indices of body condition (Brown Citation1996, Pyle Citation1997) as they represent short- and long-term measures of condition, respectively (Cherel et al. Citation1988, Jenni & Jenni-Eiermann Citation1998) and may be used to distinguish three categories of body condition for migrating birds (). We use a 14-year mark and recapture dataset for Northern Saw-whet Owls across southeastern and southcentral Pennsylvania, USA, to investigate the association between colouration (plumage and iris) and body condition of adult and juvenile female owls caught during highly contrasting years of their population cycle. We hypothesize that the extent of white feathers on the facial disk, which are laid down prior to autumnal migrations, show a stronger association with keel scores (long-term body condition) than subcutaneous fat scores (short-term body condition). However, since carotenoids (one of the eye pigments) can cause rapid colour changes, we hypothesize that eye colour shows the opposite relationship, being more strongly associated with fat scores.
Figure 1. Predicted relationship between within-individual changes in body condition and fat and keel scores of migrating Northern Saw-whet Owls, where during migration fat stores are generally utilized preferentially to protein (catabolized from muscles and indexed by keel scores). Vertical depth of shaded areas represents relative magnitude of fat (black) or keel (grey) stores. High fat scores are generally associated with high keel scores and low keel scores are generally associated with low fat scores; however, birds with low fat scores may exhibit a wide range of keel scores and birds with high keel scores may exhibit a wide range of fat scores.
Methods
Field sites and owl colour measures
Northern Saw-whet Owls were individually marked with rings, annually at five locations (three operating each year) between 1999 and 2012 across southeastern and southcentral Pennsylvania (Berry Mountain: 40°30′50.6″N 77°03′32.3″W; Hidden Valley: 40°37′18.56″N 76°16′15.99″W; King’s Gap: 40°05′34.78″N 76°16′01.15″W; Small Valley: 40°29′41″N 76°46′48″W; and South Mountain: 39°56′1.3″N 77°25′22.9″W). Owls were trapped using an array of mist nets (12 m × 2.6 m) and audio playback of a male Northern Saw-whet Owl advertisement call. Netting took place each night (except during inclement weather) from about 1 October to 30 November, starting at sunset (between 17:00 and 19:00 EST) and running at least until 23:00 EST. Nets were checked hourly and any trapped owls were removed for processing. During this period, 5761 unique Northern Saw-whet Owls were captured, of which 4453 were classified as females (77.30%). Because we captured fewer males (n = 333, 5.78% of our captures) and patterns of colouration in raptors sometimes differ between sexes (Rosenfield et al. Citation2003), we reduced our analyses of condition and colouration to include data only from female owls.
All captured owls were ringed, sexed by use of mass and wing chord measurements (Brinker et al. Citation1997, shown to correctly predict sex 97% of the time, Beckett & Proudfoot Citation2012), and aged by moult pattern (Pyle Citation1997, Weidensaul et al. Citation2011). Comparable percentages of hatch-year (HY) and after-hatch-year (AHY) female owls were captured during the study period (50.99% and 49.01%, respectively). Fat and keel, eye colour, facial and throat white, and alula barring scores were assigned during their capture by the ringer, although not all body condition and colour variables were recorded for every owl. To reduce any subjectivity, all data recorders underwent extensive training in making these determinations before contributing data to the study: usually verified by experienced personnel for several hundred owls processed over the course of at least two years. Eye colour was graded when the owl was in the hand by comparing the iris colour to four Benjamin Moore paint chips used by ringers in Project Owlnet (Classic Colour paint sample chips from darkest to lightest: 315 Oxford Gold, 322 Abstracta, 329 Golden Orchards, 336 Bold Yellow; Benjamin Moore & Co, 101 Paragon Drive, Montvale, NJ 07645 USA, www.projectowlnet.org). Similarly, facial white was also scored when the owl was in the hand and was recorded as: (1) no white encompassing eyes, (2) faint or thin rings encircling eyes, or (3) broad rings encircling eyes (). Since simple exploratory analyses indicated that facial white was by far the strongest statistical predictor of body condition of the three plumage colour measurements taken (online Supplementary Table S1), all analyses that follow report only results from models using facial white or eye colour as indices of colouration.
Figure 2. Examples of facial white scoring assigned to Northern Saw-whet Owls, with arrows indicating areas considered during score assignment. (a) Individuals with no white encompassing the eyes, (b) individuals with faint or thin rings of white encircling the eyes, and (c) individuals with broad white rings encircling the eyes.

