ABSTRACT
Capsule: Population growth rates of one-third of 68 breeding bird species in England were significantly affected by climatic variables, leading to notable (>10%) population increases in 13 species, and declines in three.
Aims: To model the impact of climate change on the abundance of breeding bird species in England.
Methods: Annual variation in population growth rates from 1966 to 2015 for English birds were modelled using a combination of climatic variables from England and along their migration routes, year and count in the previous year, and used to predict long-term trends over that time period.
Results: Population growth rates of 24/68 species (35%) were significantly correlated with climatic variables, although one relationship was driven by a single outlier and probably not robust. The majority of the effects were related to English conditions, particularly for resident species during summer and winter. Based on trends in climatic variables, the estimated magnitude of the effect of climate change upon the long-term bird population trends was sufficient to have resulted in notable (>10%) positive impacts on the abundance of 13 of the species modelled, and negative impacts on three.
Conclusion: Climate change has had a detectable impact on a sizeable proportion of the English avifauna over the last 50 years. In particular, populations of a range of resident bird species are probably significantly greater than they would otherwise have been in the absence of climate change, although some species may be less common. However, robustly attributing these trends to climate change, using a correlative approach such as this, is challenging, particularly for migrant species.
Climate change is widely regarded as a major threat to the functioning of natural systems and to the maintenance of species’ populations (Bellard et al. Citation2012). Birds are one of the taxa where climate change impacts have been most studied, and exhibit a range of documented responses (Crick Citation2004). In medium and high latitudes of North America and Europe, warmer temperatures have caused an advance in the timing of breeding and migration (Thackeray et al. Citation2010, Citation2016, Pearce-Higgins & Green Citation2014), poleward shifts in distribution (Pearce-Higgins & Green Citation2014, Gillings et al. Citation2015), and significant changes in populations, and the composition of assemblages or communities of birds (Davey et al. Citation2012, Devictor et al. Citation2012, Pearce-Higgins, Ockendon et al. Citation2015, Stephens et al. Citation2016). Whilst there is a clear fingerprint of climate change on bird distributions, assemblages and populations over recent decades, particularly from North America and Europe where they are best studied, the attribution of specific population changes to climate change remains challenging. Documenting which changes may or may not have been caused by climate change, relative to other factors which are also changing, and that may produce impacts which are difficult to distinguish from climate change, remains largely lacking (but see Jørgensen et al. Citation2016). Studies with evidence of a clear mechanism linking climate change to species’ population trends provide the strongest evidence (Frederiksen et al. Citation2004, Ludwig et al. Citation2006, Pearce-Higgins et al. Citation2010), but have generally only been undertaken for a limited number of species or populations.
Here, we use a correlative approach that can be widely applied to population time-series to synthesize the potential impacts of climate change on individual species for as wide a component of a country avifauna as possible. This enables the identification of species which appear to have been most affected by climate change so far, providing evidence to inform future conservation assessment (Pearce-Higgins et al. Citation2017, Wheatley et al. Citation2017). We also use these models to test the extent to which the modelled impact of climate change on long-term population trends is predictable by a range of ecological traits, such as habitat specialization (Sullivan et al. Citation2016), migratory distance (Pearce-Higgins, Eglington et al. Citation2015) or association with temperature (Devictor et al. Citation2012), with the expectation that habitat generalists, resident species, and warm-associated species are most likely to have increased in abundance in response to climate change.
We do this using 50 years of citizen science monitoring of bird population trends in England that provide national-level assessments of annual abundance. These data have already been used to identify the importance of particular seasons in driving trends in these species overall, and the potential for changes in climatic variables to operate over multiple years (Pearce-Higgins, Eglington et al. Citation2015), but have not yet been used to specifically identify the species whose populations have changed most in response to climate change, which is the aim of this paper.
The potentially large number of variables, relative to the length of the time-series, makes achieving this ambition difficult using standard modelling approaches. Changes in the climate can be measured in a multitude of different ways, from mean air temperature to rainfall totals, from the number of days of snow cover to wind speed. Even single components, such as temperature, can be variously summarized in many ways (Suggitt et al. Citation2017). Attempting to link potentially complex descriptions of the climate to biological data creates further statistical challenges. Multivariate techniques such as ordination can be used to summarize many different components of climate change into a small number of key changes (Martay et al. Citation2017). Alternatively, the cumulative impact of seasonal changes can be summarized into a small number of parameters, which can be modelled statistically (Sims et al. Citation2007, Thackeray et al. Citation2016, Elston et al. Citation2017). These approaches, which distil complex changes into a small number of variables, run the risk of Type II error where important correlations with specific variables are masked, whilst the naïve consideration of large numbers of predictor variables potentially inflate the risk of Type I error. As a result, we use a combination of variable filtering and model averaging to minimize the risk of over-fitting, and produce models that link annual variation in population growth to annual variation in what are intended to approximate to meaningful climate variables. These models are used to describe the likely impact that climate change has had on individual species while aiming to minimize the risk of spurious attribution to climate change.
Methods
Bird data
Data from the British Trust for Ornithology’s (BTO) Common Bird Census (CBC) and the BTO/Joint Nature Conservation Committee (JNCC) /Royal Society for the Protection of Bird (RSPB)’s Breeding Bird Survey (BBS) were used to generate annual indices of population change for England from 1966 to 2015. Within the CBC, bird territories were mapped from observations made on seven to ten visits per year to self-selected sites (Marchant et al. Citation1990). The BBS involves two parallel 1 km line-transects in 1 km2 square being surveyed during two early morning surveys between early April and late June. Despite the switch in methodology in 1994, a subsequent period of overlap between the two surveys showed that they can be combined for many species to produce joint trends of population size (Freeman et al. Citation2007). We used data for 68 species for which joint trends can be robustly produced from CBC and BBS schemes for England, including freshwater and non-native species not covered by previous analyses of these data (Pearce-Higgins, Eglington et al. Citation2015).
Annual variation in population growth rates was derived from the log-ratio of change in abundance between two consecutive years, which has an approximately normal distribution for all species. The alternative of modelling abundance in a single year as a function of the log of abundance in the previous year as an offset (see Pearce-Higgins, Eglington et al. Citation2015), using a generalized linear mixed model, was also tried and produced equivalent results.
