ABSTRACT
Capsule: Local micro-climate, as well as habitat, influences the abundance of birds within dynamically changing upland habitats.
Aims: To identify the relative importance of near-ground temperature (as an index of climate at a scale typical of the territory of breeding passerines) and habitat for some breeding birds in upland Scotland where land uses and habitats are changing.
Methods: Timed point counts sampled breeding birds within the marginal uplands of Scotland in habitats at successional stages between pasture and shrubland. Habitat composition and ambient temperatures were recorded at the same sampling points. Generalized linear mixed models were used to assess associations of bird abundance, species richness and species diversity with habitat and ambient near-ground temperature.
Results: Species richness increased with mean temperature, was greatest within areas of shrubland and was lowest in open habitats. Different species showed individual associations with temperature and habitat. Species predominantly associated with shrubland tended to have a negative association with temperature.
Conclusions: Understanding the habitat and climatic niche requirements of different species will facilitate appropriate land management to be targeted within appropriate climatic patches. For example, open habitats suitable for Whinchats Saxicola rubetra should be within warmer climate patches (typically lower elevation or southern aspects) while shrublands can support Willow Warblers Phylloscopus trochilus at cooler elevations and aspects. Management that delivers a mosaic of habitats, spanning areas of climatic heterogeneity should enhance the diversity of birds supported in the upland margins and potentially their resilience to changing environmental conditions.
Changing habitats, climate and how they interact exert a marked influence on the distribution, abundance and therefore the conservation status of many birds (Fuller et al. Citation2012a, Pearce-Higgins & Green Citation2014). In recent decades, documented range changes by birds in Britain have been predominantly northwards, consistent with predictions for a warming climate (Gillings et al. Citation2015, Massimino et al. Citation2015). Such changes are projected to continue (Massimino et al. Citation2017). As a result of these changes, numbers of some bird species that have declined elsewhere are stable or increasing in the north and west of Britain (Balmer et al. Citation2013, Morrison et al. Citation2013a). However, the north and west of Britain are distinguished from other areas not only by their latitude and longitude but also by their topography and habitat; they are more mountainous (with greater topographical variation), and have more extensive areas of semi-natural grasslands and moorlands (Thompson et al. Citation1995, Grant & Pearce-Higgins Citation2012). In particular, topographically varied landscapes have highly heterogeneous ‘climate patches’ arising from variation in elevation and aspect over relatively small spatial scales. For example, temperature decreases both with increasing elevation and more poleward-facing aspects (Franklin et al. Citation2013). Influences of topography and associated fine-scale variations in climate on distribution have been identified or implied for different taxa (Davies et al. Citation2006, Bennie et al. Citation2008, Bradbury et al. Citation2011, Calladine & Bray Citation2012, Maclean et al. Citation2015). It is, therefore, plausible that the availability of different climate patches at scales that are within expected dispersal ranges for many birds (even, in some situations, within the home ranges of individual birds) could be among the poorly understood factors influencing species distribution (Baker et al. Citation2017) and contributing to the resilience of some species within topographically variable landscapes as found in the north and west of Britain. The combination of habitat differences and finer scale heterogeneity in climatic variation has the potential to mask or exaggerate some of the apparent latitudinal trends and shifts in distribution by some birds.
Within Britain, upland grasslands and moorlands are subject to considerable and ongoing land use changes arising from altered grazing pressure from both domestic and wild herbivores (Fuller & Gough Citation1999, Evans et al. Citation2006, Thomson Citation2011) and the expansion of forests and woodlands (Calladine et al. Citation2018, Forest Research Citation2018, Thomas et al. Citation2019). In recent decades, this has led to the development of large areas of shrub woodland (or shrublands), as early young growth stages of plantations (Calladine et al. Citation2015, Citation2017) or early-succession, naturally regenerating forests (Fuller et al. Citation1999, Edwards Citation2005). Shrublands can support high densities of some bird species (Fuller Citation2012b) including some of those exhibiting more positive population trends in the upland north and west of Britain. An improved understanding of the opportunities and threats associated with changing land use in the uplands, and how they interact with small and meso-scale climatic variation, would benefit conservation and land use policies (Chamberlain et al. Citation2013, Brambilla et al. Citation2017, Jänig et al. Citation2018).
The objectives of this study were to identify the relative importance of temperature (as an index of climate at a scale typical of the territory of breeding passerines) and habitat for some breeding birds in upland Scotland, where land uses and habitats are changing. If species were found to have preferences for particular climate patches as well as habitats, then it would be possible to facilitate finer scale management of habitats within landscapes to enhance their suitability for conservation priority species by the targeting of management to be within appropriate climatic patches.