Body condition indices
Subcutaneous fat and keel scores were measured by observing subcutaneous fat deposits within the wing pit and by feeling the curvature of the pectoral muscle, respectively. Fat scores were assigned as: (1) no visible or trace fat deposit in wing pit, (2) raised, bulbous line of fat but wing pit not filled, and (3) most or all of wing pit filled, possibly bulging and spilling into surrounding areas (following DeLong & Gessaman Citation2001). Similarly, keel scores were assigned as: (1) deeply sunken pectoral muscle with a sharp keel, (2) sharp keel with concave or straight pectoral muscle, and (3) pectoral muscle protruding slightly from breast, possibly flush with the keel, or rounded with a sunken keel.
Data processing
For individuals recaptured (i.e. encountered more than once) within the same year (6.3% of all captured individuals; n = 361 owls) or a later year (0.5% of all captured individuals; n = 28 owls), we used only data collected during the first capture in our subsequent analyses.
As an index of prey availability during the summer at breeding sites, we inferred the stage in the boom–bust population cycle based on autumn capture rates. To do this, we calculated an overall annual capture rate using all individual owls (including males) during their first capture within that year and expressed this as number of owls captured per 10 m2 net per 100 h (Brinker et al. Citation1997). Since there are no previously defined categories that are consistently used between ringing sites to classify years within this population cycle, we used our capture rates (using all owls) to assign each year to one of three categories representing the stages in the boom–bust cycle: bust (0.0–3.0 owls/10 m2 net/100 h), intermediate (3.1–6.0 owls/10 m2 net/100 h), and boom (more than 6.0 owls/10 m2 net/100 h). Using this classification, four years were categorized as bust, six as intermediate, and four as boom and yielded similar assignments to boom–bust years reported for other eastern Northern Saw-whet Owl populations (Whalen & Watts Citation2002, Confer et al. Citation2014; online Supplementary Table S2). The proportion of hatch-year owls captured was significantly different within each category (Kruskal–Wallis = 7.91, df = 2, P = 0.02; online Supplementary Figure S1), and was highest during boom years, thus confirming the existence of population cycles within our data set.
Data analyses
Analyses were performed in R version 3.3.1 (R Development Core Team Citation2016). All data are reported with mean ± sd unless otherwise specified.
We tested four separate hypotheses about the association of facial white or eye colour with migration body condition. Prior to all analyses, we reduced age categories assigned during fieldwork (i.e. hatch-year, second-year, and after-second-year) to two age categories (hatch-year and after-hatch-year) after initial analyses found little difference in colouration between second-year and older owls and we were more interested in the expected larger difference between juvenile and adult owls than any improvement with age within adult owls. Additionally, because we had numerous ringers of varying experience processing owls over the course of this project, we also limited our dataset to include data only from ringers that had volunteered with the project for 11–14 years and had processed at least 100 owls. These five ringers were trained at a minimum for their first full season and were blind-tested on their assigned morphometric and condition scores by experienced ringers. We verified that inter-observer bias of eye colour and facial white scoring within the same year category was not an issue in our data (Friedman’s tests, P > 0.05 in all comparisons, details not shown). We then analysed these reduced data (n = 907 owls processed by one of five ringers) using ordered logit models as our dependent variables, fat and keel scores, were ordinal variables (R package polr, Ripley et al. Citation2015). To provide an estimate of timing of capture during the migration period, we computed a covariate, relative capture date, for every capture as: tp − tr, where tp was the date of peak capture (when the most owls were caught in that year), and tr was the capture date of a specific owl. Relative capture date was included in our ordered logit models to control for the effect of the timing of capture on body condition, as we anticipated a decrease with capture date.
Using this approach, we tested four hypotheses, examining whether for Northern Saw-whet Owls: (1) the extent of facial white is an honest short-term indicator of quality and thus increases with fat score and is greater during boom years; (2) the extent of facial white is an honest long-term indicator of quality and thus increases with keel score and is greater for adult (after-hatch-year) owls; (3) lighter eye colour is an honest short-term indicator of quality and thus increases with fat score and was lighter during boom years; and (4) lighter eye colour is an honest long-term indicator of quality and thus increases with keel score and was greater for adult (after-hatch-year) owls. Since we had no a priori reason to predict specific effects of age and year on condition or their possible interaction with the relationships between colour and condition, we screened for these before finalizing our above hypotheses. We found effects of year on fat score, and age on keel score but no support for any interactive effects (online Supplementary Table S3). For all ordered logit models, statistical significance was specified as α = 0.05.
In addition to these separate analyses of fat scores and keel scores, to aid in the visual interpretation of the association between body condition and extent of facial white or eye colour, we condensed fat and keel scores into a single measure of body condition, as migratory birds utilize fat prior to catabolizing muscle (). We then plotted the relationship between these three stages of body condition and the means of either extent of facial white or eye colour.
