 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Capsule: Non-breeding Egyptian Vultures Neophron percnopterus tracked in Oman and the Horn of Africa ranged over large areas and made regular use of anthropogenic sources of food. Tracking data provide evidence that vultures monitor the availability of food within their home ranges.
Aims: To study home range and movement patterns of non-breeding Egyptian Vultures.
Methods: Five non-breeding Egyptian Vultures were tracked using Global Positioning System technology. Home range sizes were estimated using data only from vultures tracked for more than 11 months, and movements described. Analyses sought to determine whether patterns in movement were related to the location of rubbish dump sites and landfills.
Results: Home range sizes of non-breeding Egyptian Vultures (95% kernel density estimator), ranged from 4238 to 7323 km2. The average 3-hourly step length ranged from 3106.51 ± 173.16 m (mean ± se; median: 143.72 m; interquartile range, IQR: 2237.41 m) to 6519.46 ± 224.93 m (median: 2131.40 m; IQR: 7098.73 m). Egyptian Vulture activities centred on a few large rubbish dump sites that likely provided perpetually abundant food, but they frequently left those sites and visited other dumps.
Conclusions: In line with what is known about Egyptian Vulture ecology, tracked birds ranged over large areas and made use of anthropogenic sources of food. Given the perpetual abundance of food at large rubbish dumps and in the presence of other scavengers feeding at them, there seemed little motivation for Egyptian Vultures to move away from them, unless it was to monitor food resources at other places within their home ranges. This movement behaviour likely reflects the potential benefit to vultures of knowledge about food availability in the wider environment, despite food being abundant at the site at which they are currently located.
Almost all Old and New World vultures are obligate scavengers (del Hoyo et al. Citation1994, Ruxton & Houston Citation2004), and for them food sources can be naturally scarce. However, vultures are highly adaptable, and can cope with unpredictable food resources (Houston Citation1974, Mundy et al. Citation1992, Barton & Houston Citation1993, Buckley Citation1996, Ruxton & Houston Citation2004). Many aspects of vulture ecology, especially their feeding behaviour, have been affected by human activities since the dawn of recorded history (Winter Citation1985, Dixon Citation1989, Kerrigan Citation2007). As in the past, many vulture species use anthropogenic sources of food, including carcasses of wild animals killed by humans, dead livestock, human food waste (Galushin Citation1971, Al Fazari & McGrady Citation2016) and food provided for conservation purposes at ‘vulture restaurants’ (Lee Citation2013, López-López et al. Citation2014, Schabo et al. Citation2017). Because they feed on anthropogenic waste, vultures also provide important ecosystem services, which benefit human health (Markandya et al. Citation2008). In particular, smaller vultures (e.g. Egyptian Vulture Neophron percnopterus; Hooded Vulture Necrosyrtes monachus) that feed mostly on small food items can be largely mutualistic with humans (Gangoso et al. Citation2013, Henriques et al. Citation2018). In recent decades, worldwide increases in anthropogenic food waste (Oro et al. Citation2013), the establishment of vulture feeding stations (Deygout et al. Citation2009, López-López et al. Citation2014, Schabo et al. Citation2017), contaminants (Oaks et al. Citation2004, Green et al. Citation2004, Citation2006) and other factors have affected the amount, type, distribution and quality of food for vultures in the environment (Botha et al. Citation2017). Given the reliance of many vulture species on anthropogenic food sources, it is conceivable that distribution, type and size of rubbish dumps can affect vulture ecology, including their ranging behaviour.
The Egyptian Vulture is a globally endangered scavenger that has a large geographic range, extending from Spain and Portugal to central Asia in the north, to the Indian subcontinent, Arabia and sub-Saharan Africa in the south. Breeding populations in southern parts of the range are resident, whereas those that breed in Europe, central Asia, and parts of the Middle East are migratory. Pre-breeding aged birds of migratory populations dwell for some years in southern areas before entering the breeding population (del Hoyo et al. Citation1994, Oppel et al. Citation2015, BirdLife International Citation2017, E. Buechley pers. comm.). Egyptian Vultures face a wide variety of threats throughout their annual range, including disturbance at nest sites, targeted and accidental poisoning, shooting, collisions and electrocutions at power transmission infrastructure and wind turbines (Botha et al. Citation2017). Although some countries seem to have stable or growing populations (e.g. Spain, Oman, Yemen; BirdLife International Citation2017), global and many regional population declines have been steep. In India, there was a 68% decrease in the Egyptian Vulture population between 2000 and 2003 (Cuthbert et al. Citation2006); in the Balkans, the population reportedly declined by approximately 50% between 2003 and 2011 (Velevski et al. Citation2015). Available data suggest that parts of Arabia and the Horn of Africa are strongholds for Egyptian Vultures (Porter & Suleiman Citation2012, Angelov et al. Citation2013b, Buechley et al. Citation2018a, McGrady Citation2018). Yet, the ecology of Egyptian Vultures in those regions remains poorly understood.
Vultures’ ability to range over large areas, migrate, and to use the behaviour of other scavenging birds to locate scarce food resources are important behavioural adaptations to life as an obligate scavenger (Houston Citation1974, Jackson et al. Citation2008). However, as insurance against the ephemeral nature of their food, it is probably also adaptive for vultures to be informed about food resources in their surroundings, even when immediate food needs have been met. The benefit of knowledge about food availability may be greatest for individuals that are not amongst the most efficient foragers, including juveniles and non-territory-holding older birds whose movement and ranging behaviours are not constrained by the need to maintain a territory and breed.
Movement patterns can provide insight into the strategies Egyptian Vultures employ to locate and efficiently exploit scarce food resources; these strategies may be influenced by individual age and breeding status, time of year and if an individual migrates. Some aspects of spatial ecology of Egyptian Vultures have been studied using tracking data and various mapping and modelling techniques (Meyburg et al. Citation2004, García-Ripollés et al. Citation2010 , López-López et al. Citation2013, Citation2014, Buechley et al. Citation2018a, Citation2018b), but those studies have focussed mostly on European migratory populations (but see Buechley et al. Citation2018a, Citation2018b).