Methods
Study areas
Information on birds, habitats and ambient temperature was collected from a total of 114 sampling points across seven study areas in central and southern Scotland in 2017 and 2018 (). The study areas were selected to represent contrasting and changing land uses that are typical within the Scottish uplands:
Glen Sherup – 10 sampling points (285–500 m above mean sea level (asl)), where grazing ceased in 2002 and the area was subsequently planted with broad-leaved trees to re-establish native-type woodland.
Glen Quey – 26 sampling points (250–530 m asl), grazing ceased in 2002 and the area was subsequently planted with broad-leaved trees to re-establish native-type woodland.
Menstrie Glen – 25 sampling points (125–355 m asl), grazing ceased in 2015 and the area was subsequently planted with non-native conifers to establish a commercial plantation.
Carrifran – 15 sampling points (215–370 m asl), grazing ceased in 2000 and the area was subsequently planted with broad-leaved trees to re-establish native-type woodland.
Blackhope – 8 sampling points (225–305 m asl), upland pasture grazed with sheep.
Glenrosa – 15 sampling points (15–145 m asl), grazed by wild Red Deer Cervus elaphus with some small exclosures to permit woodland regeneration.
Glenscorrosdale – 15 sampling points (110–250 m asl), grazed by sheep and wild Red Deer with some very small areas of native shrubs.
Surveys were undertaken in 2017 at Menstrie Glen (15 points) and Glen Quey and in 2018 at all other sites and the remaining 10 points at Menstrie Glen.
The study areas included three broad habitat classes: (1) open grassland or moorland with any woody vegetation restricted to dwarf ericaceous shrubs; (2) early phase of developing shrubland or woodland where developing shrub and tree species were less than 1.5 m tall and not capable of shading the field layer vegetation and (3) developed shrubland or young early successional woodland where trees and shrub species were generally more than 2 m tall and effectively shaded extensive areas of field layer vegetation ().
Table 1. Descriptive variables of habitat categories and their occurrence within the study areas sampled.
Bird surveys
Timed point counts were used to estimate breeding bird abundance within the study areas. Points were at the intersections of 200 m grids to permit representative sampling within each area while also ensuring relative independence of data collected from each point. Timed point counts (10 minutes) were undertaken twice in the breeding season (early visits were between 25 April–5 May and late visits 19 May–5 June) and most surveys were undertaken in the early mornings (92% between 04:20 and 09:30) when many bird species are most detectable (Bibby et al. Citation2000). Some later surveys were undertaken (up to 12:30) when logistics, associated with unsuitable weather, made earlier surveys impractical, but only when bird activity remained high. All birds seen and/or heard were registered and attributed to one of three distance bands (0–50 m, 50–100 m and >100 m), based on their distance from the count point when they were first detected. Birds seen or heard in flight were recorded separately and not attributed to any distance band. The exception to this rule were birds performing display flights over their breeding territories, which were recorded as if in the terrestrial distance band above which they were displaying. The 10-minute sampling interval aimed to maximize the likelihood of registering birds within the immediate vicinity while reducing the risks of counting individuals multiple times (Fuller & Langslow Citation1984, Drapeau et al. Citation1999).
Temperature and habitat recording
At each count point, a temperature logger (Thermochron ibutton) was deployed for the full breeding season (from April to July inclusive) and recorded the temperature at 4-hour intervals throughout. Loggers were strapped to the north-facing sides of wooden posts, 60 cm above ground level. Data were archived on the loggers and downloaded after the end of the season.
Habitat measures recorded for each sampling point describe both ground vegetation and the presence and structure of trees and shrubs. At each habitat sampling point, a 20 m tape was temporarily laid on the ground, centred on the point and running either north–south or east–west, the direction being selected at random for each point. At 5 m intervals along the tape, the dominant ground cover vegetation within a 1 m radius was recorded as one of seven types (Grass; Herbaceous; Bracken Pteridium aquilinum or other fern; Ericaceous dwarf shrub; Rush; Moss; Bare). The number of points (0–6) along the tape where a vegetation type was dominant was the score given to the sampling point for that ground vegetation type. At each 5 m interval along each transect, the presence of trees or shrubs directly shading the point was also recorded similarly creating an index (0–6) of shrub and tree structure and the presence of ground vegetation greater than 20 cm tall was recorded and transect scored (0–6) to provide an index of ground cover with relatively tall vegetation. Additionally, the following information was recorded along each 20 m transect: (1) the number of trees/shrubs within 5 m either side of the transect, to provide an index of tree/shrub density; (2) a list of the tree and shrub species recorded in (1); (3) the number of trees more than 10 m high within 20 m either side of the transect; (4) elevation (m asl) of the mid-point; (5) the principal facing aspect of any slope (to the nearest directional octant). Habitat cover variables were used to characterize and check consistency of the three broad habitat classes and were not used directly in the analyses.