We calculated mean fat and keel scores in relation to age and the stage in the boom–bust cycle to examine environmental and developmental effects on condition. We did the same for facial white and eye colour to quantify trends in these metrics with age and stage in the boom–bust cycle.
Finally, we examined the correlation between fat and keel scores using Spearman’s rank correlation coefficient and assessed how this correlation would change when removing individuals in the best migratory condition (i.e. fat and keel scores of 3).
Results
High fat scores were more common than either intermediate or low fat scores among owls with the highest amount of facial white (a & ). Similarly, increasing facial white was associated with higher keel scores (c & ). Light eye colour was most strongly associated with high fat scores (b & ) but the opposite was true for keel score, with the lowest scores being recorded most often among owls with light eyes (d & ).
Figure 3. Histograms of frequency distribution of facial white or eye colour categories across categories of fat score (a, b) or keel score (c, d) for 907 migrating Northern Saw-whet Owls caught in Pennsylvania. For both colour variables, significance of Z-scores (from ordered logit models; ) are given in reference to the darkest score: * P < 0.05, ** P < 0.01, *** P < 0.001.
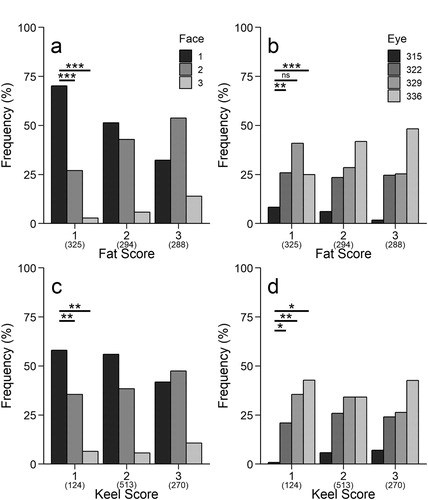
Table 1. Results of the four ordered logit models assessing relationships of fat score (a, b) or keel score (c, d) for 907 female Northern Saw-whet Owls caught in Pennsylvania during their autumnal migration between 1999 and 2012.
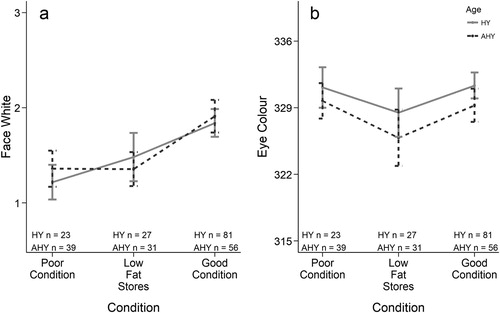
When fat and keel scores were combined into a single body condition metric, facial white increased strongly with body condition (a), whereas eye colour was lightest for owls in both poor condition and good condition but darker for intermediate owls (those with low fat stores but high keel scores; b).
Figure 4. Mean ± 95% CI (a) face white and (b) eye colour scores for hatch-year (HY) and adult (AHY) Northern Saw-whet Owls for three particular combinations of keel and fat scores that represent certain body conditions: Fat = 1/Keel = 1 (poor condition), Fat = 1/Keel = 3 (low fat stores), Fat = 3/Keel = 3 (good condition); see .
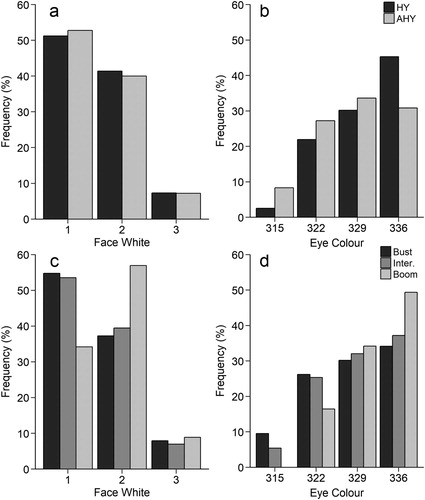
Only fat scores were higher in boom years than in bust or intermediate years () and this was the case for both hatch-year (HY) and after-hatch-year (AHY) individuals. Facial white characteristics were similar between age classes (a) but eye colour showed some variation with age: the lightest eye colour category being most common among hatch-year owls (b). The extent of facial white was also greater in boom years than in bust or intermediate years (c) and this same trend was also present for eye colour (d). However, we found no evidence of age or environmental (population cycle) effects on any of our reported relationships between colouration and body condition (online Supplementary Table S3).
Figure 5. Mean ± sd fat (Kruskal–Wallis = 36.55, df = 2, P < 0.001) and keel scores (Kruskal–Wallis = 3.02, df = 2, P = 0.22) for 907 female Northern Saw-whet Owls caught during migration in Pennsylvania between 1999 and 2012.