Little is known about the ecology and ranging behaviour of non-breeding Egyptian Vultures, due in part to lower survival rates and the fact that juvenile birds tracked from European breeding areas migrate soon after being fitted with transmitters (Meyburg et al. Citation2004, Oppel et al. Citation2015). We present data on movement patterns of non-breeding Egyptian Vultures in the Horn of Africa and the Arabian Peninsula tracked via satellite, in relation to the location of sites where food is abundant and predictable. Because the tracked birds were non-breeders and non-migratory, their ranging was not conflicted by the need to attend to a territory or the need to migrate. This information will be useful when devising waste management plans that provide continuing benefit to Egyptian Vultures and other scavenging birds of conservation concern.
Methods
Trapping and tracking
We trapped five wild Egyptian Vultures of unknown gender in Oman (4) and Djibouti (1) using nooses and leg-hold traps (Bloom et al. Citation2007) and fitted them with 33 g or 40 g solar-powered satellite transmitters as backpacks using a Teflon ribbon harness (Meyburg & Fuller Citation2007), then tracked them via satellites. The birds were weighed, measured, and aged according to methods described by Clark & Schmitt (Citation1998) and Forsman (Citation2016). gives details about the birds and the transmitters. All birds tracked in Oman were in their first plumage. The bird tracked in Djibouti-Ethiopia was in its fourth plumage (McGrady et al. Citation2014). Birds tracked in Oman could have been from migratory populations, but if so they were not yet old enough to migrate; the bird captured in the Horn of Africa remained there (M. McGrady, unpubl. data).
Table 1. Details of satellite radio tags fitted to non-breeding Egyptian Vultures in Oman and the Horn of Africa.
We used only Global Positioning System (GPS) locations for our analyses. Global Positioning System-Global System for Mobile Communications (GPS-GSM) tags were programmed to record locations every hour. GPS-Argos tags recorded data every 3 hours throughout day and night. Because all tags were solar-powered, they needed sufficient sunlight to power the acquisition of satellites, estimate locations and upload data. As a result, some data gaps occurred, most notably for the GSM tags during the night. Data gaps during daytime, when vultures would be foraging were relatively uncommon and short in duration. Data from the bird tracked in the Horn of Africa were used in the study by Buechley et al. (Citation2018a), which reports on other aspects of Egyptian Vulture movement.
Study areas
The Horn of Africa study area included mostly north-central and western Djibouti and the Djibouti-Ethiopia borderland, which is hot and mostly arid-hyper arid, with elevations ranging from −155 to approximately 2000 m above sea level. Most of the study area was part of the Ethiopian xeric grasslands and shrublands ecoregion. Djibouti and eastern Ethiopia lie at the northern end of the terrestrial part of the Rift Valley in Africa. The Bab el Mandeb Straits, which separate the Red Sea from the Gulf of Aden and Djibouti from Yemen, are an important raptor migration bottleneck in spring and autumn (Welsh & Welsh Citation1988, Citation1991, McGrady et al. Citation2014).
The region’s human population is concentrated in cities and towns; more than 75% of the approximately 1 million Djiboutians live in the capital. Nomadic pastoralism is commonly practiced. Anthropogenic waste disposal is rather unorganized across much of the area. In permanent villages and towns, waste is mostly disposed of at communal dumps, which are not far away from human habitation and are typically open and lightly regulated. The most modern waste management is practiced in the capital, which was not used by the tracked vulture.
Oman is a very arid country with ecological affinities to India (north), Arabia (middle) and Africa (south). Resident Egyptian Vultures are found mostly in the north, around the Hajar Mountains (Eriksen & Victor Citation2013), but also farther south, especially on Masirah Island (Angelov et al. Citation2013b). An unknown number of migrant Egyptian Vultures are thought to arrive in Oman in the winter, and they too settle for the non-breeding season mostly in the northern third of the country (Al Fazari & McGrady Citation2016). The Oman study area was centred on the eastern Hajar Mountains (approximately 23.2°N, 58.4°E), but extended over coastal plains and hills to the north and east mostly between Muscat and Sur, south to the Sharqiya Sands (formerly Wahiba Sands) and northern central desert, and west to the Samail Gap that separates the eastern from the western Hajars.
About 75% of Oman’s human population of over 4 million is concentrated in the capital area (Muscat), and along the northern (Batinah) coast. In more rural parts of the Oman study area, the human population is concentrated into permanent small towns and villages, and human activity occurs mostly around these and along the roads that connect them. Nomadism is non-existent or nearly so. In the past, towns and villages would each have had some sort of rubbish dump nearby. However, the number of these is declining as the Oman government centralizes waste disposal (see Discussion).
Data analysis
To enable analyses of data obtained from different tag types, we subset the data from the GSM tags to one location every 3 hours to match location data collected using the Argos tags. Location data from two of the four vultures tagged in Oman (EV4 and EV5) were insufficient (see Results) to warrant home range or movement analyses. Data from those birds were not included in formal analyses, though they provided information useful for discussion of our results.
Home ranges
Using the GPS locations from birds with at least 11 months of data, we estimated their 95%, 75%, and 50% utilization distributions using kernel density estimator (KDE; Worton Citation1989) with bivariate normal kernels. To determine appropriate bandwidth for all birds with 3-hour fixes, we calculated the average step-length (straight-line distance between successive locations) for each bird, then used the combined average as the bandwidth (4588 m). For the vulture with the Aquila GSM tag (EV3), we also estimated its home range using 1-hour fixes and used the average 1-hour step length as the bandwidth (4565 m). We also estimated the 95%, 75%, and 50% minimum convex polygon (MCP; Mohr Citation1947) for comparison with other studies. We estimated home ranges with the adehabitatHR package (Calenge Citation2006) in program R (version 3.4.4; R Core Team Citation2018).
Movement metrics
We calculated the movement descriptor of step-length as the straight-line distance between successive locations for an individual. We used locations at either 1-hour (Aquila tag) or 3-hour intervals (all birds) to calculate step-lengths; if one or more fixes were missing, we did not calculate the step-lengths for that time interval bounded by the fixes surrounding the missing data. To investigate the vultures’ extent of movement, we calculated the mean squared displacement (MSD; Kareiva & Shigesada Citation1983, Benhamou Citation2006, Codling et al. Citation2008) for each vulture, aswhere N is the number of locations for each vulture, x and y are Universal Transverse Mercator (UTM) coordinates of each respective location, and Δt represents the time interval over which the distances are being measured. MSD provides information regarding the distances that animals moved over different windows of time and about whether or not the animal exhibited movement bounded in space (Singh et al. Citation2016). Animals that move within a home range confine their movements; therefore, their MSD will reach a plateau with longer time intervals. Conversely, dispersing individuals do not confine their movements in space and their MSD will continue to increase monotonically as the time intervals increase.