Analyses
Poisson generalized linear mixed models (GLMMs: Proc Glimmix in SAS v9.4; SAS Institute Citation2013) were used to assess relationships between habitat and temperature variables and indices of species richness, diversity and the abundance of single species. Dependent variables in the different models were:
Species richness – the number of species recorded (over both visits) at each sampling point.
Species diversity – Simpson's index of species diversity for each sampling point (D = ((∑n(n − 1))/N(N − 1)), where n is the total number of registrations belonging to a given species, N is the total number of registrations of all species).
Bird density for individual species at each sampling point as calculated from point count data.
Indices of species diversity and abundance used the maximum counts from the two survey visits for each species at each sampling point. Simpson's index of diversity was calculated using registrations within 100 m of each sampling point. Bird density was estimated assuming a detectability function that was constant for all sites, species and sampling points which adjusts for decreased detectability in the outer distance bands using the formula D = loge(n/n2)*n/(πr2) (after Bibby et al. Citation1985), where D is the calculated bird density; n is the total number of birds detected (in this case, 0–100 m); n2 is the number outside of the distance band r (in this case, 50–100 m); r = 50 m. Deviation of the empirical data from the assumed detection function will tend to be greatest when sample sizes are small and generally return over-estimates of density. Towards correcting for overestimation, maximum densities per sampling point were held fixed at 6 birds per ha when the total number of contributing registrations was 60 or less and at 15 birds per ha when there were more than 60. The maximum densities were the estimates below which included at least 90% of all uncapped single point estimates.
Fixed variables in the models were:
The mean of all temperatures recorded from April to July, inclusive, at each sampling point.
The three broad habitat categories (Open, Early shrubland, Developed shrubland) with which each sampling point was located.
The number of trees >5 m high within 20 m of habitat recording transects associated with each sampling point.
Tall trees were not features of any particular habitat class and were found in all three () and therefore were included as a separate fixed variable within the models. Study area was included in the models as a random class effect to account for potential geographical effects and also year effects (noting that each sampling point was surveyed in one of two years only). Initial models included the interaction terms between mean temperature and habitat class but as none were statistically significant, these are excluded from model outputs reported.
To allow for a practical interpretation of the results from the above analyses, the relationships of mean temperature with elevation and aspect were examined to identify how near-ground temperatures were distributed within the sampled landscapes. After visual examination of the data, sampling points were assigned to two broad aspect categories: ‘north and east’ including the octants north, northeast, east and southeast; and ‘south and west’ including northwest, west, southwest and south. A normal generalized linear model (GLM: Proc Genmod in SAS v9.4) was used to assess the associations of mean temperature (the dependent variable) with elevation (a continuous variable), aspect (n = 2 classes), habitat (n = 3 classes) and their two- and three-way interaction terms.
Results
A total of 61 species was recorded from the sampling points during the surveys, of which 49 were eligible for inclusion in indices of species richness and diversity. Excluded species were those recorded exclusively at distances of more than 100 m from the sampling points or in flight. Species richness was positively associated with mean temperature and with the number of taller trees. Species richness was also greatest in developed shrubland and least in open habitats. There was a non-significant positive association between Simpson's index of diversity and mean temperature ().
Table 2. Model outputs for fixed effects from GLMMs that examine associations between habitat and temperature variables with bird species richness, diversity and the abundance of individual species. Statistically significant associations are shown in bold. The probability of marginally non-significant (0.05 > P < 0.10) associations and there mean effect estimates are also shown. No interaction terms were significant or marginally non-significant and are omitted from the table. Mean and 95% confidence intervals for indices of diversity and abundance are presented for each of the three habitat categories and are derived by back-transformation of the least square means from the GLMMs.
Twelve species were sufficiently numerous to permit convergence of models examining associations with bird density for individual species (). Six species showed a negative association with mean temperature (Tree Pipit, Meadow Pipit, Wren, Willow Warbler, Lesser Redpoll and Reed Bunting: scientific names are included in ) and two showed a positive association (Eurasian Skylark and Whinchat).