Climatic variables
Spatial and temporal variation in the climate was described by monthly averages of temperature and precipitation from the National Oceanic and Atmospheric Administration/Earth System Research Laboratory, Physical Sciences Division series (www.esrl.noaa.gov/psd/data/timeseries/). These were averaged across the following geographical areas to match locations of relevance to the bird species of interest, including key wintering locations for different migratory species (Ockendon et al. Citation2013), but also considering key stopover and wintering areas for other species (Hewson et al. Citation2016): England (5° W – 2.5° E, 50° – 55° N), Iberian Mediterranean (10° W – 5° E, 35° – 45° N), Italian Mediterranean (5 – 20° E, 35–45° N), Sahel (17° W – 9°E, 11° – 17°N), Humid zone (15° W – 9°E, 5–8° N), Congo (13–25° E, 13°S – 5°N) and South Africa (17–33° E, 23–35°S). For the purposes of analyses, we used monthly averages of mean daily temperature (°C), which have previously been shown to produce similar results to using monthly averages of daily minima or maxima (Pearce-Higgins, Eglington et al. Citation2015), and total monthly precipitation (mm). These were converted into seasonal averages for each region that match the Northern Hemisphere winter (Dec–Feb), Spring (Mar–May), Summer (Jun–Aug) and Autumn (Sept–Nov), for both the year between two consecutive surveys (yeart) and the previous year up to the first bird survey season (yeart-1) to account for potential lagged effects (Pearce-Higgins, Eglington et al. Citation2015). We also included variables describing the second bird survey season (Apr–Jun) in yeart+1 to better capture variation which may affect productivity or detectability (Pearce-Higgins, Eglington et al. Citation2015). Although wet-season rainfall (May–Oct) in the Sahel affects survival rates and populations of migratory birds that winter there (Ockendon, Johnston et al. Citation2014, Johnston et al. Citation2016), total rainfall over this period was strongly correlated with both summer (r = 0.95) and autumn (r = 0.91) rainfall, which were used instead for consistency. More sophisticated methods for analysing time-series of weather data to identify significant periods of importance (e.g. Elston et al. Citation2017) would have avoided the need to arbitrarily separate the data into seasons, but could not be used as their greater modelling complexity means that they have only been developed for use with single variables.
Analysis
The combination of two variables across eight seasons from Springt-1 to Wintert for up to seven regions generates a maximum of 112 variables, which plus precipitation and temperature during the final breeding season (Springt+1), when conditions may influence species’ settlement or detectability, produces a total of 114 potential variables for analysis. This is too many to consider in a single analysis of 50 years of data. Therefore, in an attempt to identify appropriate predictor variables whilst minimizing the risk of over-fitting and potentially spurious correlation, we have attempted to balance the need to treat all species equally with the desire not to consider inappropriate variables. Given the potential for climate change to alter interactions between species (Ockendon, Baker et al. Citation2014) and therefore to operate with time-lags (Pearce-Higgins, Eglington et al. Citation2015), we could not consider only the terms that matched the occurrence of the species at that location, but instead have included the full range of variables for each location that a species occurs in. However, we did exclude variables from regions where only a small proportion of the breeding population are thought to occur, based on Wernham et al. (Citation2002). Although it is plausible that resident breeding bird species in England could be affected by climatic conditions in Africa, for example through competition between migratory and resident species, such impacts have been rarely documented in the literature, with resident species generally out-competing migrants (Fuller & Crick Citation1992, Dugger et al. Citation2004).
This approach results in a greater number of predictor variables for migratory species than resident species, which may bias our results towards a greater likelihood of detecting impacts of climate change upon migratory species. To avoid this problem, we conducted a two-stage approach to the analysis. First, we considered all of the potential variables across all seasons for the geographical areas relevant to particular species. Following Thaxter et al. (Citation2010) and Wernham et al. (Citation2002), we considered just England for resident species; England and Iberia only for short/medium distance migrants that winter in Europe; England, Iberia and the Sahel for arid-zone Sahelian migrants, England, Iberia, Sahel, Humid zone and the Congo for Humid-zone migrants; and additionally, South Africa for Barn Swallow Hirundo rustica. Italy was considered as an additional passage area, where appropriate, based on ringing recoveries or subsequent tracking studies (Wernham et al. Citation2002, Hewson et al. Citation2016). Single models of annual variation in population growth were run for each of these variables in turn, to determine their potential predictive power in accounting for annual changes in bird abundance. These models also considered the potential for non-linear relationships by including quadratic terms for the climatic variables.
It is important to also account for the potential for non-climatic variables to affect long-term trends in bird populations (Eglington et al. Citation2012), particularly as these may correlate with the long-term trends in some climatic variables. Whilst this was not directly possible in a manner that would treat all species equally, we instead included YEAR and YEAR2 terms in all the models, allowing population growth rates to vary in relatively complex ways through the time-series, for example in response to other, non-climatic factors. Count in the previous year (COUNT) was also included to account for density-dependence (Pearce-Higgins, Eglington et al. Citation2015). Given the potential for temporal autocorrelation in both the abundance and climate data, we used a mixed model, accounting for any first-order autoregressive covariance. Variables were considered for the second analytical stage if they were significant with a P < 0.10 threshold, after applying the Bonferonni correction (e.g. P < 0.0056 for resident species in England (0.10/18 climatic predictor variables – 8 seasonal temperature and rainfall variables across two years, plus two for Springt+1), P < 0.0020 for arid-zone migrants (0.10/50 variables – 16 climatic variables for three regions plus Springt+1 variables from England only) and P < 0.0012 for humid-zone migrants (0.10/82 variables – 16 climatic variables for five regions plus Springt+1 variables from England)). On the very small (<1%) number of occasions there were convergence problems with these models, significance was tested using a Generalized Linear Model (GLM).
In order to summarize the patterns of the effects of climatic variables upon population growth rates, we modelled variation in the probability of significant (P < 0.10) linear associations with location, season, climatic variable and migratory distance. This was undertaken using a Generalized Linear Mixed Model (GLMM) with genus and family as random effects, and specifying a binomial error structure and logit link function. All two-way interactions were tested, with a final model determined by backward deletion of non-significant (P > 0.05) terms.
At the second stage, all possible combinations of variables that exceeded the significance threshold in stage one were considered using a multi-model approach. Given the constraints of selecting mixed models using Akaike information criteria corrected for small sample size (AICc) values, we used GLMs, considering YEAR, YEAR2, COUNT and up to 3 climatic variables together, the maximum number of variables that we considered could be used given our sample size of 50 years of data. Significant quadratic terms were considered together with their linear term irrespective of the stage-one significance of the linear term, whilst the same linear terms were also considered in isolation if identified as significant in the stage-one models. On occasions where more than three climatic variables were selected from Stage 1, these were considered with all possible combinations of up to three climatic variables, but models with more than three such variables were not run.
All variables included within the top (ΔAICc < 2) models were then included within a final mixed model that included a first-order autoregressive covariance function, to produce the final parameter estimates of population growth for that species. By accounting for temporal autocorrelation, this generated more realistic parameter estimates than model-averaged outputs from the second stage models, although the two sets of parameters were closely correlated.