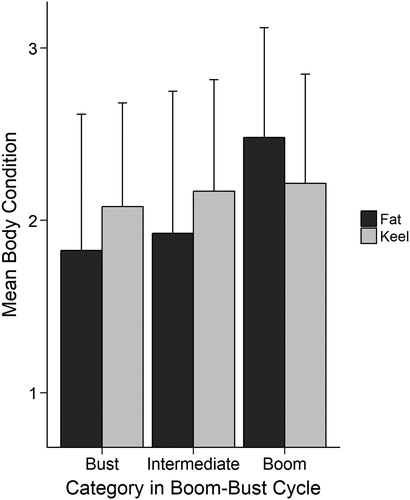
Figure 6. Per cent of female Northern Saw-whet Owls in each age category (a, b; n = 907, 48.18% HY, 51.82% AHY) and stage in the boom–bust cycle (c, d; n = 907, 13.89% bust, 77.40% intermediate, 8.71% boom). (a) Face white in relation to age from least white to most white, X2 = 0.21, df = 2, P = 0.90. (b) Eye colour in relation to age from darkest to lightest, X2 = 29.61, df = 3, P < 0.001. (c) Face white in relation to stage in the boom–bust cycle, from least white to most white, X2 = 11.53, df = 4, P < 0.05. (d) Eye colour in relation to stage in the boom–bust cycle, from darkest to lightest, X2 = 14.02, df = 6, P < 0.05.
Fat and keel scores were weakly but positively correlated (Spearman’s ρ = 0.28); after removing individuals in the highest condition (fat and keel scores of 3), this correlation was virtually non-existent (Spearman’s ρ = −0.02).
Discussion
In our study, firstly, the extent of facial white plumage was strongly associated with both short- and long-term body condition indices for Northern Saw-whet Owls during their autumnal migrations, contrary to our hypothesis that, being laid down prior to migration, it should only reflect long-term condition. Secondly, the relationship between eye colour and body condition, although strong, appeared to be more complex, with the lightest eye colours being found among owls with either poor keel scores or high fat scores. These relationships, despite being more complex than anticipated, can be reconciled in reference to individual quality and the hormonal control of avian colouration.
Since some wing and most body feathers are moulted prior to migration in this species (Rasmussen et al. Citation2008), plumage scores reflect breeding season condition and thus the extent of facial white plumage should show stronger relationships with keel score (a long-term measure of condition, Cherel et al. Citation1988, Jenni & Jenni-Eiermann Citation1998) than with the shorter-term, fat score index. However, in our data, the relationship with keel and fat scores was similar in strength (compare significance in a, c). This strong relationship with fat score appears to primarily be driven by more extensive facial white for owls in the best condition (both highest fat and keel scores) with all other owls having lower, but similar scores (compare the confidence intervals in a). Thus, the owls in best body condition prior to migration (when feathers are laid down) manage to maintain the highest migratory fuel reserves (fat), presumably as these represent the best quality individuals. This also helps explain the positive correlation between our body condition scores and why this correlation is weak: this relationship is primarily driven by those owls in good migratory condition, with little correlation between lower scores. A mechanistic link between nutritional stress and plumage colouration was also supported by the lower face white scores for owls caught in bust years (regardless of age), suggesting that face white plumage corresponds with large-scale changes in prey availability across the breeding range, presumably in response to the lower body condition recorded in these years.
The relationship between eye and plumage colouration and body condition we detected in Northern Saw-whet Owls (a, b) is exciting, but not unexpected. Given the relationships that have been demonstrated in other birds between colouration and quality during the breeding season (Andersson Citation1982), food availability and condition, and that both condition and colouration are regulated by similar hormonal control (Newton & Marquiss Citation1982, Rosenfield et al. Citation2003 Jouventin et al. Citation2005), it stands to reason that inter-annual variability in food availability could be reflected in colouration. This should be expected for carotenoid-based pigmentation, which can only be obtained through diet (Møller et al. Citation2000, McGraw & Hill Citation2006, Benito et al. Citation2011); however, melanin-based pigmentation appears to be more tightly controlled by genetic mechanisms and is not known to vary with environmental conditions (Badyaev & Hill Citation2000). Here, we provide evidence that variation in prey population cycles could indeed be influencing the extent of melanization in a wild population of a small, migratory owl. Future work for this species should evaluate the strength of melanization controlled by genetics versus that which we propose to be controlled by prey availability across the breeding range.