Four species (Wren, Willow Warbler, Great Tit and Chaffinch) were positively associated with the presence of relatively tall trees, and three (Eurasian Skylark, Tree Pipit and Meadow Pipit) were negatively associated. Seven species tended to be more abundant within increasingly developed shrubland compared to open habitats (Tree Pipit, Whitethroat, Willow Warbler, Great Tit, Chaffinch, Lesser Redpoll and Reed Bunting) and just one tended to be more abundant in open habitats (Eurasian Skylark). Whinchat and Song Thrush tended to be more abundant in early developing shrubland than in developed shrubland. Wren tended to be most abundant in open habitats and least abundant in early developing shrubland.
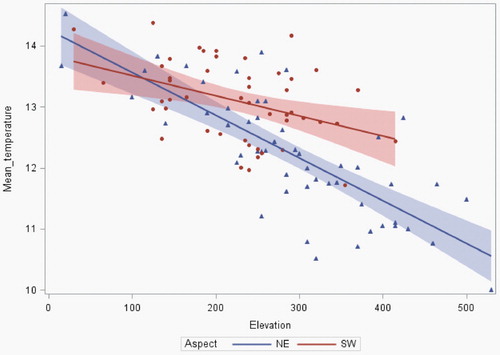
The mean of the mean temperatures recorded at the sampling points between April and July inclusive was 12.6°C (range 10.0–14.5°C). Mean temperature was negatively associated with elevation (χ2 = 46.4, P < 0.001) and the nature of that relationship differed between aspects (aspect × elevation interaction term Χ2 = 7.31, P = 0.006). There was no significant association with habitat class (χ2 = 2.34, df = 2, P = 0.31) and all interaction terms including habitat were not significant. Although the mean temperature was similar at lower altitudes between the two aspect classes, the rate of decline generally diverged above about 200 m asl with ‘north and east’ aspects being cooler than ‘south and west’ aspects ().
Figure 2. Mean near-ground temperature (April to July inclusive) recorded at sampling points at different elevations within study areas across southern Scotland. The fitted linear regression lines (and associated confidence intervals) are plotted for points on slopes with North and East aspects and with South and West aspects.
Discussion
Species richness and diversity within dynamically changing habitats of the upland margins tended to increase with mean near-ground temperature. However, bird density for all species that were associated with shrubland habitats, and were sufficiently numerous to model the association, tended to be less abundant in relatively warm areas. Species predominantly associated with open habitats showed both positive and negative associations with near-ground temperature. Presumably, temperature interacts with habitats and their structure to influence food availability (Kurht et al. Citation2006) and potentially other determinants of species occurrence. Species richness was greatest in the more developed shrubland sampled and least in the most open habitats.
Seral development from open habitats through woodlands will inevitably be associated with changes in bird assemblages. Within the uplands (and their margins) of Scotland, this typically involves birds of open grassland and moorland (cultural plagio-climax habitats; Brooker Citation2011) being progressively replaced first by shrubland species and then, where conditions permit, by forest birds (Fuller et al. Citation1999, Fuller Citation2012b). In this study, examples of such change are illustrated by reduced densities of Eurasian Skylark and increased densities of Willow Warbler in more developed shrubland (). Comparable shifts in semi-natural open habitats and associated avifauna resulting from changes in grazing management and/or climate, have also been described in ecotone habitats between mountain timberlines and treelines (Chamberlain et al. Citation2013, Jänig et al. Citation2018). In general, species density will be positively related to factors that increase the availability of ecological niches, such as the occurrence of shrubs and taller trees in the present study. However, some species or assemblages that are considered conservation priorities are supported by non-climax stages (Fuller Citation2012b) and the maintenance of conditions for such species may require intervention by land managers (Pearce-Higgins et al. Citation2007, Chamberlain et al. Citation2013). Understanding of habitat requirements and the climatic conditions that best suit target species will improve effectiveness of management in delivering conditions to benefit those species or assemblages. For example, habitats and structures favoured by a target species could be established or managed for within areas where micro-climatic conditions are also best suited to those species. Among the six species with a negative association with near-ground temperature, five are listed as birds of conservation concern in Britain, based on reductions in range and abundance (Tree Pipit, Meadow Pipit, Willow Warbler, Lesser Redpoll and Reed Bunting; Eaton et al. Citation2015) of which only one (Meadow Pipit) was not associated with developing shrubland (). Tree Pipit, Willow Warbler and Lesser Redpoll are also species having shown marked contrast in population trends with latitude across Britain having increased in the northern uplands contrasting with decreases in the southern lowlands (Balmer et al. Citation2013). Northward shifts in distribution are consistent with predictions for a warming climate (Morrison et al. Citation2013a, Massimino et al. Citation2017), however in Britain, it is also consistent with preferences cooler elevations and aspects (as found within undulating and mountainous landscapes) and for some species with an increased availability of shrubland habitats (Morrison et al. Citation2013a, Gillings et al. Citation2015). Species-specific examples can illustrate the point.