Revistation analysis
Oftentimes, animals will return to certain areas multiple times throughout their lifetime. These areas may be critical foraging sites and knowledge of such sites can provide important information for management (Barraquand & Benhamou Citation2008, Benhamou & Riotte-Lambert Citation2012, Van Moorter et al. Citation2016, Bracis et al. Citation2018). Therefore, we calculated the number of revisits vultures made within a 4 km radius of each of their locations, the time they spent within these areas (residence time), and time since their last visit to the area (Bracis et al. Citation2018). We also calculated revisits made to dump sites in Oman, again using a 4 km radius around each dump site, and calculated average residence time for each visit to the five most visited sites for each Oman vulture as well as the time since the last visit. Dump site locations were obtained from be’ah, the government authority for waste management. For the revisitation analysis, we were primarily interested in foraging sites so it was essential to use a large enough radius to encompass potential foraging activities. We calculated the number of revisits to dump sites for each vulture at different radii (100–10,000 m in increments of 100 m). We then averaged the number of revisits for all locations at each respective radius and plotted the results. The curves generally reached an approximate asymptote around 4 km, indicating that the number of revisits at that radius was not sensitive to further changes (online Figure S1).
We calculated step-lengths using the adehabitatLT (Calenge Citation2006) package in program R (version 3.4.4; R Core Team Citation2018). All revisitation analyses were performed using the R package recurse (Bracis et al. Citation2018).
Results
Home ranges and movements
Vulture EV1 was tracked as it ranged in Djibouti and southeastern Ethiopia for 388 days (2492 total fixes; , ). This vulture’s KDE was larger than one of the Oman vultures, but smaller than the other (). We calculated 2354 3-hour step-lengths; the mean (±se) 3-hour step-length was 3106.51 ± 173.16 m (median: 143.72 m; interquartile range, IQR: 2237.41 m). The vulture moved the longest distances during midday (). The MSD for this vulture indicated that it exhibited bounded movements, and it covered its home range in approximately 21 days ().
Figure 1. Locations (3-hourly), and 95%, 75%, and 50% KDE home ranges for (A) Horn of Africa vulture EV1; (B) Oman vulture EV2; and (C) Oman vulture EV3. See for detailed information on the individual vultures.
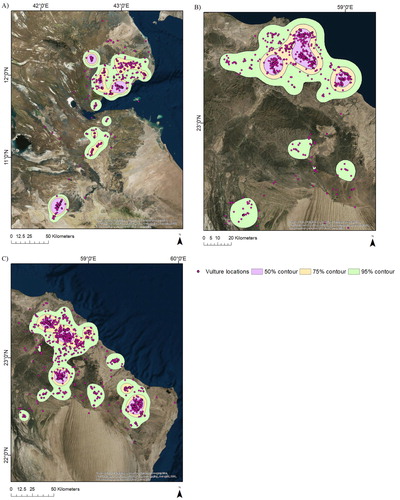
Figure 2. Average 3-hour step-lengths throughout the diel period for the Egyptian Vultures tracked during this study: (A) Horn of Africa vulture EV1, (B) Oman vulture EV2, and (C) Oman vulture EV3. See for details regarding the individual vulture.
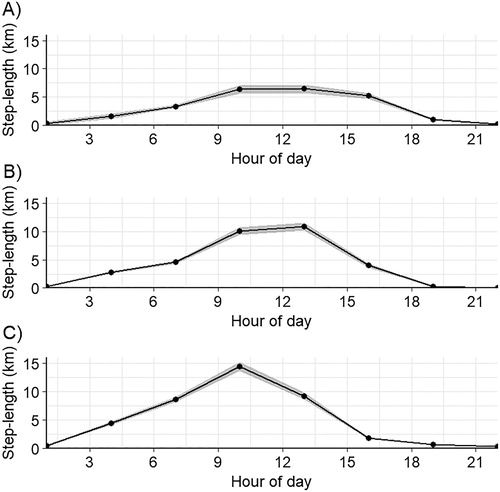
Figure 3. Mean squared displacement (MSD) for Egyptian Vultures tracked during this study: (A) Horn of Africa vulture EV1, (B) Oman vulture EV2, and (C) Oman vulture EV3. See for details regarding the individual vulture.
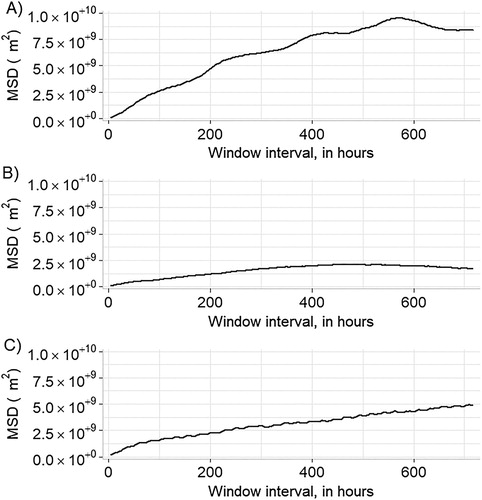
Table 2. Home range sizes in km2 for non-breeding Egyptian Vultures captured in Djibouti (N = 1) and Oman (N = 2) at different contours, based on the Kernel Density Estimator (KDE) and on Minimum Convex Polygon (MCP).
Vulture EV2 was tracked in Oman over 338 days (2423 total fixes; , ). This vulture’s 95% KDE was the largest of the three vultures at the 3-hour interval (). We calculated 1977 3-hour step-lengths; the mean (±se) 3-hour step-length was 4137.48 ± 162.72 m (median: 1076.95 m; IQR: 4811.52 m). This vulture moved the longest distances during midday (). The MSD for this vulture indicated that it exhibited bounded movements and covered its home range in approximately 17 days ().
