ABSTRACT
Capsule: The existence of clinal variation in the colour polymorphism of the Booted Eagle Hieraaetus pennatus in its breeding area in the Palaearctic is probably caused by the influence of precipitation and the detectability of the two morphs in different light conditions.
Aims: To test whether Booted Eagles shows clinal variation in colour polymorphism along its breeding range in the Palaearctic and to test if there was selective or/and environmental pressure in the polymorphism throughout its breeding range in the Palaearctic and South Africa.
Methods: Published data were obtained on the proportion of colour morphs of seven study populations within the Palaearctic and South Africa, as well as those of 11 populations on the Iberian Peninsula, and the variation was examined in relation to longitude, latitude, and environmental and meteorological variables.
Results: There was a strong relationship between the proportion of the dark morph and longitude from west to east. In the Palaearctic and South Africa, there was a strong positive relationship between the proportion of the dark morph and the amount of rainfall during the period of chick growth.
Conclusion: There is clinal variation in colour polymorphism in the Booted Eagle. The variation is probably maintained by disruptive selection due to climatic factors such as rain and cloud cover, which influence the detectability of the different colour morphs to their prey.
Colour polymorphism is a common phenomenon in many animal taxa and consists of the occurrence of two or more different genetically determined colour morphs in an animal population (Huxley Citation1955). It is very frequent among raptors, affecting approximately 30% of the species, and 22% of the diurnal raptors (Fowlie & Krüger Citation2003, Galeotti et al. Citation2003, Chakarov et al. Citation2011). Clinal variation can occur in the ratio of different morphs found within a polymorphic species, and this phenomenon can occur at several scales: a continental scale, with gradual longitudinal or latitudinal changes (Roulin Citation2003, Mundy Citation2005), a medium scale (Amar et al. Citation2014) or a very small scale (Wunderle Citation1981, Bourgeois et al. Citation2017). In raptors, this phenomenon has already been mentioned in previous research from decades ago (Brown & Amadon Citation1968, Cramp & Simmons Citation1980, Ferguson-Lees & Christie Citation2001) but its occurrence has been little studied and few studies have documented the relationship with biotic and environmental factors (Amar et al. Citation2014).
The causes and evolution of colour polymorphism in wild bird populations have been intensively studied, but until now these issues and their mechanisms are still poorly understood (Galeotti et al. Citation2003). Three main mechanisms have been described to explain the maintenance of colour polymorphism: apostatic, disruptive and sexual selection (Galeotti et al. Citation2003). The apostatic and disruptive selection hypotheses postulate differential foraging efficiencies for the morphs, either in the form of an avoidance image (Paulson Citation1973, Rohwer & Paulson Citation1987) or based on the advantages of foraging in different habitats and/or light conditions and preying upon different species (Furness Citation1987, Roulin & Wink Citation2004, Tate & Amar Citation2017), while the sexual selection hypothesis proposes that the maintenance of different colour morphs depends on the preferences at the time of mating (Galeotti et al. Citation2003).
However, Preston (Citation1980) argued against the apostatic selection mechanism and comparative studies, controlling for phylogeny, did not support this hypothesis to explain the maintenance of colour polymorphism in birds in general (Fowlie & Krüger Citation2003, Galeotti et al. Citation2003) or within raptors and nightjars (Galeotti & Rubolini Citation2004).
Roulin & Wink (Citation2004), in a comparative analysis focused on predator–prey relationships, suggested that polymorphic raptor species tended to prey more frequently on mammals. However, independent contrasts made by Fowlie & Krüger (Citation2003) found that species hunting mainly insects, amphibians and reptiles were more likely to be polymorphic than those that preyed upon birds and mammals. This may also indicate the importance of population size in colour polymorphism, since species that hunt small prey and have a generalist diet and wider ecological niches tend to reach larger population sizes than the species that hunt larger prey and have a more specialized diet (Newton Citation1979, Fowlie & Krüger Citation2003). The results of other authors suggest that colour polymorphism in raptors is related to larger areas of distribution and consequently with larger population sizes, wider ecological niches, strong migratory habits but is little influenced by climatic regimes (Galeotti & Rubolini Citation2004).