Whinchat and Eurasian Skylark were the two species positively associated with mean temperature (). Both are listed as a species of conservation concern (Eaton et al. Citation2015) and both are associated with open habitats and were formerly widespread in southern and lowland Britain where their decline was associated with agricultural intensification (Chamberlain & Crick Citation1999, Henderson et al. Citation2014). A retreat to upland marginal habitats is perhaps most marked for Whinchats where inhospitable conditions at higher elevations and exposed aspects limit opportunities for the species (Calladine & Bray Citation2012). A negative association with temperature is consistent with a squeezing of suitable niches within their upland refugia.
Changing land use and climate change are together leading to encroachment by woodland onto open and upland habitats globally (Myers-Smith et al. Citation2011, Stevens et al. Citation2016, Mollet et al. Citation2018, Calladine et al. Citation2018). This presents a threat to species reliant on open habitats but also creates potential opportunities for shrub and forest birds. The creation or expansion of suitable habitats within areas that are (or could be in the future) within suitable climate envelopes has been advocated as a means of countering range losses due to changing climates (Vos et al. Citation2008). Contrasting trends for breeding Willow Warbler within Britain (Morrison et al. Citation2010, Balmer et al. Citation2013) appear to be a non-deliberate example of such mitigation; expansion of shrubland and woodland has likely contributed to population increases in the northern uplands, potentially compensating for losses in the southern lowlands. However, the more varied topography of northern areas of Britain will mean that suitable habitat and climatic conditions suitable for some species may overlap in relatively small and scattered patches; mean breeding season temperatures varied by 4.5°C within the study areas reported here. Habitat diversity can contribute to the resilience of areas to support species and communities (Both Citation2012) and it is likely that micro-climate heterogeneity will also contribute to that resilience. Promoting habitat heterogeneity in areas of climatic heterogeneity could therefore be a conservation strategy that enhances the diversity and persistence of birds supported in the upland margins.
Some species will require more targeted management that focuses on specific requirements within appropriate climatic patches. For example, more open habitats for Whinchats would be more suitable if maintained within warmer areas, at lower elevations and with more southern aspects. Shrublands and woodlands for Willow Warblers would be most appropriate at relatively higher elevations and more northern aspects. Understanding how climate patches are determined by topography () would facilitate spatial targeting of management (e.g. grazing or its exclusion and tree planting) to deliver and maintain habitats within appropriate climatic conditions for target species.
Assemblages of shrubland birds in upland Scotland tend to be less diverse than those from further south (Gillings et al. Citation1998), a latitudinal gradient also apparent across Europe (Mikusiński et al. Citation2018), and it follows that the relationships found in our study areas would not necessarily relate directly to elsewhere. There is a need to better understand how habitats, land uses, topography and climate interact to determine species distributions and how such mechanisms operate in different areas. Further work identifying mechanisms for these associations and how they translate to breeding success (Ockenden et al. Citation2013), migration strategies (Morrison et al. Citation2013a, Citation2013b, Hewson et al. Citation2016) and survival would help further refine options for conservation management.
Acknowledgements
We are grateful to the individuals and organizations who facilitated access to study areas; The Woodland Trust, Tillhill Forestry, The Borders Forest Trust, National Trust for Scotland, John Barker, John Savory and Terry Southall. The manuscript was improved by comments from two anonymous referees.
Additional information
Funding
References
- Baker, D.J., Hartley, A.J., Pearce-Higgins, J.W., Jones, R.G. & Willis, S.G. 2017. Neglected issues in using weather and climate information in ecology and biogeography. Divers. Distrib. 23: 329–340. doi: 10.1111/ddi.12527
- Balmer, D., Gillings, S., Caffrey, B., Swann, B., Downie, I. & Fuller, R.J. 2013. Bird Atlas 2007–11: the breeding and wintering birds of Britain and Ireland. BTO, Thetford.
- Bennie, J., Huntley, B., Wiltshire, A., Hill, M.O. & Baxter, R. 2008. Slope, aspect and climate: spatially explicit and implicit models of topographic microclimate in chalk grassland. Ecol. Modell. 216: 47–59. doi: 10.1016/j.ecolmodel.2008.04.010
- Bibby, C.J., Phillips, B.N. & Seddon, A.J.E. 1985. Birds of restocked conifer plantations in Wales. J. Appl. Ecol. 22: 619–633.