These final models were used to predict annual estimates of abundance (M) for individual species, using the free-running approach of Eglington et al. (Citation2012). This approach uses the parameters in the final model to predict abundance in any one year from the values of the predictor variables. In order to make these predictions ‘free-running’, values of COUNT in the previous year were based upon predicted values of abundance from the model, rather than observed abundance, with the exception of predicted abundance in year 2 which was predicted from the initial observed count in year one. For species whose models included one or more climatic variables, two version of M were estimated. Firstly, using the observed values of climate variables that describe the conditions experienced (Mc) and secondly, replacing the observed climatic variables with detrended values (Md) produced by regressing the climatic variables against year, and then subtracting the effect of the modelled trend through time from the observed climatic values.
In order to describe the long-term changes in abundance of each species, and the extent to which that is consistent with modelled climate change impacts, we averaged observed (O) or modelled (M) abundance values over the final five (2011–15) years of the time-series, thus reducing the effect of individual years upon our measure of change. These were summarized as the natural log of abundance so that population increases and decreases to 2011–15 were comparable. The potential impact of climate change upon species’ populations (C) was estimated by subtracting mean predictions for 2011–15 based on detrended climatic variables from those based on observed climatic variables: C = ln(Mc2011–2015) – ln(Md2011–2015). For the purposes of this paper, we assume that the difference between observed and detrended climatic variables approximates to a description of climate change. Positive values are indicative of species predicted to have a greater abundance in 2011–15 as a result of linear trends in climatic predictor variables. For convenience, we regarded differences of 0.1 (which approximates to a 10% difference in modelled abundance between 2011 and 2015 for the two predictions) as biologically important.
To generalize differences between species, we tested the following hypotheses. First, that climatic changes have resulted in more positive population trajectories for resident species than migrant species (Pearce-Higgins & Green Citation2014). Second, that climatic changes have resulted in more positive population trends for warm-associated than cold-associated species, as measured by the species temperature index (STI) which describes the spatial association of each species and breeding season temperature across Europe (Devictor et al. Citation2012). Third, that habitat generalists have responded more positively to climate change than habitat specialists (Davey et al. Citation2012), as measured by the species specialization index (SSI; Sullivan et al. Citation2016). These hypotheses were testsed by modelling variation in the observed trends (ln(O)), and the effect of climate change (C) in a GLMM model with genus and family specified as random effects, and migratory status, SSI and STI as predictor variables. Dependent variables had a normal distribution.
Results
Of 2056 combinations of linear climatic variables and population changes, 226 (10.8%) were below the P < 0.10 threshold for selection, of which 123 (6.0%) were statistically significant at the P < 0.05 threshold; slightly greater than the 206 and 103 respectively expected by chance. Twenty-five of these were significant at the appropriate Bonferonni-corrected threshold for that species, based upon the number of variables considered, approximately 3 times the 8 expected by chance. Using the distribution of significant (P < 0.10) effects of climatic variables upon population growth as a measure of relative sensitivity of populations to climatic variables, the proportion of significant effects differed with season and migratory status (season * migratory status: F8,2070 = 3.79, P = 0.0002), but not as a result of other two-way interactions. Neither location (F6,2064 = 1.66, P = 0.12) nor variable-type (F1,2069 = 0.02, P = 0.89) had significant explanatory power, whilst after excluding the significant two-way interaction, a significant impact of season (F8,2079 = 2.02, P = 0.041) but not migratory status (F1,60.06 = 0.28, P = 0.60) remained. In summary, only climatic variables relating to summert and wintert seasons for resident species linearly affected population growth rates with a ‘probability of significance’ significantly greater than 0.10, the value expected by chance (). It is worth noting that as a result of this combination, both seasons relate to descriptions of climatic variables in England.
Figure 1. The proportion of analysed species for which each variable had a significant (P < 0.10) relationship between population growth, which varies by season and migratory status (migrant – dark grey; resident – light grey). Bars represent the 95% confidence intervals around the estimated proportion, and where these do not overlap the 0.10 value expected by chance (dashed line), that season is significantly more important than expected by chance.
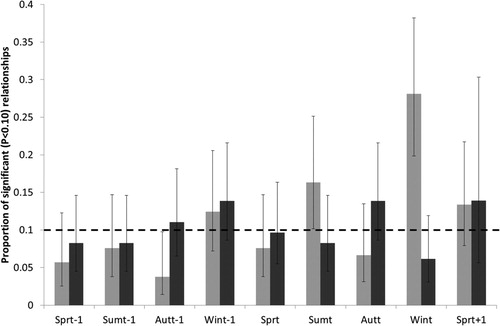
Following this, of the 25 linear tests identified as significant at the lowest Bonferonni-corrected threshold, 23 were from England of which 11 were from the wintert, 4 the summert, 5 the breeding seasont+1 and one each from autumnt, wintert-1 and springt. The remaining variables were from autumnt-1 in Spain and autumnt in the Sahel.
When the models of population growth, including significant quadratic terms, were used to produce free-running model predictions of abundance through time (e.g. . Graphs for all species are shown in online supplementary material, Figure S1), they showed good descriptive power, with a correlation between observed trends ln(O) and modelled trends over the same time-period ln(M) of r = 0.93, although much of which could have been driven by the underlying trend (intercept) and effect of year upon population growth rate. Overall, population growth rates of 24 species were predicted to have been significantly impacted by climatic variables (), with the equivalent correlation between observed and predicted trends of r = 0.98 for these species alone, suggesting that for these species, the inclusion of climatic variables had a significant impact on their long-term trend. Additionally, these species showed a significantly stronger correlation between modelled and observed annual variation in population growth than species whose models did not include climatic variables (F1,66 = 50.69, P < 0.0001; r = 0.503 ± 0.036 vs r = 0.188 ± 0.026). However, the model for one species, Nuthatch Sitta europaea, which included a quadratic relationship between population growth rate and a lagged effect of breeding season rainfall (UKRBRt-1), appeared to be driven by data from a single year that resulted in a negative correlation between observed and modelled population growth rates (). This model was therefore not considered to be robust and, given no other climatic predictors were significantly correlated with Nuthatch population growth rates, this species was subsequently regarded as unaffected by climatic variables.
Figure 2. Observed (thick line), and modelled trend based on observed climate variables (Mc – dashed line) and detrended climate data (Md – dotted line) for English populations of (a) Goldcrest where Mc > Md and therefore warmer, wetter winters () have increased abundance, and (b) Cuckoo where Mc < Md and warmer springs () are associated with declines in abundance. Equivalent graphs for all species are provided in Figure S1. There is no observed data for 2001, when access restrictions to the English countryside in response to the foot-and-mouth disease outbreak prevented data collection.