Vulture EV3 was tracked in Oman for 622 days (, online Figure S2; 5318 fixes at 1-hour intervals). This vulture’s 95% KDE was the smallest of the three vultures when estimated using only one location every 3 hours (, ); its 95% KDE estimated using the hourly locations was larger (). We calculated 3390 hourly step-lengths, and 2282 3-hour step-lengths. The mean (±se) hourly step-length was 4771.89 ± 126.15 m (median: 1497.04 m; IQR: 5641.75 m) and the mean 3-hour step-length was 6519.46 ± 224.93 m (median: 2131.40 m; IQR: 7098.73 m). This vulture also moved the longest distances during midday (), exhibited bounded movements and covered its home range in approximately 25 days ().
Vulture EV4 transmitted only from 10 January to 4 February 2015 (25 days), during which 170 locations were received. The reason for the abrupt cessation of transmission is not known, nor is the fate of the bird. Prior to cessation of transmissions, the bird appeared to be behaving normally, and had travelled (approximately 60 km) between active rubbish dumps (Al Mataqa and Al Hamar). The plot of MSD suggested that the tracking period was too short to describe the bounds of ranging.
Vulture EV5 transmitted 174 locations only from 19 January to 25 February 2016 (36 days), during which it appeared to be behaving normally. EV5 was found dead two days after its last transmission near human habitation (after roosting for the night on a high voltage electricity pylon). The cause of death could not be determined because the corpse had decayed. The plot of MSD suggested that the tracking period was too short to describe the bounds of ranging.
Revisitation analysis
The number of revisits to GPS locations ranged from 1 to 114 for vulture EV1 in Djibouti, from 1 to 94 for Oman vulture EV2, and from 1 to 102 for Oman vulture EV3 (). The number of revisits to dump sites ranged from 0 to 79 for Oman vulture EV2 and from 0 to 86 for Oman vulture EV3 (). Considering only the 5 most visited sites, vulture EV2 had a mean (±se) residence time of 9.29 ± 3.3 hours (median: 5.33 hours; IQR: 13.29 hours) within 4 km of the 5 dump sites it visited most, and an mean (±se) time between visits of 349.14 ± 220.80 hours (median: 129.37 hours; IQR: 156.79 hours), that is 14.55 ± 9.20 days. Oman vulture EV3 had an average residence time of 14.50 ± 2.92 hours (median: 14.10 hours; IQR: 7.90 hours) within 4 km of the 5 dump sites it visited most, and a mean (±se) time between visits of 368.20 ± 128.98 hours (median: 202.79 hours; IQR: 369.44 hours), i.e. 15.34 ± 5.37 days. The average residence time and the average time spent between visits to the top 5 dump sites are presented in .
Figure 4. Locations (3-hourly), and the number of revisits to the most frequently visited locations (circles) and dump sites (squares) by (A) the Horn of Africa vulture EV1; (B) Oman vulture EV2; and (C) Oman vulture EV3. See for detailed information on the individual vultures and for detailed information on residence times to the most visited dump sites.
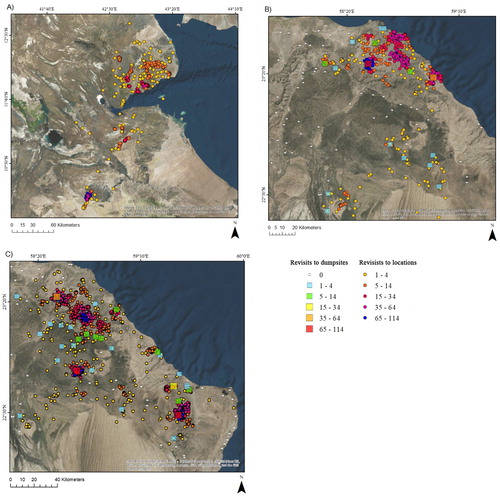
Table 3. Average residence times and average times between visits to dump sites in Oman by non-breeding Egyptian Vultures (A) EV2 and (B) EV3 at their respective 5 most-visited dump sites.
Close examination of satellite imagery showed that in both Oman and the Horn of Africa most vulture locations were near human activity centres, often along roads and near permanent and temporary settlements. They seemed also to frequent areas that could have been moister than the general landscape, in wadis and near recharge dams.
Discussion
In line with what is already known about the species (Cramp & Simmons Citation1980, Mundy et al. Citation1992, Eriksen & Victor Citation2013, Al Fazari & McGrady Citation2016), non-breeding Egyptian Vultures in our study ranged over large areas and made regular use of anthropogenic food sources, the most obvious being landfills, rubbish dumps of various sizes and abattoirs. Furthermore, tracked birds made almost daily use (with many other avian scavengers) of large dump sites with perpetually available, super-abundant food; they would leave these sites from time to time and move to distant (60–200 km) sources of food, which were sometimes also large dump sites being used by many other vultures. At these new food sources, vultures would dwell for variable amounts of time before making another move, either to a new, similar site or back to the original site. Tracking data suggested that in making the moves from one site of predictable, abundant food to the next, vultures sometimes visited smaller food sources (e.g. village dumps or road-killed animals) of which we had no direct knowledge. Visits to smaller sources of food were more pronounced in the northern Djibouti part of their range in the Horn of Africa and probably resulted from the human population in this area being highly dispersed at low density and being more nomadic. Movements by the vultures in this study found some concordance with the searching behaviour of Egyptian Vultures during the non-breeding season in West Africa (López-López et al. Citation2013). However, differences in the age and migratory status of the vultures, and most likely the abundance and distribution of food in the two studies, mean that more work is needed before conclusions about Egyptian Vulture search strategies can be drawn.
Information about food is important to vultures (Buckley Citation1996, Citation1997), enabling them to forage more efficiently (Houston Citation1974, Jackson et al. Citation2008), and can be either personal information gained from direct experience or social information gained from observing the activity of other scavenging birds (Schmidt et al. Citation2010, Cortés-Avizanda et al. Citation2014). Personal information can be transferred to others, and so is the basis of socially acquired information (Danchin et al. Citation2004). In the apparent absence of depleting food at large food sources (e.g. landfills and rubbish dumps) as motivation to move and the presence of other scavengers to encourage them to stay (via social facilitation), we hypothesize that the vultures we tracked were monitoring their extensive ranges for food, and gathering both personal and social information. Monitoring was characterized by visiting sites known to them (or probably so) to have had food in the past, and while moving between those sites the vultures gathered information on the location of other sources of food, and sometimes used them.