- Bibby, C.J., Burgess, N.D., Hill, D.A. & Mustoe, S. 2000. Bird Census Techniques. Academic Press, London.
- Both, C. 2012. Insufficient adaptation to climate change alters avian habitat quality and thereby changes habitat selection. In Fuller, R.J. (ed) Birds and Habitat: relationships in changing landscapes, 432–452. Cambridge University Press, Cambridge.
- Bradbury, R.B., Pearce-Higgins, J.W., Wotton, S.R., Conway, G.J. & Grice, P.V. 2011. The influence of climate and topography in patterns of territory establishment in a range-expanding bird. Ibis 153: 336–344. doi: 10.1111/j.1474-919X.2011.01106.x
- Brambilla, M., Caprio, E., Assandri, G., Scridel, D., Bassi, E., Bionda, R., Celada, C., Falco, R., Bogliani, G., Pedrini, P., Rolando, A. & Chamberlain, D. 2017. A spatially explicit definition of conservation priorities according to population resistance and resilience, species importance and level of threat in a changing climate. Biodivers. Res. 23: 727–738.
- Brooker, R. 2011. The changing nature of Scotland’s uplands – an interplay of processes and timescales. In Mars, S.J., Foster, S., Hendrie, C., Mackey, E.C. & Thompson, D.B.A. (eds) The Changing Nature of Scotland, 381–396. TSO, Edinburgh.
- Calladine, J. & Bray, J. 2012. The importance of altitude and aspect for breeding Whinchats Saxicola rubetra in the uplands: limitations of the uplands as a refuge for a declining, formerly widespread species? Bird Study 59: 43–51. doi: 10.1080/00063657.2011.623767
- Calladine, J., Bray, J., Broome, A. & Fuller, R.J. 2015. Comparison of breeding bird assemblages in conifer plantations managed by continuous cover forestry and clearfelling. For. Ecol. Manage. 344: 20–29. doi: 10.1016/j.foreco.2015.02.017
- Calladine, J., Jarrett, D., Wilson, M. & Edwards, C. 2017. Stand structure and breeding birds in managed Scots pine forests: some likely long-term implications for continuous cover forestry. For. Ecol. Manage. 397: 174–184.
- Calladine, J., Díaz, M., Reino, L., Jardine, D. & Wilson, M. 2018. Plantations of non-native tree species. In Mikusiński, G., Roberge, J.M. & Fuller, R.J. (eds) Ecology and Conservation of Forest Birds, 350–386. Cambridge University Press, Cambridge. doi: 10.1017/9781139680363.013
- Chamberlain, D.E. & Crick, H.Q.P. 1999. Population declines and reproductive performance in skylarks Alauda arvensis in different regions and habitats of the United Kingdom. Ibis 141: 38–51. doi: 10.1111/j.1474-919X.1999.tb04261.x
- Chamberlain, D.E., Negro, M., Caprio, E. & Rolando, A. 2013. Assessing the sensitivity of alpine birds to potential future changes in habitat and climate to inform management strategies. Biol. Conserv. 167: 127–135. doi: 10.1016/j.biocon.2013.07.036
- Davies, Z.G., Wilson, R.J., Coles, S. & Thomas, C.D. 2006.Changing habitat associations of a thermally constrained species, the silver spotted skipper butterfly, in response to climate warming. J. Anim. Ecol. 75: 247–256. doi: 10.1111/j.1365-2656.2006.01044.x
- Drapeau, P., Bergeron, Y. & Harvey, B. 1999. Refining the use of point counts at the scale of individual points in studies of bird-habitat relationships. J. Avian Biol. 30: 367–382. doi: 10.2307/3677009
- Eaton, M.A., Aebischer, N.J., Brown, A.F., Hearn, R.D., Lock, L., Musgrove, A.J., Noble, D.G., Stroud, D.A. and Gregory, R.D. 2015 Birds of conservation concern 4: the population status of birds in the United Kingdom, Channel Islands and Isle of Man. Br. Birds 108: 708–746.
- Edwards, M.E. 2005. Landscape history and biodiversity conservation in the uplands of Norway and Britain: comparisons and contradictions. In Thompson, D.B.A., Price, M.F & Galbraith, C.A. (eds) Mountains of Northern Europe: conservation, management, people and nature, 163–178. TSO Scotland, Edinburgh.