On the other hand, in the Black Sparrowhawk Accipiter melanoleucus Amar et al. (Citation2014) found that the high frequency of birds of the dark morph (76%), in the recently established population of the Cape Town region, South Africa, could be related to the higher rainfall during the breeding season than in the drier eastern regions where the light morph birds prevail. This phenomenon also occurs on a smaller scale, with the Bananaquits on the islands of Grenada and St. Vincent (Wunderle Citation1981), following Gloger’s rule, who predicted that dark birds should be more frequent in wet environments (with higher rainfall regimes) and that the light ones should appear more frequently in the dryer areas.
The Booted Eagle Hieraaetus pennatus is a medium-sized migratory raptor that arrives at its Palearctic breeding range from late March to early May. This species is monogamous and shows a sophisticated aerial activity in the nesting area during courtship (Cramp & Simmons Citation1980, Ferguson-Lees & Christie Citation2001). From mid-April to mid-May, females lay one or two eggs, occasionally three, and usually raise two chicks (exceptionally three) per breeding event (Cramp & Simmons Citation1980, Ferguson-Lees & Christie Citation2001). The Booted Eagle shows two discrete morphs, light and dark (Ferguson-Lees & Christie Citation2001, Génsbøl Citation2008, Forsman Citation2016), with some light brown individuals, also called ‘rufous’ morph, and classified by some authors as a different morph (Clark Citation1999, García-Dios Citation2017), which looks like a variation of the dark morph with differences in the degree of deposition of melanin in feathers, perhaps genetically determined (Bosch et al. Citation2019). In some raptor species, as in other bird taxa (Bourgeois et al. Citation2017), the colour morphs apparently follow Mendel’s inheritance pattern with a single locus and two allele system, with the allele that characterizes the light morph being dominant (Amar et al. Citation2013) or recessive (Schmutz & Schmutz Citation1981, Briggs et al. Citation2010, Chang et al. Citation2010). A recent investigation on the inheritance pattern of colour polymorphism in the Booted Eagle concluded that there was Mendelian inheritance but composed of two loci with epistasis and two alleles per locus (Bosch et al. Citation2019). However, these authors stressed the need for future studies to develop analysis using genetic markers to check the genotypes of adults and their offspring.
For decades, previous research has mentioned clinal variation in the wing length of Booted Eagles along its breeding range in the Palearctic, from west to east, which supposedly would not affect the plumage, other than a possible greater incidence of the dark morph towards the east (Brown & Amadon Citation1968, Cramp & Simmons Citation1980, Ferguson-Lees & Christie Citation2001). In this study, I tested if Booted Eagles show a clinal variation in the colour polymorphism along its breeding range in the Palearctic and also on the Iberian Peninsula. To assess if there was a selective or/and environmental pressure in the colour polymorphism, I also checked whether the colour morph ratio was related to several environmental variables, throughout its breeding range in the Palearctic and South Africa and also on the Iberian Peninsula.
Methods
Data collection
On a continental scale, data were obtained on the morph ratio of eight different groups that varied longitudinally in the Palearctic from west to east, including the average value of the Spanish populations (). The mean (±sd) sample size was 560.6 ± 1085.4 eagles, ranging from 10 western Siberian eagles to 2651 eagles of Spanish populations. The data from France and Greece were obtained from Cramp & Simmons (Citation1980) and Ferguson-Lees & Christie (Citation2001), who did not provide any sample sizes. The populations of the Russian Federation were monitored from 1996 to 2006 and the population of Ukraine from 2006 to 2013. The eagles of the Russian populations, classified as intermediate morphs by Karyakin (Citation2007), have been considered dark morphs in this study, since the so-called intermediate or ‘rufous’ morph is not universally recognized and seems to be a pale variety of the more variable dark morph (Ferguson-Lees & Christie Citation2001, Bosch et al. Citation2019). Information from South Africa was obtained from Steyn & Grobler (Citation1981) (, ).