Although data from two other Egyptian Vultures radio-tagged in Oman were insufficient for quantitative analyses, their behaviour appeared similar to those tracked over longer periods – they flew between active dump sites and roosted on power infrastructure. Their home ranges were large and growing when transmissions ceased.
On average, the vultures we tracked had a home range (95% KDE) of 5670 km2 ± 1079. Home range sizes were similar to those reported by Buechley et al. (Citation2018a) for vultures wintering in the Horn of Africa, but home ranges of Egyptian Vultures reported from western Sahelian areas (Meyburg et al. Citation2004, García-Ripollés et al. Citation2010) were consistently larger than those found in this study (). The home ranges in our study areas were more similar in size to one another than either of them were to any of the other studies. Caution should be used in drawing conclusions from such comparisons because the studies differed as to the age of the tracked birds and whether they were migratory or not. Also, the likely variation in food availability between areas and years, driven by factors such as precipitation (Buontempo et al. Citation2010), would affect both the movement and space use patterns of Egyptian Vultures (Newton Citation1979).
Table 4. Mean 11-month home range sizes (km2) for Egyptian Vultures tracked by us compared to published mean winter home range sizes of Egyptian Vultures from migratory Eurasian populations.
In the Djibouti portion of the Horn of Africa study area, where human nomadism is common, satellite imagery showed that vulture locations were often near remains of small, nomadic settlements (i.e. tent rings). We do not know if the settlements were inhabited when visited by the vulture, but it reinforces the link between vulture ranging behaviour and human activity/human waste dump sites. Associations between bird locations and signs of such nomadic settlements were not seen in Ethiopia and Oman, largely because the human populations in those places are not nomadic.
In recent years and during the study period, the number of small rubbish dumps in Oman declined as part of a country-wide upgrade of waste management that will eventually see the closing of over 300 small dumps, and a consolidation of dumping at 12 modern engineered landfills (S. Al Touqi pers. comm.). Currently, those modern landfills are either operating or soon will be, and they feature increased segregation of waste types, and compacting and covering of waste with earth on at least a daily basis. Katzenberger et al. (Citation2017) found no short-term effects of closing rubbish dumps on Egyptian Vulture reproductive parameters in Turkey, but no such reproductive information is available for vultures in Oman. The study by Katzenberger et al. (Citation2017) and the large numbers of Egyptian Vultures seen at landfills and rubbish dumps in Oman (Eriksen & Victor Citation2013, Al Fazari & McGrady Citation2016) during a time when the number of dump sites is decreasing suggests that any negative effects of dump site closures may be buffered by the ability of vultures to take advantage of small, ephemeral food items (e.g. road killed animal or roadside biological litter). However, closing small dumps and concentrating of dumping at a few landfill sites will affect the distribution of vulture food, and this could potentially influence movement and ranging patterns. Special handling of toxic waste could improve the quality of food available to vultures and also reduce the potential of accidental poisoning. Covering waste with earth will likely reduce the overall amount of available food, although this might have little effect on vultures because there is such a huge surplus. Food will likely still be available for vultures during most daylight hours because covering rubbish typically occurs near the end of the day and new rubbish starts arriving at daybreak, and also because covering is rarely complete.
Our knowledge of predictable food sources for the Horn of Africa bird is very limited. However, it is highly likely that only the most temporary of settlements will not have a nearby rubbish dump; semi-permanent and permanent settlements of any size will have rubbish dumps and larger villages and towns will also have a regularly used abattoir. The lack of organized waste management in the Horn of Africa study area means that small amounts of food for Egyptian Vultures will also be available around permanent and non-permanent settlements, not within any organized dumping site. Although the vulture tracked in Djibouti-Ethiopia was never again located at the abattoir where it was trapped and where vultures congregate when animals are being killed, on eight occasions it was located at the large open rubbish dump outside the town, dwelling there up to three days at a time. Because of the lack of specific information on the location of dump sites in the African study area, we do not know if it visited rubbish dumps or abattoirs elsewhere, but clustering of transmitted locations around towns and villages suggest as much. Small clusters of vulture locations near stone circles indicate that nomad camps were also attractive to vultures.
It is common for Egyptian Vultures to occur singly or in small groups (Cramp & Simmons Citation1980, Mundy et al. Citation1992, del Hoyo et al. Citation1994). In both study areas, small numbers (less than 5) of Egyptian Vultures were frequently seen feeding at small settlements, places where livestock gathered, rubbish dumps serving small villages, and along the shoreline (M. McGrady unpublished data). However, the large number of Egyptian Vultures at Omani rubbish dumps (260 Mundy et al. Citation1992, 400+ Al Fazari & McGrady Citation2016, 640 J. Eriksen pers. comm.) are amongst the largest reported globally (Arkumarev et al. Citation2014, Katzenberger et al. Citation2017), though roosts of nearly 1000 birds have been observed at a communal roost on Socotra (JM Thiollay pers. comm.).
Egyptian Vultures are globally endangered and face a variety of threats across their range (Botha et al. Citation2017). At least two of the four tracked in Oman died within a short period (about one month). This is a worrying mortality even when one considers our small sample size, and emphasizes the need for more work to understand the risks to vultures in these stronghold areas.
Conservation activities for Egyptian Vultures in our study areas (apparent strongholds) can affect resident and migrant populations. Food availability is an important factor in determining the distribution and density of species, including vultures (Newton Citation1979); less food can have a negative impact on vulture numbers and range extent (Botha et al. Citation2017). Throughout much of the Egyptian Vulture’s European distribution, the number of wild mammals is declining, husbandry of domestic animals has improved and anthropogenic waste is carefully managed to promote human health. This has resulted in less food being available for scavenging birds (Margalida et al. Citation2010, Donázar et al. Citation2009, Citation2010), negative effects on populations (Velevski et al. Citation2015), and a diminution of the value of ecosystem services provided by vultures (Deygout et al. Citation2009). Waste management in Djibouti and Ethiopia, on the other hand, is not very developed, and in Oman it has developed rapidly only in the past two decades. The relatively low level of human-related development in our study areas presents opportunities for an integrated approach to waste management and vulture conservation. Certainly, the large number of vultures using the most modern of landfills in Oman suggests that changes in waste management to benefit human health might not be detrimental to Egyptian Vultures in that region.