- Evans, D.M., Redpath, S.M., Evans, S.A., Elston, D.A., Gardner, C.J., Dennis, P. & Pakeman, R.J. 2006. Low intensity, mixed livestock grazing improves the breeding abundance of a common insectivorous passerine. Biol. Lett. 2: 636–638. doi: 10.1098/rsbl.2006.0543
- Forest Research. 2018. Woodland Area, Planting and Publicly Funded Restocking. Forest Research, Edinburgh.
- Franklin, J., Davis, F.W., Ikegami, M., Syphard, A.D., Lorraine, E.F., Flint, A.L. & Hannah, L. 2013. Modelling plant species distributions under future climates: how fine scale do climate projections need to be? Glb. Chg. Bio. 19: 473–483. doi: 10.1111/gcb.12051
- Fuller, R.J. (ed) 2012a. Birds and Habitat: relationships in changing landscapes. Cambridge University Press, Cambridge.
- Fuller, R.J. 2012b. Avian responses to transitional habitats in temperate cultural landscapes: woodland edges and young growth. In Fuller, R.J. (ed) Birds and Habitat: relationships in changing landscapes, 125–149. Cambridge University Press, Cambridge.
- Fuller, R.J. & Gough, S.J. 1999. Changes in sheep numbers in Britain: implications for bird populations. Biol. Conserv. 91: 73–89. doi: 10.1016/S0006-3207(99)00039-7
- Fuller, R.J. & Langslow, D.R. 1984. Estimating numbers of birds by point counts: how long should counts last? Bird Study 31: 195–202. doi: 10.1080/00063658409476841
- Fuller, R.J., Gillings, S. & Whitfield, D.P. 1999. Responses of breeding birds to expansion of scrub in the eastern Scottish Highlands: preliminary implications for conservation strategies. Vogelwelt 120: 53–62.
- Gillings, S., Fuller, R.J. & Henderson, A.C.B. 1998. Avian community composition and patterns of bird distribution within birch-heath mosaics in north-east Scotland. Ornis Fenn. 75: 27–37.
- Gillings, S., Balmer, D.E. & Fuller, R.J. 2015. Directionality of recent bird distribution shifts and climate change in Great Britain. Glb. Chg. Bio. 21: 2155–2168. doi: 10.1111/gcb.12823
- Grant, M.C. & Pearce-Higgins, J.W. 2012. Spatial variation and habitat relationships in moorland bird assemblages: a British perspective. In Fuller, R.J. (ed) 2012. Birds and Habitat: relationships in changing landscapes, 207–236. Cambridge University Press, Cambridge.
- Henderson, I., Calladine, J., Massimino, D., Taylor, J. & Gillings, S. 2014. Evidence for contrasting causes of population change in two closely related, sympatric breeding species the Whinchat Saxicola rubetra and Stonechat Saxicola torquata in Britain. Bird Study 61: 553–565. doi: 10.1080/00063657.2014.962482
- Hewson, C.M., Thorup, K., Pearce-Higgins, J.W. & Atkinson, P.W. 2016. Population decline is linked to migration route in the Common Cuckoo, a long-distance nocturnally-migrating bird. Nat. Commun. 7: 12296. doi: 10.1038/ncomms12296
- Jänig, J., Alba, R., Vallino, C., Rosselli, D., Pittarello, M., Rolando, A. & Chamberlain, D. 2018. The contribution of broadscale and finescale habitat structure to the distribution and diversity of birds in an Alpine forest-shrub. J. Ornithol. 159: 747–759. doi: 10.1007/s10336-018-1549-9
- Kuhrt, U., Samietz, J. & Dorn, J. 2006. Effect of plant architecture and hail nets on temperature of codling moth habitats in apple orchards. Entomol. Exp. Appl. 118: 245–259. doi: 10.1111/j.1570-7458.2006.00385.x
- Maclean, I.M.D., Hopkins, J.J., Bennie, J., Lawson, C.R. & Wilson, R.J. 2015. Microclimates buffer the responses of plant communities to climate change. Glob. Ecol. Biogeogr. 24: 1340–1350. doi: 10.1111/geb.12359
- Massimino, D., Johnston, A., Pearce-Higgins, J.W. 2015. The geographical range of British birds expands during 15 years of warming. Bird Study 62: 523–534. doi: 10.1080/00063657.2015.1089835
- Massimino, D., Johnston, A., Gillings, S., Jiguet, F. & Pearce-Higgins, J.W. 2017. Projected reductions in climatic suitability for vulnerable British birds. Clim. Change 145: 117–130. doi: 10.1007/s10584-017-2081-2
- Mikusiński, G., Villero, D., Herrando, S. & Brotons, L. 2018. Macroecological patterns in forest bird diversity in Europe. In Mikusiński, G., Roberge, J.M. & Fuller, R.J. (eds) Ecology and Conservation of Forest Birds, 137–164. Cambridge University Press, Cambridge.