The link we report between facial white plumage and body condition is consistent with studies showing that plumage colour depends on both individual quality and nutritional status (Svensson & Merilä Citation1996, Fairhurst et al. Citation2014), especially for white feathers (Saino et al. Citation2015). In fact, whether fat and keel scores were considered separately or condensed meaningfully into a single metric of condition, there was always a strong, positive relationship between the extent of facial white plumage and body condition ( & ). As white plumage is believed to be a costly trait since it is more prone to degradation than pigmented feather tissue (Burtt Citation1986), we suggest that the extent of facial white plumage may have strong potential to be used as an indicator of good body condition in migrating owls.
Eye colour in Northern Saw-whet Owls was also associated with body condition in our separate analyses of fat and keel scores. Even so, the direction of the relationships with fat and keel scores were opposite to one another, because light eyes were associated with high fat scores but low keel scores (). However, when fat and keel scores were condensed into a single metric of condition based on migratory physiology (), it was clear that lighter eye colours were found more commonly in both owls in poor and good condition than in those in intermediate body condition (b). The mechanism behind these responses is likely to be complex, since eye colour in Northern Saw-whet Owls is a product of three pigments (carotenoids, pteridines, and purines) and little is known about how these respond to changing nutritional status (McGraw & Hill Citation2006). However, carotenoids cannot be synthesized directly but must be obtained through diets (Møller et al. Citation2000, McGraw & Hill Citation2006, Benito et al. Citation2011) and these also play a role in immunocompetence (Blount et al. Citation2003) and are often stored in the fat (Negro et al. Citation2001). Thus, one possible explanation is that when fat stores are mobilized during migration the concentration of carotenoids in other tissues, possibly including the epithelial tissue of the iris, necessarily increases (Negro et al. Citation2001, Costantini et al. Citation2008), causing owls with the lowest fat scores to have the darkest eyes ( & b). Once fat stores are depleted, migratory birds, including owls, catabolize muscle to provide the energy required for migration (Cherel et al. Citation1988, Jenni & Jenni-Eiermann Citation1998). In these emaciated individuals, carotenoids may be used extensively for immunocompetency (Negro et al. Citation2001, Blount et al. Citation2003) and thus mobilized from tissues such as the eyes, depleting colour intensity and leading to our finding that owls with both the lowest fat and keel scores, those in the poorest condition, had light coloured eyes (b). This may also help explain why hatch-year owls generally had lighter eyes (b) as they may have a greater need to utilize carotenoids for immunocompetency during the summer or insufficient time to shunt pigments into tissues such as the iris prior to migration (Negro et al. Citation2001, Blount et al. Citation2003). Alternatively, hatch-year owls may be in generally better condition during migration (Young & Proudfoot Citation2014), as a result of parental provisioning during the summer, and thus maintain fat deposits (with carotenoid stores) to a greater extent than after-hatch-year birds. Even so, little is known of the mechanism behind changes in pteridine- and purine-based pigmentation and thus, because of the contrasting responses to fat and keel scores and possible developmental constraints for hatch-year owls, eye colour alone may be too complex to be reliable indicator of body condition in this species. However, given that carotenoid-based pigmentation has been implicated in Northern Saw-whet Owl eye colour (Oliphant Citation1987) and that carotenoids can only be obtained through diet (Møller et al. Citation2000, McGraw & Hill Citation2006, Benito et al. Citation2011), future work should consider the role of both circulating carotenoids and body condition in determining eye colour.
Although both body condition and colouration were found to vary with age and environment (boom–bust cycles), the relationships between them did not. Thus, regardless of age or environment, strong relationships existed between plumage colour or eye colour and body condition of migratory owls. Furthermore, lower keel scores recorded for Northern Saw-whet owls during intermediate and bust years of the population cycle () suggest that environmental changes that impact prey availability across the breeding range will have carry-over effects, reducing long-term condition during migration, a critical life-history stage with high energetic demands where birds are most likely to incur fitness consequences (Moreno & Møller Citation2011, Klaassen et al. Citation2012). Plumage colouration has much potential to be used as a simple index to monitor stress caused by environmental fluctuations (Svensson & Merilä Citation1996, Saino et al. Citation2015). We find the extent of facial white plumage during migration to be a strong candidate for such an index of nutritional stress because it distinguishes the individuals most capable of maintaining good body condition in both the short- and long-term.
Supplemental Material
Download MS Word (48.1 KB)Acknowledgements
We thank the many volunteer banders and interns who have collected data used in this project, especially site coordinators Sandra J. Lockerman and Gary L. Shimmel, under the auspices of the Ned Smith Center for Nature and Art. S. Gibson and R. Wails provided technical support. All banding activities were performed under a federal bird banding permit issued to S. Weidensaul (#22918).