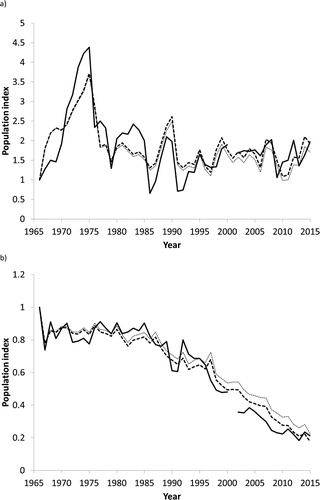
Table 1. Observed and modelled abundance averaged from 2011 to 2015 (see Figure S1 for the species-specific time-series from which these figures are derived). For species with a significant relationship between population growth and a climatic variable (bold), the significant climatic variables in the final model, and the form of relationships between population growth and that variable are given. Climate variables are summarized as follows, with the first two letters denoting location (UK, England; SP, Spain; SA, Sahel; Hu, Humid zone), the next letter separating temperature (T) from rainfall (R) and the final two letters and subscript summarizing the season and year (WI, winter, SP, spring, SU, summer, AU, autumn).
Four species with population growth rates significantly correlated with climatic variables appeared to be negatively impacted by climate change, although for only three (Cuckoo Cuculus canorus, Little Owl Athene noctua and Reed Warbler Acrocephalus scirpaceus) was the difference in mean predicted abundance for 2011–15 as a result of potential climate change impacts (C) greater than 10% (Mallard Anas platyrhynchos was the fourth species). Modelled impacts of climate change were predicted to be positive for 19 species, suggesting that observed trends in climatic variables have resulted in either more positive or less negative population trends than otherwise would have occurred. For 13 of these, C > 0.1, and therefore the modelled climate change impacts are regarded as important (Corn Bunting Emberiza calandra, Goldcrest Regulus regulus, Greenfinch Chloris chloris, Grey Partridge Perdix perdix, Kestrel Falco tinnunculus, Linnet Linaria cannabina, Long-tailed Tit Aegithalos caudatus, Moorhen Gallinula chloropus, Pheasant Phasianus colchicus, Pied Wagtail Motacilla alba, Red-legged Partridge Alectoris rufa, Song Thrush Turdus philomelos and Wren Troglodytes troglodytes).
Migratory species were relatively less-abundant in 2011–15 than residents (F1,19.9 = 5.01, P = 0.037; migrant ln(O2011–2015)= −0.91 ± 0.34; resident ln(O2011–2015) = −0.047 ± 0.19). There was no evidence that variation in abundance between species was correlated with either SSI (F1,55.6 = 1.38, P = 0.25) or STI (F1,42.7 = 0.04, P = 0.84). Contrary to expectation, there was also no evidence from our models that climate change had a strong role to play in determining the variation in these trends, with no significant difference in the modelled effect of climate change (C) with migratory distance (F1,23.9 = 1.94, P = 0.18), SSI (F1,62.2 = 1.36, P = 0.18) or STI (F1,63.9 = 2.51, P = 0.12).
Discussion
We estimate that the population growth rates of 24/68 species (35%) have been significantly impacted by climatic variables, although we regard one of these species’ models as being driven by a single outlier with poor descriptive power, and not robust. The majority of the effects were related to English conditions, particularly for resident species during summer and winter. The resulting magnitude of the effect of climate change upon the long-term population trend of these species, as measured by mean abundance from 2011 to 2015, was dependent upon a combination of the magnitude of long-term trends in the relevant climatic variables, and in the strength of their impact upon the species of interest. These results suggest that climate change, as described by our models and long-term trends in climatic variables, has had a measurable impact upon the population fluctuations of about 1/3 of the UK breeding species considered, resulting in notable (>10%) positive impacts on the abundance of 13 of the species modelled, and notable negative impacts on three.
These findings support previous analyses of the same joint population trends for England that highlighted the role of winter temperature as a key driver of resident bird populations (Pearce-Higgins, Eglington et al. Citation2015). The latter study considered mean effects across 59 species, rather than the frequency of highly significant effects analysed in this study. By predicting the impacts of climate change on long-term trends we show that warmer winter temperatures, in combination with other aspects of climate change, have a positive effect upon population growth rates of a range of resident bird species (Baillie & Greenwood Citation1991), probably by improving over-winter survival rates (Robinson et al. Citation2007). Specifically, we identify a range of species most positively affected by warmer winters (, Figure S1).
However, there are some differences between this and the Pearce-Higgins, Eglington et al. (Citation2015) study, which found stronger associations with spring temperature, but fewer positive effects of summer temperature. Pearce-Higgins, Eglington et al. (Citation2015) also found more equivocal effects of English weather upon migrant species and a negative lagged response of migrant populations to summer drought conditions, which did not come out as important for any of the species that we considered. This could be because we used seasonal rather than monthly variables, or because such effects, whilst being consistent across species, were not of sufficient magnitude in affecting individual species to be identified by our modelling approach which for Sahelian migrants required P < 0.00125 for effects to reach significance.
Unlike the Pearce-Higgins, Eglington et al. (Citation2015) analyses of these data, we also considered the effects of weather conditions at migratory stop-over locations and wintering areas. Despite previous analysis highlighting consistent positive effects of Sahelian rainfall upon migrant survival (Johnston et al. Citation2016) and population change (Ockendon, Johnston et al. Citation2014), we identified only Common Whitethroat Sylvia communis population trends as being significantly affected by Sahel rainfall, although three other species, Common Redstart Phoenicurus phoenicurus, Reed Warbler and Sedge Warbler Acrocephalus schoenobaenus all exhibited positive associations between Sahel rainfall and population growth at the P < 0.05 significance level, but these were not strong enough to survive our variable selection process. There was not strong evidence for significant effects of climatic conditions on other migratory species, apart from a negative effect of autumn temperature in Spain upon Reed Warbler population growth, and quadratic effects of humid zone rainfall in the spring and Sahelian temperature in the autumn, upon Spotted Flycatcher Muscicapa striata population growth rates. Given the likely importance of weather conditions in the tropics, particularly precipitation, affecting ecological systems there (Pearce-Higgins, Ockendon et al. Citation2015), and therefore long-distance migrant populations (Ockendon, Johnston et al. Citation2014, Johnston et al. Citation2016), our relative failure to detect such effects probably illustrates the difficulty of assessing the impacts of climate change on migratory species (Small-Lorenz et al. Citation2013), rather than being evidence that they are not important. Any such under-estimation is probably a function of: (1) uncertainty about precise wintering locations which will reduce the likelihood of detecting significant effects; (2) variation in the extent to which breeding populations have discrete wintering locations (Gilroy et al. Citation2016), with reduced probability of detection of effects on wintering populations if those populations are dispersed; (3) a greater number of potential variables to be considered, increasing the statistical barrier for significance and (4) more accurate weather and climatological data being available from England, than other parts of the world (Baker et al. Citation2017). These four considerations combine to mean that effects of climate change on breeding long-distance migrant populations are more likely to be detected if they occur through impacts on the breeding grounds where the geographical extent of the population can be tightly linked to geographically appropriate weather data than elsewhere, which is a challenge when attempting to identify the causes of decline in long-distance migratory bird populations. More detailed studies of migratory movements (Hewson et al. Citation2016), coupled with bespoke analyses of relevant environmental predictors (Beale et al. Citation2006), are probably required to better track potential impacts of climate change on these species. For species with mixing between resident and non-breeding populations, such analyses might not be possible, and separate models may be required to detect impacts on breeding and non-breeding populations.