Figure 1. Location map of the study areas in Africa and Eurasia for studied Booted Eagle populations. Spain (1), France (2), Greece (3), South Africa (4), Ukraine (5), Volga Ural (6), Western Siberia (7) and Altai Sayan (8). The percentages of the dark morph are in bold. Source: World_location_map.svg.
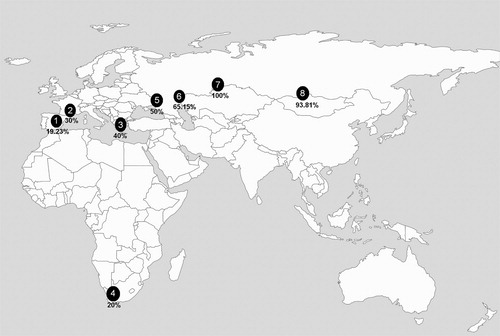
Table 1. Percentage of dark morph Booted Eagles at each study area across the Palearctic Region and South Africa. With sample size (n): * indicates reproductive birds and floaters together; ♦ only reproductive birds; + quantitative prey data available.
In addition, I obtained all the available information on the morph ratio during field work to study the biology of the species in central Catalonia from 1995 to 2017 (for more details see Bosch Citation2003, Citation2011, Bosch et al. Citation2015). I have also collected all available information on the morph ratio of 10 other populations on the Iberian Peninsula, published in research papers. The mean (±sd) sample size was 294.6 ± 224.9 eagles, ranging from 69 eagles of Plaséncia (Cáceres) to 806 eagles of the Murcia region (, ). The study periods for the Spanish populations were mainly from 1995 to 2017, except for the population of Navarre, which was studied in the 1970s and 1980s (Iribarren Citation1975, Iribarren & Rodríguez Arbeloa Citation1988).
Figure 2. Location map of the study areas for Booted Eagles in the Iberian Peninsula: Murcia district (1), Central Catalonia (2), Ports Natural Park (3), Doñana National Park (4), Ávila district (5), Tiétar valley (6), Guadarrama Sierra (7), Navarra district (8), Plaséncia (Cáceres) (9), Gredos Sierra (10) and Majorca (11). The percentages of the dark morph are in bold. Source: Stamen cartography (http://maps.stamen.com/), drawn in R version 3.5.1 (https://www.Rproject.org/) by using the rosm library (https://CRAN.Rproject.org/package=rosm).
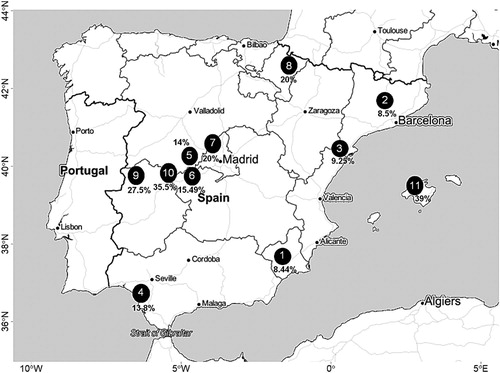
Table 2. Percentage of dark morph Booted Eagles at each study area in Spain. With sample size (n): * indicates reproductive birds and floaters together; ♦ only reproductive birds; + quantitative prey data available.
Biological data
The dates of laying were similar for all the populations studied in the Palearctic; varying from the second half of April to mid-May (Bosch Citation2003, Díaz Citation2006, Karyakin Citation2007, Moroz & Vetrov Citation2013, Baigés Citation2014, Bosch et al. Citation2015, Martínez et al. Citation2016, García-Dios Citation2017).
There were no significant differences in breeding success between pairs that exhibited different combinations in plumage colour (Martínez et al. Citation2016). Data from three populations studied in Spain suggest that there was temporal stability in the morph frequencies (Bosch et al. Citation2019).