Additional information
Funding
References
- Andersson, M. 1982. Sexual selection, natural selection and quality advertisement. Biol. J. Linn. Soc. 17: 375–393. doi: 10.1111/j.1095-8312.1982.tb02028.x
- Avilés, J.M. & Parejo, D. 2013. Colour also matters for nocturnal birds: owlet bill colouration advertises quality and influences parental feeding behaviour in Little Owls. Oecologia 173: 399–408. doi: 10.1007/s00442-013-2625-8
- Badyaev, A.V. & Hill, G.E. 2000. Evolution of sexual dichromatism: contribution of carotenoid- versus melanin-based coloration. Biol. J. Linn. Soc. 69: 153–172. doi: 10.1111/j.1095-8312.2000.tb01196.x
- Beckett, S.R. & Proudfoot, G.A. 2011. Large-scale movement and migration of Northern Saw-whet Owls in eastern North America. Wilson J. Ornithol. 123: 521–535. doi: 10.1676/10-130.1
- Beckett, S.R. & Proudfoot, G.A. 2012. Sex-specific migration trends of Northern Saw-whet Owls in eastern North America. J. Raptor Res. 46: 98–108. doi: 10.3356/JRR-11-10.1
- Benito, M.M., González-Solís, J. & Becker, P.H. 2011. Carotenoid supplementation and sex-specific trade-offs between colouration and condition in Common Tern chicks. J. Comp. Physiol. B 181: 539–549.
- Biard, C., Gil, D., Karadaş, F., Saino, N., Spottiswoode, C.N., Surai, P.F. & Møller, A.P. 2009. Maternal effects mediated by antioxidants and the evolution of carotenoid-based signals in birds. Am. Nat. 174: 696–708. doi: 10.1086/606021
- Blount, J.D., Metcalfe, N.B., Birkhead, T.R. & Surai, P.F. 2003. Carotenoid modulation of immune function and sexual attractiveness in Zebra Finches. Science 300: 125–127. doi: 10.1126/science.1082142
- Bortolotti, G.R., Smits, J.E. & Bird, D.M. 2003. Iris colour of American Kestrels varies with age, sex, and exposure to PCBs. Physiol. Biochem. Zool. 76: 99–104. doi: 10.1086/345485
- Brinker, D.F., Duffy, K.E., Whalen, D.M., Watts, B.D. & Dodge, K.M. 1997. Autumn migration of Northern Saw-whet Owls (Aegolius acadicus) in the middle Atlantic and northeastern United States: what observations from 1995 suggest. In Duncan, J.R., Johnson, D.H. & Nicholls, T.H. (eds.) Biology and Conservation of Owls of the Northern Hemisphere: 2nd International Symposium, 74–89. USDA Forest Service, St. Paul.
- Brittain, R.A., Meretsky, V.J., Gwinn, J.A., Hammond, J.G. & Riegel, J.K. 2009. Northern Saw-whet Owl (Aegolius acadicus) autumn migration magnitude and demographics in south-central Indiana. J. Raptor Res. 43: 199–209. doi: 10.3356/JRR-08-51.1
- Brown, M.E. 1996. Assessing body condition in birds. In Nolan, V. & Ketterson, E.D. (eds.) Current Ornithology, Vol. 13: 67–135. Plenum Press, New York, NY.
- Burtt, E.H. 1986. An analysis of physical, physiological, and optical aspects of avian coloration with emphasis on wood-warblers. Ornithol. Monogr. 38: 1–126.
- Burton, N.H.K., Rehfisch, M.M., Clark, N.A. & Dodd, S.G. 2006. Impacts of sudden winter habitat loss on the body condition and survival of Redshank Tringa totanus. J. Appl. Ecol. 43: 464–473. doi: 10.1111/j.1365-2664.2006.01156.x
- Cherel, Y., Robin, J.-P. & Maho, Y.L. 1988. Physiology and biochemistry of long-term fasting in birds. Can. J. Zool. 66: 159–166. doi: 10.1139/z88-022
- Confer, J.L., Kanda, L.L. & Ireyena, L. 2014. Northern Saw-whet Owl: regional patterns for fall migration and demographics revealed by banding data. Wilson J. Ornithol. 126: 305–320. doi: 10.1676/13-011.1
- Costantini, D., Fanfani, A. & Dell'Omo, G. 2008. Effects of corticosteroids on oxidative damage and circulating carotenoids in captive adult kestrels (Falco tinnunculus). J. Comp. Physiol. B 178: 829–835. doi: 10.1007/s00360-008-0270-z
- Cresswell, W. 2009. The use of mass and fat reserve measurements from ringing studies to assess body condition. Ring. Migr. 24: 227–232. doi: 10.1080/03078698.2009.9674396
- DeLong, J.P. & Gessaman, J.A. 2001. A comparison of noninvasive techniques for estimating total body fat in Sharp-shinned and Cooper’s Hawks. J. Field Ornithol. 72: 349–364. doi: 10.1648/0273-8570-72.3.349
- Fairhurst, G.D., Dawson, R.D., van Oort, H. & Bortolotti, G.R. 2014. Synchronizing feather-based measures of corticosterone and carotenoid-dependent signals: what relationships do we expect? Oecologia 174: 689–698. doi: 10.1007/s00442-013-2830-5
- Fox, D.L. 1976. Animal Biochromes and Structural Colors. University of California Press, Berkeley, CA.