- Mollet, P., Bollmann, K., Braunisch, V. & Artellaz, R. 2018. Subalpine coniferous forests of Europe. In Mikusiński, G., Roberge, J.M. & Fuller, R.J. (eds) Ecology and Conservation of Forest Birds, 231–252. Cambridge University Press, Cambridge.
- Morrison, C.A., Robinson, R.A., Clark, J.A., & Gill, J.A. 2010. Spatial and temporal variability on population trends of a long-distance migratory bird. Divers. Distrib. 16: 620–627. doi: 10.1111/j.1472-4642.2010.00663.x
- Morrison, C.A., Robinson, R.A., Clark, J.A., Risely, K. & Gill, J.A. 2013a. Recent population declines in afro-palearctic migratory birds: the influence of breeding and non-breeding season. Divers. Distrib. 19: 1051–1058. doi: 10.1111/ddi.12084
- Morrison, C.A., Robinson, R.A., Clark, J.A., Marca, A.D., Newton, J. & Gill, J.A. 2013b. Using stable isotopes to link breeding population trends to winter ecology in Willow Warblers, Phylloscopus trochilus. Bird Study 60: 211–220. doi: 10.1080/00063657.2013.767773
- Myers-Smith, I.H., Forbes, B.C, Wilmking, M., Hallinger, M., Lantz, T., Blok, D., Tape, K.D., Macias-Fauria, M., Sass-Klaassen, U., Levesque, E., Boudreau, S., Ropars, P., Hermanutz, L., Trant, A., Collier, L.S., Weijers, S., Rozema, J., Rayback, S.A., Schmidt, N.M., Schaepman-Strub, G., Wipf, S., Rixen, C., Ménard, C.B., Venn, S., Goetz, S., Andreu-Hayles, L., Elmendorf, S., Ravolainen, V., Welker, J., Grogan, P., Epstein, H.E. & Hik, D.S. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ. Res. Lett. 6: 045509. doi: 10.1088/1748-9326/6/4/045509
- Ockenden, N., Leech, D. & Pearce-Higgins, J.W. 2013. Climatic effects on breeding grounds are more important drivers of breeding phenology in migrant birds than carry-over effects from wintering grounds. Biol. Lett. 9: 20130669. doi: 10.1098/rsbl.2013.0669
- Pearce-Higgins, J.W. & Green, R.E. 2014. Birds and Climate Change: impacts and conservation responses. Cambridge University Press, Cambridge.
- Pearce-Higgins, J.W., Grant, M.C., Robinson, M.C. & Haysom, S.L. 2007. The role of forest maturation causing the decline of Black Grouse Tetrao tetrix. Ibis 149: 143–155. doi: 10.1111/j.1474-919X.2006.00623.x
- SAS Institute 2013. SAS Version 9.4. Cary, NC.
- Stevens, N., Erasmus, B. F., Archibald, S., & Bond, W. J. 2016. Woody encroachment over 70 years in South African savannahs: overgrazing, global change or extinction aftershock? Philos. Trans. R. Soc., B 371: 20150437. doi: 10.1098/rstb.2015.0437
- Thomas, H.J.D., Paterson, J.S., Metzger, M.J. & Sing, L. 2019. An evaluation of Scottish woodland grant schemes using site suitability modelling. Land Use Policy. 80: 309–317. doi: 10.1016/j.landusepol.2016.03.030
- Thompson, D.B.A., MacDonald, A.J., Marsden, J.H. & Galbraith, C.A. 1995. Upland heather moorland in Great Britain: A review of international importance, vegetation change and some objectives for nature conservation. Biol. Conserv. 71: 163–178. doi: 10.1016/0006-3207(94)00043-P
- Thomson, S. 2011. Response from the Hills: Business as Usual or a Turning Point? Scottish Agricultural College, Edinburgh.
- Vos, C.C., Berry, P., Opdam, P., Baveco, H., Nijhof, B., O’Hanley, J., Bell, C. & Kuipers, H. 2008. Adapting landscapes to climate change: examples of climate-proof ecosystem networks and priority adaptation zones. J. Appl. Ecol. 45: 1722–1731. doi: 10.1111/j.1365-2664.2008.01569.x