A key aim of this study was to provide species-level descriptions of how climate change may have affected breeding bird populations in England over the last five decades. Of the 23 species with projected impacts, we note some species where the observed population trends, which have previously been attributed to non-climatic drivers, are also consistent with the modelled effect of climatic variables. For example, the recent decline in Greenfinch populations, which our model suggests could be linked to climate change through a quadratic relationship between breeding season temperature (UKTBRt) and population growth rate, has previously been attributed with a high degree of confidence to the disease finch trichomonosis (Lawson et al. Citation2018). Interestingly, an effect of summer drought on Greenfinch survival rates has also previously been documented (Robinson et al. Citation2007), and it is plausible that the effect of disease may be exacerbated by hot, dry spring and summer conditions. Our models also suggest a significant role of warming in driving population increases in two gamebird species which are the subject of extensive artificial releases: Pheasant and Red-legged Partridge. Whilst these predictions may, therefore, also appear to be spurious, the fact that both species have southerly distributions (Balmer et al. Citation2013) and, in common with the ecologically similar Grey Partridge, which shows the same positive effect of summer temperature, are Galliformes, known to be sensitive to cold, wet breeding season conditions (Pearce-Higgins & Green Citation2014), means these modelled effects cannot be completely disregarded. Warm summer weather could play a role in maintaining established populations of these species by enhancing their breeding success, or for Pheasants, could enhance the normally low autumn survival of released poults which may then recruit into the subsequent breeding population (Madden et al. Citation2018). We also note the apparently spurious relationship between Nuthatch population growth rates and breeding season rainfall, due to a single outlier, that resulted in a worse than expected by chance correlation between observed and modelled population growth rates. Despite evidence for the Nuthatch having increased significantly in abundance and range extent in recent decades (Massimino et al. Citation2015) and projected to benefit from future warming (Renwick et al. Citation2012, Massimino et al. Citation2017), we could not directly link observed population growth to our measures of climate change.
The strong positive effect of climate change upon population trends of small insectivorous passerines such as Goldcrest, Long-tailed Tit and Dunnock Prunella modularis are in line with expectations from previous studies or known effects on survival rates (Robinson et al. Citation2007). Thus, although one of our models (Nuthatch) is probably spurious (1% of all models or 4% of models with significant terms), and another three (Greenfinch, Pheasant and Red-legged partridge) may be uncertain due to potentially confounding factors, predictions for ten, Blackbird Turdus merula (Robinson et al. Citation2012), Coot Fulica atra (Cavé & Visser Citation1985), Dunnock (Robinson et al. Citation2007, Martay et al. Citation2017), Goldcrest (Greenwood & Baillie Citation1991), Long-tailed Tit (Greenwood & Baillie Citation1991), Mallard (Krapu et al. Citation1983), Song Thrush (Martay et al. Citation2017), Tawny Owl Strix aluco (Francis & Saurola Citation2004), Common Whitethroat (Ockendon, Johnston et al. Citation2014) and Wren (Martay et al. Citation2017, Robinson et al. Citation2007) each have a degree of independent validation in terms of the results of species or population-specific studies, or the findings of similar analyses of long-term data. However, there are probably a small number of other species which previous studies have suggested appear sensitive to cold-weather events, but which our models failed to link statistically to winter weather, such as Lapwing Vanellus vanellus (Robinson et al. Citation2014), Blue Tit Cyanistes caeruleus (Robinson et al. Citation2007), Great Tit Parus major (Robinson et al. Citation2007) and Treecreeper Certhia familiaris (Peach et al. Citation1995). As outlined above for migrants, a lack of association for any one species does not indicate that it has been unaffected by climate change.
We noted a lack of significant association between both STI and SSI and our modelled impacts of climate change. There is good evidence that warm-associated species have indeed increased in abundance relative to cold-associated species, both across Europe and in the UK (Devictor et al. Citation2012, Oliver et al. Citation2017), whilst increases in the relative abundance of habitat generalists have also been linked to temperature (Davey et al. Citation2012). Although the effect of temperature upon species’ population growth rates is also correlated with STI and SSI, being more positive for warm-associated species and habitat generalists (Pearce-Higgins, Eglington et al. Citation2015), the fact that we failed to find strong associations between either STI and SSI, and modelled impacts of climate change (P = 0.12 and 0.18, respectively) may result from the multiple drivers that combine to generate long-term population trends. Other studies have shown that changes in bird communities are related to intensification of land use (Doxa et al. Citation2012, Gámez-Virués et al. Citation2015) and habitat fragmentation (Devictor et al. Citation2008), which in many circumstances may be more important than climate change as a driver of long-term population trend (Eglington et al. Citation2012). Further, our models probably only capture a subset of components of climate change impacts on species, as the already discussed lack of strong climatic relationships for Nuthatch, migrants and some resident species demonstrates. It is also worth noting that our approach to quantifying climate change impacts, based on linear trends in climatic variables, is a relatively simplistic one. Longer-term changes in species’ distributions and patterns of abundance (Gillings et al. Citation2015, Massimino et al. Citation2015) driven by warmer temperatures may not simply occur as a result of the relatively direct effects of temperature on demographic parameters, but potentially also through longer-term changes in dispersal and territorial settlement patterns, perhaps driven by more gradual changes in habitat quality, which are more difficult to model and predict.