Apart from changes in food availability, electrocution represents a major threat to Egyptian Vultures, with incidents being documented both in Oman (EV2 in this study) and the Horn of Africa (Angelov et al. Citation2013b), and at least one of the vultures we tracked was electrocuted. Examination of satellite imagery showed that tagged vultures in both countries made regular use of high voltage electricity pylons for roosting, particularly along roads and near rubbish dumps during day light hours, mirroring the findings of Arkumarev et al. (Citation2014) and Buechley et al. (Citation2018a) in Ethiopia. Vultures also perch along medium voltage power distribution lines, which are typically the most dangerous (Lehman et al. Citation2007, Angelov et al. Citation2013a). However, no detailed analysis could be undertaken because digital information on power distribution and transportation infrastructure (e.g. roads, powerlines) is limited in both countries. Also, potentially important human-related features (e.g. some settlements) and environmental conditions (e.g. rain events) are ephemeral and not reliably represented on available satellite imagery.
Electricity networks are expanding in both study areas (although Oman has a more extensive and more rapidly developing network), and we know of no consideration being given to the possibility of bird electrocution. Because distribution networks in both countries are currently not very extensive, installing vulture-safe infrastructure can be part of new network development, and making existing power infrastructure safer is more easily achieved. Because vultures and other large scavenging birds aggregate near dumps and landfills, prioritizing improvements to electricity infrastructure in the vicinity of dump sites and landfills would likely to be most effective in terms of conservation and cost.
The results presented in this paper are from a few non-territorial birds and should be interpreted with caution. Indeed, older birds (some of which appear to be breeders) that we are currently tracking in Oman appear to range differently (M. McGrady and B. Meyburg unpublished). Data from more non-territorial and territorial birds are needed to better understand patterns of Egyptian Vulture ranging behaviour.
Supplemental Material
Download MS Word (633.1 KB)Acknowledgements
Aquila Telemetry and Geotrak kindly provided some transmitters for the study, and Hawk Mountain Sanctuary provided funding for some satellite time and data handling services. In Oman, the Environment Society of Oman, Arid Lands and Sita-Suez provided in-kind and logistical support, the Ministry of Environment and Climate Affairs provided permission to capture birds, and be’ah provided access to landfills. The Ministry of Housing, Urban Affairs and Environment granted permission to capture the bird in Djibouti. The authors would like to personally thank E. Abdillahi, A. Al Balushi, W.A. Al Fazari, F. Al Lamki, D. Barber, A.M. Darar, M. Sarrouf-Willson, and B. Zaitoon for their help. We are grateful to I. Hartley, and an anonymous associate editor, P. López-López and S. Oppel for many thoughtful comments and corrections that improved the manuscript.
Additional information
Funding
References
- Al Fazari, W.A. & McGrady, M.J. 2016. Counts of Egyptian Vultures Neophron percnopterus and other avian scavengers at Muscat’s municipal landfill, Oman, November 2013 – March 2015. Sandgrouse 38: 99–105.
- Angelov, I., Hashim, I. & Oppel, S. 2013a. Persistent electrocution mortality of Egyptian Vultures Neophron percnopterus over 28 years in East Africa. Bird Conserv. Int. 23: 1–6. doi: 10.1017/S0959270912000123
- Angelov, I., Yotsova, T., Sarrouf, M. & McGrady, J.M. 2013b. Large increase of the Egyptian Vulture Neophron percnopterus population on Masirah Island, Oman. Sandgrouse 34: 140–152.
- Arkumarev, V., Dobrev, V., Abebe, Y.D., Popgeorgiev, G. & Nikolov, S.C. 2014. Congregations of wintering Egyptian Vultures Neophron percnopterus in Afar, Ethiopia: present status and implications for conservation. Ostrich 85: 139–145. doi: 10.2989/00306525.2014.971450
- Barraquand, F. & Benhamou, S. 2008. Animal movements in heterogeneous landscapes: identifying profitable places and homogeneous movement bouts. Ecology 89: 3336–3348. doi: 10.1890/08-0162.1
- Barton, N.W.A. & Houston, D.C. 1993. A comparison of digestive efficiency in birds of prey. Ibis 135: 363–371. doi: 10.1111/j.1474-919X.1993.tb02107.x
- Benhamou, S. 2006. Detecting an orientation component in animal paths when the preferred direction is individual-dependent. Ecology 87: 518–528. doi: 10.1890/05-0495
- Benhamou, S. & Riotte-Lambert, L. 2012. Beyond the utilization distribution: identifying home range areas that are intensively exploited or repeatedly visited. Ecol. Model. 227: 112–116. doi: 10.1016/j.ecolmodel.2011.12.015
- BirdLife International. 2017. Species factsheet: Neophron percnopterus. http://www.birdlife.org on 04/12/2017.
- Bloom, P.H., Clark, W.S. & Kidd, J.W. 2007. Capture techniques. In Bird, D.M. & Bildstein, K.L. (eds) Raptor Research and Management Techniques Manual, 193–220. Hancock House, Surrey, CA.
- Botha, A. J., Andevski, J., Bowden, C.G.R., Gudka, M., Safford, R.J., Tavares, J. & Williams, N.P. 2017. Multi-species Action Plan to Conserve African-Eurasian Vultures. CMS Raptors MOU Technical Publication No. 5. CMS Technical Series No. 35. Coordinating Unit of the CMS Raptors MOU, Abu Dhabi, United Arab Emirates.
- Bracis, C., Bildstein, K.L. & Mueller, T. 2018. Revisitation analysis uncovers spatio-temporal patterns in animal movement data. Ecography 41: 1801–1811. doi: 10.1111/ecog.03618
- Buckley, N.J. 1996. Food finding and the influence of information, local enhancement, and communal roosting on foraging success of North American vultures. Auk 113: 473–488. doi: 10.2307/4088913
- Buckley N.J. 1997. Experimental tests of the information-center hypothesis with Black Vultures (Coragyps atratus) and Turkey Vultures (Cathartes aura). Behav. Ecol. Sociobiol. 41: 267–279. doi: 10.1007/s002650050388
- Buechley, E.R., McGrady, M.J., Çoban, E. & Şekercioğlu, Ç.H. 2018a. Satellite tracking a wide-ranging endangered vulture species to target conservation actions in the Middle East and East Africa. Biodivers. Conserv. 27: 2293–2310. doi: 10.1007/s10531-018-1538-6
- Buechley, E.R., Oppel, S., Beatty, W.S., Nikolov, S.C., Dobrev, V., Arkumarev, V., Saravia, V., Bougain, C., Bounas, A., Kret, E. & Skartsi, T. 2018b. Identifying critical migratory bottlenecks and high-use areas for an endangered migratory soaring bird across three continents. J. Avian Biol. doi:10.1111/jav.01629.