- Griffith, S.C., Örnborg, J., Russell, A.F., Andersson, S. & Sheldon, B.C. 2003. Correlations between ultraviolet coloration, overwinter survival and offspring sex ratio in the Blue Tit. J. Evol. Biol. 16: 1045–1054. doi: 10.1046/j.1420-9101.2003.00550.x
- Heise, C.D. & Moore, F.R. 2003. Age-related differences in foraging efficiency, molt, and fat deposition of Gray Catbirds prior to autumn migration. Condor 105: 496–504. doi: 10.1650/7183
- Jaspers, V.L.B., Voorspoels, S., Covaci, A. & Eens, M. 2006. Can predatory bird feathers be used as a non-destructive biomonitoring tool of organic pollutants? Biol. Lett. 2: 283–285. doi: 10.1098/rsbl.2006.0450
- Jenni, L. & Jenni-Eiermann, S. 1998. Fuel supply and metabolic constraints in migrating birds. J. Avian Biol. 29: 521–528. doi: 10.2307/3677171
- Jouventin, P., Nolan, P.M., Örnborg, J. & Dobson, F.S. 2005. Ultraviolet beak spots in King and Emperor Penguins. Condor 107: 144–150. doi: 10.1650/7512
- Klaassen, M., Hoye, B.J., Nolet, B.A. & Buttemer, W.A. 2012. Ecophysiology of avian migration in the face of current global hazards. Phil. Trans. R Soc. B 367: 1719–1732. doi: 10.1098/rstb.2012.0008
- Labocha, M.K. & Hayes, J.P. 2012. Morphometric indices of body condition in birds: a review. J. Ornithol. 153: 1–22. doi: 10.1007/s10336-011-0706-1
- Martínez-Padilla, J., Mougeot, F., García, J.T., Arroyo, B. & Bortolotti, G.R. 2013. Feather corticosterone levels and carotenoid-based coloration in Common Buzzard (Buteo buteo) nestlings. J. Raptor Res. 47: 161–173. doi: 10.3356/JRR-12-41.1
- McGraw, K.J. & Ardia, D.R. 2003. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 162: 704–712. doi: 10.1086/378904
- McGraw, K.J. & Hill, G.E. 2006. Bird Colouration Volume 1: Mechanisms and Measurements. Harvard University Press, Cambridge.
- Møller, A.P., Biard, C., Blount, J.D., Houston, D.C., Ninni, P., Saino, N. & Surai, P.F. 2000. Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult. Biol. Rev. 11: 137–159.
- Moreno, J. & Møller, A.P. 2011. Extreme climatic events in relation to global change and their impact on life histories. Curr. Zool. 57: 375–389. doi: 10.1093/czoolo/57.3.375
- Negro, J.J., Figuerola, J., Garrido, J. & Green, A.J. 2001. Fat stores in birds: an overlooked sink for carotenoid pigments? Funct. Ecol. 15: 297–303. doi: 10.1046/j.1365-2435.2001.00526.x
- Newton, I. & Marquiss, M. 1982. Eye colour, age and breeding performance in Sparrowhawks Accipiter nisus. Bird Study 29: 195–200. doi: 10.1080/00063658209476757
- Norris, D.R., Marra, P.P., Montgomerie, R., Kyser, T.K. & Ratcliffe, L.M. 2004. Reproductive effort, molting latitude, and feather color in a migratory songbird. Science 306: 2249–2250. doi: 10.1126/science.1103542
- Oliphant, L.W. 1987. Pteridines and purines as major pigments of the avian iris. Pigm. Cell Res. 1: 129–131. doi: 10.1111/j.1600-0749.1987.tb00401.x
- Pap, P.L., Vágási, C.I., Czirják, G.Á. & Barta, Z. 2008. Diet quality affects postnuptial molting and feather quality of the House Sparrow (Passer domesticus): interaction with humoral immune function? Can. J. Zool. 86: 834–842. doi: 10.1139/Z08-060
- Préault, M., Chastel, O., Cézilly, F. & Faivre, B. 2005. Male bill colour and age are associated with parental abilities and breeding performance in blackbirds. Behav. Ecol. Sociobiol. 58: 497–505. doi: 10.1007/s00265-005-0937-3
- Pyle, P. 1997. Flight-feather Molt Patterns and Age in North American Owls. American Birding Association, Colorado Springs.