To conclude, despite the limitations of a correlative study such as this, we have documented that population trends of one-third of breeding bird species considered may have been affected by long-term trends in climatic variables, and therefore have potentially been affected by climate change. Such effects for many resident species are likely to have been positive. This suggests that even for species which have experienced significant long-term population declines over this period, such as warm-associated farmland birds like the Corn Bunting and Grey Partridge, those declines might have been significantly less negative than they otherwise might have been in the absence of climate change (Eglington et al. Citation2012). Importantly, our study also highlights the limitations of a large-scale multi-species correlative approach for detecting climate change impacts. A small proportion of our models may have produced spurious predictions, whilst we also failed to detect what we regard as likely biologically important relationships between Sahel rainfall and migrant population growth trends for a number of species (Ockendon, Johnston et al. Citation2014), and positive impacts of milder winter weather on some others (Robinson et al. Citation2007). This emphasizes that the strongest evidence for climatic impacts on species must also include a mechanistic understanding of the potential links between climatic variables and the population of interest (Pearce-Higgins et al. Citation2010). Ideally, a combination of such ecological understanding from detailed site-based studies and analyses of the large-scale and long-term data provide the greatest confidence and ability to identify meaningful climate change impacts across large-scales. This means that of the findings presented here, we have the greatest confidence in the apparently positive responses to winter temperature (or correlated positive effects of winter rainfall) detected here in ten resident species, that have driven a more positive long-term population trajectory, because they are supported by demographic information linking cold winter weather to increases in mortality (Robinson et al. Citation2007). This relaxation of cold-weather constraints is likely to be one of the mechanisms underpinning significant range expansion in many resident species (Massimino et al. Citation2015) and in projected future increases in many others (Pearce-Higgins et al. Citation2017), although other components of climate change, such as direct impacts on breeding success or post-fledging survival, or longer-term impacts on species interactions and habitat quality (Ockendon, Baker et al. Citation2014), are also likely to be important, the latter particularly over longer timescales.
Supplemental Material
Download MS Word (3.7 MB)Additional information
Funding
References
- Baker, D.J., Hartley, A.J., Pearce-Higgins, J.W., Jones, R.G. & Willis, S.G. 2017. Neglected issues in using weather and climate information in ecology and biogeography. Divers. Distrib. 23: 329–340. doi: 10.1111/ddi.12527
- Balmer, D.E., Gillings, S., Caffrey, B.J., Swann, R.L., Downie, I.S. & Fuller, R.J. 2013. Bird Atlas 2007–11: the Breeding and Wintering Birds of Britain and Ireland. BTO, Thetford.
- Beale, C.M., Burfield, I.J., Sim, I.M.W., Rebecca, G.W., Pearce-Higgins, J.W. & Grant, M.C. 2006. Climate change may account for the decline in British ring ouzels Turdus torquatus. J. Anim. Ecol. 75: 826–835. doi: 10.1111/j.1365-2656.2006.01102.x
- Bellard, C., Bertelsmeier, C., Leadley, P., Thuiller, W. & Courchamp, F. 2012. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15: 365–377. doi: 10.1111/j.1461-0248.2011.01736.x
- Cavé, A. J. & Visser, J. 1985. Winter severity and breeding bird numbers in a Coot population. Ardea 73: 129–138.
- Crick, H.Q.P. 2004. The impacts of climate change on birds. Ibis 146(S1): 48–56. doi: 10.1111/j.1474-919X.2004.00327.x
- Davey, C.M., Chamberlain, D.E., Newson, S.E., Noble, D.G. & Johnston, A. 2012. Rise of the generalists: evidence for climate driven homogenization in avian communities. Glob. Ecol. Biogeogr. 21: 568–578. doi: 10.1111/j.1466-8238.2011.00693.x
- Devictor, V., Julliard, R., Clavel, J., Jiguet, F., Lee, A., & Couvet, D. 2008. Functional biotic homogenization of bird communities in disturbed landscapes. Glob. Ecol. Biogeogr. 17: 252–261. doi: 10.1111/j.1466-8238.2007.00364.x
- Devictor, V., van Swaay, C., Brereteon, T., Brotons, L., Chamberlain, D., Heliölä, J., Herrando, S., Julliard, R., Kuussaari, M., Linström, Å., Reif, J., Roy, D.B., Schweiger, O., Settele, J., Stefanescu, C., Van Strien, A., Van Turnhout, C., Vermouzek, Z., De Vries, M.W., Wynhoff, I. & Jiguet, F. 2012. Differences in the climate debts of birds and butterflies at a continental scale. Nat. Clim. Change 2: 121–124. doi: 10.1038/nclimate1347
- Doxa, A., Paracchini, M. L., Pointereau, P., Devictor, V., & Jiguet, F. 2012. Preventing biotic homogenization of farmland bird communities: the role of High Nature Value farmland. Agric. Ecosyst. Environ. 148: 83–88. doi: 10.1016/j.agee.2011.11.020
- Dugger, K.M., Faaborg, J., Arendt, W.J. & Hobson, K.A. 2004. Understanding survival and abundance of overwintering warblers: does rainfall matter? Condor 106: 744–760 doi: 10.1093/condor/106.4.744
- Eglington, S.M. & Pearce-Higgins, J.W. 2012. Disentangling the relative importance of changes in climate and land-use intensity in driving recent bird population trends. PLoS ONE 7: e30407. doi: 10.1371/journal.pone.0030407
- Elston, D.A., Brewer, M.J., Martay, B., Johnston, A., Henrys, P.A., Bell, J.R., Harrington, R., Monteith, D., Brereton, T.M., Boughey, K.L. & Pearce-Higgins, J.W. 2017. A new approach to modelling the relationship between annual population abundance indices and weather data. Journal of Agricultural, Biological and Environmental Statistics 22: 427–445. doi: 10.1007/s13253-017-0287-4
- Francis, C.M. & Saurola, P. 2004. Estimating components of variance in demographic parameters of Tawny Owls, Strix aluco. Anim. Biodivers. Conserv. 27: 489–502.
- Frederiksen, M., Wanless, S., Harris, M.P., Rothery, P. & Wilson, L.J. 2004. The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes. J. Appl. Ecol. 41: 1129–1139. doi: 10.1111/j.0021-8901.2004.00966.x
- Freeman, S.N., Noble, D.G., Newson, S.E. & Baillie, S.R. 2007. Modelling population changes using data from different surveys: the Common Bird Census and the Breeding Bird Survey. Bird Study 54: 61–72. doi: 10.1080/00063650709461457
- Fuller, R.J. & Crick, H.Q.P. 1992. Broad-scale patterns in geographical and habitat distribution of migrant and resident passerines in Britain and Ireland. Ibis 134(S1): 14–20.