- Buontempo, C., Booth, B. & Moufouma-Okia, W. 2010. Sahelian Climate: Past, Current, Projections. Met Office Hadley Centre, Devon, UK.
- Calenge, C. 2006. The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Model. 197: 516–519. doi: 10.1016/j.ecolmodel.2006.03.017
- Clark, W.S. & Schmitt, N.J. 1998. Aging Egyptian Vultures. Alula 4: 122–127.
- Codling, E.A., Plank, M.J. & Benhamou, S. 2008. Random walk models in biology. J. Roy. Soc. Interface 5: 813–834. doi: 10.1098/rsif.2008.0014
- Cortés-Avizanda, A., Jovani, R., Donázar, J.A. and Grimm, V. 2014. Bird sky networks: how do avian scavengers use social information to find carrion? Ecology 95: 1799–1808. doi: 10.1890/13-0574.1
- Cramp, S. & Simmons, K.E.L. (eds) 1980. Handbook of the Birds of Europe, the Middle East and North Africa – the Birds of the Western Palearctic. Oxford University Press, Oxford.
- Cuthbert, R., Green, R.E., Ranade, S., Saravanan, S., Pain, D.J., Prakash, V. & Cunningham, A.A. 2006. Rapid population declines of Egyptian vulture (Neophron percnopterus) and red-headed vulture (Sarcogyps calvus) in India. Anim. Conserv. 9: 349–354.
- Danchin, E., Giraldeau, L.-A., Valone, T.J. & Wagner, R.H. 2004. Public information: from nosy neighbors to cultural evolution. Science 305: 487–491. doi: 10.1126/science.1098254
- del Hoyo, J., Elliot, A. & Sargatal, J. (eds.) 1994. Handbook of Birds of the World. Vol. 2. New World Vultures to Guineafowl. Lynx Edicions, Barcelona.
- Deygout, C., Gault, A., Sarrazin, F. & Bessa-Gomes, C. 2009. Modeling the impact of feeding stations on vulture scavenging service efficiency. Ecol. Modell. 220: 1826–1835. doi: 10.1016/j.ecolmodel.2009.04.030
- Dixon, D.M. 1989. A note on some scavengers of ancient Egypt. World. Archaeol. 21: 193–197. doi: 10.1080/00438243.1989.9980101
- Donázar, J., Margalida, A., Carrete, M. & Sánchez-Zapata, J.A. 2009. Too sanitary for vultures. Science 326: 664. doi: 10.1126/science.326_664a
- Donázar, J.A., Cortés-Avizanda, A. and Carrete, M. 2010. Dietary shifts in two vultures after the demise of supplementary feeding stations: consequences of the EU sanitary legislation. Eur. J. Wildlife Res. 56: 613–621. doi: 10.1007/s10344-009-0358-0
- Eriksen, J. & Victor, R. 2013. Oman Bird List. Edn 7. Center for Environmental Studies and Research, Muscat.
- Forsman, D. 2016. Flight Identification of Raptors of Europe, North Africa and the Middle East. Christopher Helm, London.
- Galushin, V.M. 1971. A huge urban population of birds of prey in Delhi, India. Ibis 113: 522.
- Gangoso, L., Agudo, R., Anadón, J.D., de la Riva, M., Suleyman, A.S., Porter, R. & Donázar, J.A. 2013. Reinventing mutualism between humans and wild fauna: insights from vultures as ecosystem services providers. Conserv. Lett. 6: 172–179. doi: 10.1111/j.1755-263X.2012.00289.x
- García-Ripollés, C., López-López, P. & Urios, V. 2010. First description of migration and wintering of adult Egyptian Vultures Neophron percnopterus tracked by GPS satellite telemetry. Bird Study 57: 261–265. doi: 10.1080/00063650903505762
- Green, R. E., Newton, I., Shultz, S., Cunningham, A. A., Gilbert, M., Pain, D. J. & Prakash, V. 2004. Diclofenac poisoning as a cause of vulture population declines across the Indian subcontinent. J. Appl. Ecol. 41: 793–800. doi: 10.1111/j.0021-8901.2004.00954.x
- Green, R. E., Taggart, M. A., Das, D., Pain, D. J., Sashi Kumar, C., Cunningham, A. A. & Cuthbert, R. 2006. Collapse of Asian vulture populations: risk of mortality from residues of the veterinary drug diclofenac in carcasses of treated cattle. J. Appl. Ecol. 43: 949–956. doi: 10.1111/j.1365-2664.2006.01225.x
- Henriques, M., Granadeiro, J.P., Monteiro, H., Nuno, A., Lecoq, M., Cardoso, P., Regalla, A. & Catry, P. 2018. Not in wilderness: African vulture strongholds remain in areas with high human density. PloS One 13: e0190594. doi: 10.1371/journal.pone.0190594
- Houston, D.C. 1974. Food searching in Griffon Vultures. E. Afr. Wildl. J. 12: 63–77. doi: 10.1111/j.1365-2028.1974.tb00107.x
- Jackson, A.L., Ruxton, G.D. & Houston, D.C. 2008. The effect of social facilitation on foraging success in vultures: a modelling study. Biol. Lett. 4: 311–313. doi: 10.1098/rsbl.2008.0038
- Kareiva, P.M. & Shigesada, N. 1983. Analyzing insect movement as a correlated random walk. Oecologia 56: 234–238. doi: 10.1007/BF00379695
- Katzenberger, J., Tabur, E., Şen, B., İiyaroğlu, S., Erkol, I.L. & Oppel, S. 2017. No short-term effect of closing a rubbish dump on reproductive parameters of an Egyptian Vulture population in Turkey. Bird Conserv. Int. 27: 1–12. doi: 10.1017/S0959270917000326
- Kerrigan, M. 2007. The History of Death: Burial Customs and Funeral Rites, From the Ancient World to Modern Times. Amber Books, London.