From the data of the eagles tagged with conventional radio-tracking in Spain (Sánchez-Zapata & Calvo Citation1999, Díaz Citation2006, Martínez et al. Citation2007, Díaz & Cebollada Citation2011) and later with satellite transmitters (Bosch et al. Citation2016, López-López et al. Citation2016), Booted Eagles prefer to build their nests in forests but to forage in ecotones, agro-forestry habitats with scrub, crops and pastures, with some eagles also foraging in urban areas and close to rivers and water bodies (Carlon Citation1996, Palomino & Carrascal Citation2007, Bosch et al. Citation2016). The home ranges of these tagged eagles varied from 20 to 253 km2.
In the Russian Federation, breeding pairs preferred to settle mainly in two types of habitat: flood-land forests and river terraces surrounded by steppe pastures. Breeding density was negatively correlated with forest fragmentation and birch dominance in the canopy forest cover, and nests were preferentially placed in river valleys (Karyakin Citation2007).
In Ukraine (Donetsk and Lugansk region) breeding pairs preferentially inhabited old forests of flood-lands and forests surrounded by steppe, meadows and reservoirs (Moroz & Vetrov Citation2013).
In South Africa, Booted Eagles nested preferentially on cliffs surrounded by dry scrubland or also on hill ledges located near dry or discontinuous riverbeds (Steyn & Grobler Citation1981).
Foraging activities were carried out mainly during the central hours of the day, coinciding with the maximum air temperature and solar radiation (Bosch et al. Citation2016, López-López et al. Citation2016).
The diet of the Booted Eagle showed strong variation throughout its breeding range in the Palearctic (online supplementary Tables S1 and S2).
Environmental data
Meteorological data were obtained from the Agéncia Española de Meteorologia (AEMET), http:/www.climatemps.com and http:/www.climate-data.org (for more details see online supplementary Tables S3 and S4). The weather information on these websites covered the early 1980s to the current date, so overlapped with almost the entire period in which the data on morph ratios were collected. Meteorological data were obtained from meteorological stations within or close to the study area for each Booted Eagle study population based on location information provided by the authors of the research articles ( and ). The following independent meteorological variables were selected: the mean daily rainfall (RBreed) and the mean daily temperature (TBreed) during the breeding period, from April to August for the Palearctic populations and September to January for South Africa; and the mean daily rainfall (RChicks) and the percentage of daylight cloudy hours (Cloudy) during the period of growth of the chicks, when the eagles needed to provide the maximum number amount of prey, during June and July in the Palearctic and November and December in South Africa. Mean altitude above sea level (Alt) was included for each study area, calculated from: the altitude of each known nest of the study area, the mean altitude of the known nests of the Sierra de Guadarrama, Madrid, population (unpublished data provided by J. Díaz), the mean altitudes of the meteorological stations and at least five random points within the study areas of each of the studied Booted Eagle populations. In the populations of the Altai-Sayan region, apart from the meteorological stations, the altitudes were obtained directly from the nests mapped by Karyakin (Citation2007), using Google Earth software. Finally, as an independent explanatory variable I also included the proportion of mammals in the diet (Mammals), for the populations in which the diet was known ().
Statistical analysis
To test for clinal variation in the proportion of dark morph Booted Eagles in the Palearctic and Iberian Peninsula, general linear models (GLMs) were used to relate the proportion of dark morphs of each study population with the geographical variables (longitude and latitude). Data from South Africa were not used to test clinal variation. GLMs were also used to test the relationship between meteorological variables, altitude and the proportion of mammals in the diet with the proportion of dark morph eagles in each study area. I used simple regression models because of the low sample sizes (n = 8, for the populations of the Palearctic and South Africa; n = 11, for the Spanish populations). Previously, I tested for collinearity between the predictive variables, four of which were correlated with each other, therefore, the variables Cloudy, RBreed and TBreed were excluded from the GLM analysis, which used only RChicks.