- R Development Core Team. 2016. R: a language and environment for statistical computing, ver. 3.3.1. Vienna: R Foundation for Statistical Computing. Available from: https://www.r-project.org/
- Rasmussen, J.L., Sealy, S.G. & Cannings, R.J. 2008. Northern Saw-whet Owl (Aegolius acadicus). In Poole, A. (ed.) The Birds of North America Online. Cornell Lab of Ornithology, Ithaca, NY. Available from: https://doi.org/10.2173/bna.42.
- Ripley, B., Venables, B., Bates, D.M., Hornik, K., Gebhardt, A. & Firth, D. 2015. MASS: support functions and datasets for Venables and Ripley's MASS. R package ver. 7.3-45. Available from: https://cran.r-project.org/web/packages/MASS/.
- Rosenfield, R.N., Bielefeldt, J., Rosenfield, L.J., Stewart, A.C., Murphy, R.K., Grosshuesch, D.A. & Bozek, M.A. 2003. Comparative relationships among eye color, age, and sex in three North American populations of Cooper's Hawks. Wilson Bull. 115: 225–230. doi: 10.1676/03-012
- Roulin, A., Almasi, B., Rossi-Pedruzzi, A., Ducrest, A.-L., Wakamatsu, K., Miksik, I., Blount, J.D., Jenni-Eiermann, S. & Jenni, L. 2008. Corticosterone mediates the condition-dependent component of melanin-based coloration. Anim. Behav. 75: 1351–1358. doi: 10.1016/j.anbehav.2007.09.007
- Saino, N., Romano, M., Romano, A., Rubolini, D., Ambrosini, R., Caprioli, M., Parolini, M., Scandolara, C., Bazzi, G. & Costanzo, A. 2015. White tail spots in breeding Barn Swallows Hirundo rustica signal body condition during winter moult. Ibis 157: 722–730. doi: 10.1111/ibi.12278
- Stock, S.L., Heglund, P.J., Kaltenecker, G.S., Carlisle, J.D. & Leppert, L. 2006. Comparative ecology of the Flammulated Owl and Northern Saw-whet Owl during fall migration. J. Raptor Res. 40: 120–129. doi: 10.3356/0892-1016(2006)40[120:CEOTFO]2.0.CO;2
- Svensson, E. & Merilä, J. 1996. Molt and migratory condition in Blue Tits: a serological study. Condor 98: 825–831. doi: 10.2307/1369863
- Tellería, J.L., De La Hera, I. & Perez-Tris, J. 2013. Morphological variation as a tool for monitoring bird populations: a review. Ardeola 60: 191–224. doi: 10.13157/arla.60.2.2013.191
- Velando, A., Beamonte-Barrientos, R. & Torers, R. 2006. Pigment-based skin colour in the Blue-footed Booby: an honest signal of current condition used by females to adjust reproductive investment. Oecologia 149: 353–542. doi: 10.1007/s00442-006-0457-5
- Weidensaul, C.S., Colvin, B.A., Brinker, D.F. & Huy, J.S. 2011. Use of ultraviolet light as an aid in age classification of owls. Wilson J. Ornithol. 123: 373–377. doi: 10.1676/09-125.1
- Weidensaul, S., Stoffel, M., Monroe, M.S., Okines, D., Lane, B., Gregoire, J., Gregoire, S. & Kita, T. 2015. Plumage aberrations in Northern Saw-whet Owls (Aegolius acadicus). J. Raptor Res. 49: 84–88. doi: 10.3356/jrr-13-00073.1
- Whalen, D.M. & Watts, B.D. 2002. Annual migration density and stopover patterns of Northern Saw-whet Owls (Aegolius acadicus). Auk 119: 1154–1161. doi: 10.1642/0004-8038(2002)119[1154:AMDASP]2.0.CO;2
- Wiehn, J. 1997. Plumage characteristics as an indicator of male parental quality in the American Kestrel. J. Avian Biol. 28: 47–55. doi: 10.2307/3677093
- Young, E.I. & Proudfoot, G.A. 2014. Prevalence of haematozoa in migrating Northern Saw-whet Owls (Aegolius acadicus) of eastern North America. Wilson J. Ornithol. 126: 746–753. doi: 10.1676/13-124.1