- Gámez-Virués, S., Perović, D.J., Gossner, M.M., Börschig, C., Blüthgen, N., De Jong, H., Simons, N.K., Klein, A.M., Krauss, J., Maier, G. & Scherber, C. 2015. Landscape simplification filters species traits and drives biotic homogenization. Nat. Commun. 6: 8568. doi: 10.1038/ncomms9568
- Gillings, S., Balmer, D.E. & Fuller, R.J. 2015. Directionality of recent bird distribution shifts and climate change in Great Britain. Glb. Chg. Bio. 21:2155–2168 doi: 10.1111/gcb.12823
- Gilroy, J.J., Gill, J.A., Butchart, S.H.M., Jones, V.R. & Franco, A.M.A. 2016. Migratory diversity predicts population declines in birds. Ecol. Lett. 19: 308–317. doi: 10.1111/ele.12569
- Greenwood, J.D. & Baillie, S.R. 1991. Effects of density-dependence and weather on population changes of English passerines using a non-experimental paradigm. Ibis 133(S1): 121–133.
- Hewson, C.M., Thorup, K., Pearce-Higgins, J.W. & Atkinson, P.W. 2016. Population decline is linked to migration route in the Common Cuckoo. Nat. Commun. 7: 12296. doi: 10.1038/ncomms12296
- Johnston, A., Robinson, R.A., Gargallo, G., Julliard, R., van der Jeugd, H. & Baillie, S.R. 2016. Survival of Afro-Palearctic passerine migrants in Western Europe and the impacts of seasonal weather variables. Ibis 158: 465–480. doi: 10.1111/ibi.12366
- Jørgensen, P.S., Böhning-Gaese, K., Thorup, K., Tøttrup, A.P., Chylarecki, P., Jiguet, F., Lehikoinen, A., Noble, D.G., Reif, J., Schmid, H., Van Turnhout, C., Burfield, I.J., Foppen, R., Voříšek, P., Van Strien, A., Gregory, R.D. & Rahbek, C. 2016. Continent-scale global change attribution in European birds - combining annual and decadal time scales. Glb. Chg. Bio. 22: 530–543. doi: 10.1111/gcb.13097
- Krapu, G.L., Klett, A.T. & Jorde, D.G. 1983. The effect of variable spring water conditions on mallard reproduction. Auk 100: 689–698.
- Lawson, B., Robinson, R.A., Toms, M.P., Risely, K., MacDonald, S. & Cunningham, A.A. 2018. Health hazards to wild birds and risk factors associated with anthropogenic food provisioning. Philos. Trans. Royal Soc. Lond. Ser. B 373: 1745.
- Ludwig, G.X., Alatalo, R.V., Helle, P., Lindén, H., Lindström, J. & Siitari, H. 2006. Short- and long-term population dynamical consequences of asymmetric climate change in black grouse. Proceedings of the Royal Society B: Biological Sciences 273: 2009–2016. doi: 10.1098/rspb.2006.3538
- Madden, J.R., Hall, A. & Whiteside, M.A. 2018. Why do many pheasants released in the UK die, and how can we best reduce their natural mortality? Eur J Wildl Res 64: 40. doi: 10.1007/s10344-018-1199-5
- Marchant, J.H., Hudson, R., Carter, S.P. & Whittington, P.A. 1990. Population Trends in British Breeding Birds. BTO, Tring.
- Martay, B., Brewer, M.J., Elston, D.A., Bell, J.R., Harrington, R., Brereton, T.M., Barlow, K.E., Botham, M.S. & Pearce-Higgins, J.W. 2017. Impacts of climate change on national biodiversity population trends. Ecography 40: 1139–1151. doi: 10.1111/ecog.02411
- Massimino, D., Johnston, A. & Pearce-Higgins, J.W. 2015. The geographical range of British birds expands during 15 years of warming. Bird Study 62: 523–534. doi: 10.1080/00063657.2015.1089835
- Massimino, D., Johnston, A., Gillings, S., Jiguet, F. & Pearce-Higgins, J.W. 2017. Projected reductions in climate suitability for vulnerable British birds. Clim. Change 145: 117–130. doi: 10.1007/s10584-017-2081-2
- Ockendon, N., Baker, D.J., Carr, J.A., Almond, R.E.A., Amano, T., Bertram, E., Bradbury, R.B., Bradley, C., Butchart, S.H.M., Doswald, N., Foden, W., Gill, D.J.C., Green, R.E., Sutherland, W.J., Tanner, E.V.J. & Pearce-Higgins, J.W. 2014. Mechanisms underpinning climatic impacts on natural populations: altered species interactions are more important than direct effects. Glb. Chg. Bio. 20: 2221–2229. doi: 10.1111/gcb.12559
- Ockendon, N., Johnston, A. & Baillie, S.R. 2014. Rainfall on wintering grounds affects population change in many species of Afro-Palaearctic migrants. J. Ornith 155: 905–917. doi: 10.1007/s10336-014-1073-5
- Ockendon, N., Leech, D. & Pearce-Higgins, J.W. 2013. Climate effects on breeding grounds are more important drivers of breeding phenology in migrant birds than carry-over effects from wintering grounds. Biol. Lett. 9: 20130669. doi: 10.1098/rsbl.2013.0669
- Oliver, T.H., Gillings, S., Pearce-Higgins, J.W., Brereton, T., Crick, H.Q.P., Duffield, S., Morecroft, M.D. & Roy, D.B. 2017. Large extents of intensive land use limit community reorganisation during climate warming. Glb. Chg. Bio. 23: 2272–2283.
- Peach, W.J., du Feu, C. & McMeeking, J. 1995. Site tenacity and survival rates of Wrens troglodytes troglodytes and Treecreepers Certhia familiaris in a Nottinghamshire wood. Ibis 137: 497–507. doi: 10.1111/j.1474-919X.1995.tb03259.x
- Pearce-Higgins, J.W., Beale, C.M., Oliver, T.H., August, T.A., Carroll, M., Massimino, D., Ockendon, N., Savage, J., Wheatley, C.J., Ausden, M.A., Bradbury, R.B., Duffield, S.J., Macgregor, N.A., McClean, C.J., Morecroft, M.D., Thomas, C.D., Watts, O., Beckmann, B.C., Fox, R., Roy, H.E., Sutton, P.G., Walker, K.J. & Crick, H.Q.P. 2017. A national-scale assessment of climate change impacts on species: assessing the balance of risks and opportunities for multiple taxa. Biol. Conserv. 213: 124–134. doi: 10.1016/j.biocon.2017.06.035
- Pearce-Higgins, J.W., Dennis, P., Whittingham, M.J. & Yalden, D.W. 2010. Impacts of climate on prey abundance account for fluctuations in a population of a northern wader at the southern edge of its range. Glb. Chg. Bio. 16: 12–23. doi: 10.1111/j.1365-2486.2009.01883.x
- Pearce-Higgins, J.W., Eglington, S.M., Martay, B. & Chamberlain, D.E. 2015. Drivers of climate change impacts on bird communities. J. Anim. Ecol. 84: 943–954. doi: 10.1111/1365-2656.12364
- Pearce-Higgins, J.W. & Green, R.E. 2014. Birds and Climate Change: Impacts and Conservation Responses. Cambridge University Press, Cambridge.