The models were ranked using the MuMIn statistical package for their Akaike Information Criterion scores corrected for low sample sizes (AICc), where n/K < 40 (K = number of parameters estimated). The best models were selected for the differences of AICc (ΔAICc) and for the AICc weights (Wi) and also with the evidence ratio (ER) of the best model with respect to the others. All statistical analysis was performed using the R statistical package, version 3.5.2 (R Development Core Team Citation2018).
Results
The percentage of Booted Eagles of the dark morph varied throughout their range in the Palearctic from 19% in Spain to 94% in the Altai-Sayan mountains (). There was a strong positive relationship between the proportion of dark morph eagles and longitude (, ). According to the Akaike weights, the longitude had a support of 98% relative to the latitude and the null model. The morph ratio also varied latitudinally, with increasingly more dark morphs from south to north, but the evidence ratio (ER) showed 61.1 times less support with respect to longitude. The dark morph ratio of Spanish populations varied from 8.44% to 39% () but, within this region, the regression models showed no relationship between morph ratio and the longitude or latitude, with the null model being the best.
Figure 3. Scatter plot and regression line of the model showing the association between the proportion of dark morph Booted Eagles with longitude (°) in the Palearctic Region.
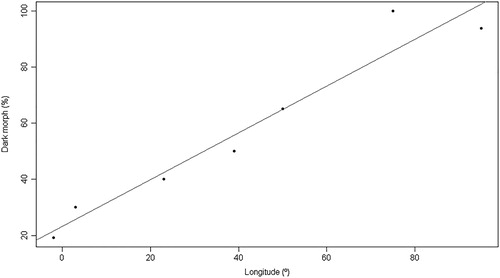
Table 3. Results of GLM analysis between the proportion of dark morph Booted Eagles and the longitude and latitude of the studied populations in the Palearctic. K = number of parameters, LogLik = Log likelihood, ΔAICc = change in AICc relative to the best model, Wi = AICc weight, ER = evidence ratio.
On a continental scale, only one of the three GLMs performed between the proportion of dark morph eagles and the predictive variables yielded significant results (). This model showed a significant positive relationship between the proportion of dark morphs and the amount of rainfall in the period of growth of the chicks ( and ). According to the Akaike weight scores, it had 83% support compared to the other models and the 95% confidence intervals of the parameter coefficients did not overlap with zero, suggesting the strong positive influence of this variable (). For the data from the Iberian Peninsula, the models did not show a significant relationship between the proportion of dark eagles and the meteorological variables, the altitude and the proportion of mammals in the diet.
Figure 4. Scatter plot and regression line of the best model showing the association between the proportion of dark morph Booted Eagles with the amount of rainfall during the period of growth of chicks for the Palearctic and South African populations.
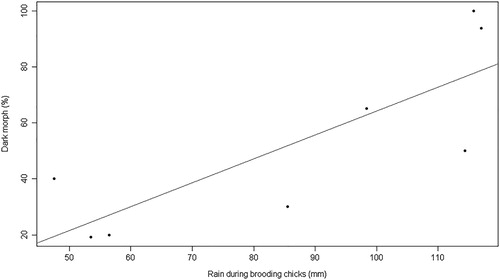
Table 4. Results of GLM analysis between the proportion of dark morph Booted Eagles and environmental variables in the Palearctic Region and South Africa: RChicks = amount of rainfall during the period of growth of chicks (June/July and November/December in the Palearctic and South Africa respectively), Mammals = % of mammals in diet. K = number of parameters, LogLik = Log likelihood, ΔAICc = change in AICc relative to the best model, Wi = AICc weight, ER = evidence ratio.
Discussion
The results show a strong positive relationship between the proportion of dark morph Booted Eagles within a population and the longitude, giving clear evidence for the existence of a cline along the breeding range in the Palearctic. Contrarily, in the Iberian Peninsula, where the proportion of the dark morph varies from 8.44 to 39%, no such relationship was found between the proportion of eagles of the dark morph and the longitude, nor with the latitude.