- Lehman, R.N., Kennedy, P.L. & Savidge, J.A. 2007. The state of the art in raptor electrocution research: a global review. Biol. Conserv. 136: 159–174. doi: 10.1016/j.biocon.2006.09.015
- Lee, S. 2013. Distribution and abundance of wintering raptors in the Korean peninsula. Journal of Ecology and Environment 36: 211–216. doi: 10.5141/ecoenv.2013.211
- López-López, P., Benavent-Corai, J., García-Ripollés, C. & Urios, V. 2013. Scavengers on the move: behavioural changes in foraging search patterns during the annual cycle. PLoS One 8: e54352. doi:10.1371/journal.pone.0054352.
- López-López, P., García-Ripollés, C. & Urios, V. 2014. Food predictability determines space use of endangered vultures: implications for management of supplementary feeding. Ecol. Appl. 24: 938–949. doi: 10.1890/13-2000.1
- Margalida, A., Donázar, J.A., Carrete, M. & Sánchez-Zapata, J.A. 2010. Sanitary versus environmental policies: fitting together two pieces of the puzzle of European vulture conservation. J. Appl. Ecol. 47: 931–935. doi: 10.1111/j.1365-2664.2010.01835.x
- Markandya, A., Taylor, T., Longo, A., Murty, M.N., Murty, S. & Dhavala, K. 2008. Counting the cost of vulture decline—an appraisal of the human health and other benefits of vultures in India. Ecol. Econ. 67: 194–204. doi: 10.1016/j.ecolecon.2008.04.020
- McGrady, M.J. 2018. Review and current status of diurnal raptor migration and wintering on the Arabian Peninsula. Sandgrouse Suppl. 4: 85–104.
- McGrady, M.J., Rayaleh, H. A., Dara, A. M. & Abdillahi, E. 2014. Migration of raptors across the Bab el Mandeb Straits during 2–10 March 2013. Bulletin of the African Bird Club 21: 65–72.
- Meyburg, B.-U. & Fuller, M.R. 2007. Satellite tracking. In Bird D.M. & Bildstein, K.L. (eds) Raptor Research and Management Techniques Manual, 242–248. Hancock House, Surrey, CA.
- Meyburg, B.-U., Gallardo, M., Meyburg, C. & Dimitrova, E. 2004. Migrations and sojourn in Africa of Egyptian Vultures (Neophron percnopterus) tracked by satellite. J. Ornithol. 145: 273–280. doi: 10.1007/s10336-004-0037-6
- Mohr, C. 1947. Table of equivalent populations of North American small mammals. Am. Midl. Nat. 37: 223–249. doi: 10.2307/2421652
- Mundy, P., Butchart, D., Ledger, J. & Piper, S. 1992. The Vultures of Africa. Academic Press, London.
- Newton, I. 1979. Population Ecology of Raptors. T. & A.D. Poyser, London.
- Oaks, J.L., Gilbert, M., Virani, M.Z., Watson, R.T., Meteyer, C.U., Rideout, B.A., Shivaprasad, H.L., Ahmed, S., Chaudhry, M.J.I., Arshad, M. & Mahmood, S. 2004. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427: 630–633. doi: 10.1038/nature02317
- Oppel, S., Dobrev, V., Arkumarev, V., Saravia, V., Bounas, A., Kret, E., Velevski, M., Stoychev, S. & Nikolov, S.C. 2015. High juvenile mortality during migration in a declining population of a long-distance migratory raptor. Ibis 157: 545–557. doi: 10.1111/ibi.12258
- Oro, D., Genovart, M., Tavecchia, G., Fowler, M.S. & Martínez-Abraín, A. 2013. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 16: 1501–1514. doi: 10.1111/ele.12187
- Porter, R. & Suleiman, A.S. 2012. The Egyptian Vulture Neophron percnopterus on Socotra, Yemen: population, ecology, conservation and ethno-ornithology. Sandgrouse 34: 44–62.
- R Core Team. 2018. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/.
- Ruxton, G.D. & Houston, D.C. 2004. Obligate vertebrate scavengers must be large soaring fliers. J. Theor. Biol. 228: 431–436. doi: 10.1016/j.jtbi.2004.02.005
- Schabo, D.G., Heuner, S., Neethling, M.V., Rösner, S., Uys, R. & Farwig, N. 2017. Long-term data indicates that supplementary food enhances the number of breeding pairs in a Cape Vulture Gyps coprotheres colony. Bird Conserv. Int. 27: 140–152. doi: 10.1017/S0959270915000350
- Schmidt, K.A., Dall, S.R.X. & van Gils, J.A. 2010. The ecology of information: an overview on the ecological significance of making informed decisions. Oikos 119: 304–316. doi: 10.1111/j.1600-0706.2009.17573.x
- Singh, N.J., Allen, A.M. & Ericsson, G. 2016. Quantifying migration behaviour using net squared displacement approach: clarifications and caveats. PloS One 11: e0149594. doi: 10.1371/journal.pone.0149594
- Van Moorter, B., Rolandsen, C.M. & Bastille, M. 2016. Movement is the glue connecting home ranges and habitat selection. J. Anim. Ecol. 85: 21–31. doi: 10.1111/1365-2656.12394
- Velevski, M., Nikolov, S.C., Hallmann, B., Dobrev, V., Sidiropoulos, L., Saravia, V., Tsiakiris, R., Arkumarev, V., Galanaki, A., Kominos, T. & Stara, K. 2015. Population decline and range contraction of the Egyptian Vulture Neophron percnopterus in the Balkan Peninsula. Bird Conserv. Int. 25: 440–450. doi: 10.1017/S0959270914000343
- Welsh, G. & Welsh, H. 1988. The autumn migration of raptors and other soaring birds across the Bab el Mandeb Straits. Sandgrouse 10: 26–50.
- Welsh, G. & Welsh, H. 1991. Spring raptor observations from Djibouti. Ornithological Society of the Middle East Bulletin 26: 25–27.
- Winter, I.J. 1985. After the battle is over: the ‘Stele of the Vultures’ and the beginning of historical narrative in the art of the ancient Near East. In Kessler, H.L. & Simpson, M.S. (eds) Pictorial Narrative in Antiquity and the Middle Ages. Center for Advanced Study in the Visual Arts, Symposium Series IV, 11–32. National Gallery of Art, Washington, DC.
- Worton, B. 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70: 164–168. doi: 10.2307/1938423