- Pearce-Higgins, J.W., Ockendon, N., Baker, D.J., Carr, J., White, E.C., Almond, R.E.A., Amano, T., Bertram, E., Bradbury, R.B., Bradley, C., Butchart, S.H.M., Doswald, N., Foden, W., Gill, D.J.C., Green, R.E., Sutherland, W.J. & Tanner, E.V.J. 2015. Geographical variation in species’ population responses to changes in temperature and precipitation. Proc. Royal Soc., Ser. B. 282: 20151561. doi: 10.1098/rspb.2015.1561
- Renwick, A.R., Massimino, D., Newson, S.E., Chamberlain, D.E., Pearce-Higgins, J.W. & Johnston, A. 2012. Modeling changes in species’ abundance in response to projected climate change. Divers. Distrib. 18: 121–132. doi: 10.1111/j.1472-4642.2011.00827.x
- Robinson, R.A., Baillie, S.R. & Crick, H.Q.P. 2007. Weather-dependent survival: implications of climate change for passerine population processes. Ibis 149: 357–364. doi: 10.1111/j.1474-919X.2006.00648.x
- Robinson, R.A., Baillie, S.R. & King, R. 2012. Population processes in European Blackbirds Turdus merula: a state-space approach. J Ornith 152(S2): 419–433. doi: 10.1007/s10336-010-0612-y
- Robinson, R.A., Morrison, C.A., Baillie, S.R. & Francis, C. 2014. Integrating demographic data: towards a framework for monitoring wildlife populations at large spatial scales. Methods Ecol Evol 5: 1361–1372. doi: 10.1111/2041-210X.12204
- Sims M., Elston D.A., Larkham A., Nussey D.H. & Albon S.D. 2007. Identifying when weather influences life history traits of grazing herbivores. J. Anim. Ecol. 76:761–770. doi: 10.1111/j.1365-2656.2007.01251.x
- Small-Lorenz, S.L., Culp, L.A., Brandt Ryder, T., Will, T.C. & Marra, P.P. 2013. A blind spot in climate change vulnerability assessments. Nat. Clim. Change 3: 91–93. doi: 10.1038/nclimate1810
- Stephens, P.A., Mason, L.R., Green, R.E., Gregory, R.D., Sauer, J.R., Alison, J., Aunins, A., Brotons, L., Butchart, S.H.M., Campedelli, T., Chodkiewicz, T., Chylarecki, P., Crowe, O., Elts, J., Escandell, V., Foppen, R.P.B., Heldbjerg, H., Herrando, S., Husby, M., Jiguet, F., Lehikoinen, A., Linström, A., Noble, D.G., Praquet, J.-Y., Reif, J., Sattler, T., Szép, T., Teufelbauer, N., Trautmann, S., van Strien, A.J., van Turnhout, C.A.M., Vorisek, P. & Willis, S.G. 2016. Consistent response of bird populations to climate change on two continents. Science 352: 84–87. doi: 10.1126/science.aac4858
- Sullivan, M.J.P., Newson, S.E., Pearce-Higgins, J.W. & Rutz, C. 2016. Changing densities of generalist species underlie apparent homogenization of UK bird communities. Ibis 158: 645–655 doi: 10.1111/ibi.12370
- Suggitt, A.J., Platts, P.J., Barata, I.M., Bennie, J.J., Burgess, M.D., Bystraikova, N., Duffield, S., Ewing, S.R., Gillingham, P.K., Harper, A., Hartley, A.J., Hemming, D.L., Maclean, I.M.D., Maltbym K., Marshall, H.H., Morecroft, M.D., Pearce-Higgins, J.W., Pearce-Kelly, P., Phillimore, A.B., Price, J.T., Pyke, A., Steward, J.E., Warren, R. & Hill, J.K. 2017. Conducting robust ecological analyses with climate data. Oikos 126: 1533–1541. doi: 10.1111/oik.04203
- Thackeray, S.J., Henrys, P.A, Hemming, D., Bell, J.R., Botham, M.S., Burthe, S., Halaouet, P., Johns, D.G., Jones, I.D., Leech, D.I., Mackay, E.B., Massimino, D., Atkinson, S., Bacon, P.J., Brereton, T.M., Carvalho, L., Clutton-Brock, T.H., Duck, C., Edwards, M., Elliott, J.M., Hall, S.J.G., Harrington, R., Pearce-Higgins, J.W., Hoye, T.T., Kruuk, L.E.B., Pemberton, J.M., Sparks, T.H., Thompson, P.M., White, I., Winfield, I.J. & Wanless, S. 2016. Phenological sensitivity to climate across taxa and trophic levels. Nature 535: 241–245. doi: 10.1038/nature18608
- Thackeray, S.J., Sparks, T.H., Frederiksen, M., Burthe, S., Bacon, P.J., Bell, J.R., Botham, M.S., Brereton, T.M., Bright, P.W., Carvalho, L.C., Clutton-Brock, T., Dawson, A., Edwards, M., Elliott, M., Harrington, R., Johns, D., Jones, I.D., Jones, J.T., Leech, D.I., Roy, D.B., Scott, W.A., Smith, M., Smithers, R.J., Winfield, I.J. & Wanless, S. 2010. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glb. Chg. Bio. 16: 3304–3313. doi: 10.1111/j.1365-2486.2010.02165.x
- Thaxter, C., Joys, A., Gregory, R., Baillie, S. & Noble, D. 2010. Hypotheses to explain patterns of population change among breeding bird species in England. Biol. Conserv. 143: 2006–2019. doi: 10.1016/j.biocon.2010.05.004
- Wernham, C.V., Toms, M.P., Marchant, J.H., Clark, J.A., Siriwardena, G.M., Baillie, S.R. (Eds.). 2002. The Migration Atlas: Movements of the Birds of Britain and Ireland. T&AD Poyser, London.
- Wheatley, C.J., Beale, C.M., Bradbury, R.B., Pearce-Higgins, J.W., Critchlow, R., & Thomas, C.D. 2017. Climate change vulnerability for species – assessing the assessments. Glb. Chg. Bio. 23: 3704–3715. doi: 10.1111/gcb.13759