The results also show a significant relationship between the proportion of dark morph Booted Eagles within a population and the amount of rainfall during the growth period of chicks. This could be an adaptive response of this species to the increase of rainfall, following Gloger’s rule (Gloger Citation1833), which predicts that dark birds should be more frequent in wet places and lighter birds should be more frequent in drier areas. For example, Wunderle (Citation1981) found that dark morph Bananaquits Coereba flaveola were more abundant in wet, rainy forests and light morphs in lower, open and drier areas of the island of Grenada. There was also a strong positive relationship between the proportion of Black Sparrowhawks of the dark morph and the amount of rainfall during the breeding season in the Cape Town region of South Africa (Amar et al. Citation2014, Tate et al. Citation2016, Tate & Amar Citation2017). Contrarily, a comparative analysis showed that the polymorphism in raptors was related to wider distribution areas, larger habitat niches and a strong migratory behaviour (Galeotti & Rubolini Citation2004), factors that all occur in the Booted Eagle, and only marginally with the climate regime. However, the rainfall regimes are very different in the Mediterranean region compared to the Eurasian continental zones. In the Iberian Peninsula and in South Africa, where Booted Eagles of the light morph are most common, rainfall is higher during the periods of pre-laying and incubation than in the period of chick growth, while contrarily, from the Ukraine to the east the rainfall increases, being approximately double that of the Iberian Peninsula when the chicks are growing and the adults, mainly the males, must increase the supply of prey to the nest. The increase in the amount of precipitation is closely related to the number of daylight cloudy hours, which increased progressively from 28% in the Iberian Peninsula to 50.5% in the Altai-Sayan region. Accordingly, a comparative analysis by Galeotti et al. (Citation2003) highlighted the probability that the evolution of the polymorphism could be driven by the selective pressure related to the detectability of the birds in different light conditions and maintained by disruptive selection, in which different colour morphs obtained different advantages in different habitats and against different prey.
Karyakin (Citation2007) described the increase in the proportion of mammals in the diet of the Booted Eagle towards the east, from 42% in the Volga region to 89% in the Altai-Sayan region, due to an increase in the abundance of colonial steppe mammals, such as Daurian Pikas Ochotona dauurica, Long-tailed Ground Squirrels Urocitellus undulatus and Naped Ground Squirrels Spermophilus dauricus. This relationship may be a result of the greater availability of mammals in the east and an adaptive response of the Booted Eagle, in which the dark morph individuals may be more cryptic than light morphs under conditions of cloudy skies. In open land, such as the steppes, the Booted Eagles look for prey mainly by quartering and soaring at mid-height, stooping suddenly towards the ground when they discover prey (Ferguson-Lees & Christie Citation2001). We do not know the ability of different mammal species to recognize and distinguish aerial predators, but light morph Booted Eagles, with their characteristic and striking plumage, may be easier to detect from the ground by mammals, compared to the dark morph eagles and other aerial predators, especially when cloudy skies prevail. On the other hand, this selective advantage for the dark morph might not be so evident when the eagles hunt birds, since these are mainly caught by surprise in fast and agile chase flights at wood edges or in open woods (Ferguson-Lees & Christie Citation2001).
Another theory, also related to Gloger’s rule, states that individuals of the dark morph should be more resistant to parasites than those of the light morph. Several studies on different animal taxa, including raptors, have found a relationship between different colour morphs and parasite load, with dark morphs having lower loads than pale morphs (Chakarov et al. Citation2008, Jacquin et al. Citation2011, Lei et al. Citation2013, MacColl et al. Citation2013). In this way, if in more rainy environments there are higher parasite loads, the individuals of the dark morph have a selective advantage with respect to those of the light morph and could be more abundant in these wetter environments. However, in the eastern Booted Eagle populations, the lower average temperatures during the breeding season may restrain the activity of parasite transmission vectors and, as a consequence, their impact and propagation.
We must take into account that the selective pressure acts throughout the year and not only during the breeding season. The eastern populations of Booted Eagle mainly migrate to the south in September, reaching the wintering areas, mostly in the Indian subcontinent, in October, staying there until March of the following year. During the wintering period, in the lower third of the Indian subcontinent, where most of the eagles winter, the average temperature reaches 25°C and precipitation 298.6 mm (India.climatemps.com), values considerably higher than in the breeding areas, where the average temperature only reach 14°C and the rain 218.3 mm during the breeding season (Russia.climatemps.com). These warmer and wetter environmental conditions could have a considerable influence not only on transmission vectors and parasite load, but also on detectability and other selective pressures, which can influence the survival rates of the different colour morphs. On the contrary, the populations of western Europe and South Africa, where the majority of birds are of the light morph, spend the winter in the sub-Saharan Sahel and in Namibia and southern Angola, respectively, in a very dry environment.
Amar et al. (Citation2014) also found a significant relationship between the interaction of temperature and rainfall and the proportion of dark morph Black Sparrowhawks, which negatively correlated with temperature in places with high rainfall. They suggested that the negative relationship between temperature and the proportion of dark morph birds supported the idea that thermoregulation could play a role in the selection of morph type. Walsberg (Citation1983) suggested that darker birds should better absorb the energy of solar radiation and thus can reduce their resting basal metabolic rates in places with lower temperatures (Hamilton & Heppner Citation1967). This theory has been confirmed by recent studies in ectotherms (amphibians and reptiles) (Forsman & Hagman Citation2009, Pizzato & Dubey Citation2012), with the colour polymorphic species having larger ranges, broader niches and less risk of being endangered, because they are more adaptable and resilient to environmental changes. However, Delhey et al. (Citation2013) suggested that it is not clear that these patterns are also applicable to endotherms such as birds, in which the direct physiological effects of colour polymorphism are less important and the colonization of new habitats with different thermal conditions or expansions in areas with different climatic regimes may have less influence than in ectotherms. In the Booted Eagle, the role of temperature, a variable negatively correlated with the amount of rainfall, is less clear and may be merely coincident and as a consequence of the increase in the latitudinal range of the Booted Eagle populations towards the east and the progressive decrease in solar radiation and the average temperature in these areas. However, recently Amar et al. (Citation2019) have found that the clinal variation of the Swainson’s Hawk Buteo swainsoni in North America, with a gradual greater abundance of the dark morph towards the west, could be related to the greater energy needs of individuals in drier and cooler areas.
Surprisingly, in the Spanish populations, where the proportion of the dark morph varies from 8.44% to 39%, the models did not show any significant relationship between that proportion of dark morph Booted Eagles and the meteorological and environmental explanatory variables. Within the Iberian Peninsula there is no clinal variation of the colour morph in the populations of Booted Eagle, as in the Black Sparrowhawk in South Africa (Amar et al. Citation2014), but it would be expected that the differences found in the proportion of the dark morph could be explained by differences in altitude, the amount of rain or temperature. It is possible, given that the variation in the proportion of the dark morph and the explanatory variables is smaller than on a continental scale. Perhaps more accurate data and a smaller scale are needed to explain the differences in the morph ratio on the Iberian Peninsula.
To conclude, the results suggest that clinal variation of the colour polymorphism in the Booted Eagle is maintained by disruptive selection from climatic factors such as rain and the associated presence of cloudy skies. The clinal increase in wing length in the Palearctic, from west to east (Brown & Amadon Citation1968, Cramp & Simmons Citation1980, Ferguson-Lees & Christie Citation2001, Clark & Davies Citation2018), may also be due to the gradual increase of rain and cloudy skies, and may give the eagles a lower wing load in less favourable environments for a typically gliding species.
The bias found in the proportion of the colour morphs of offspring of mixed pairs may also play a role in maintaining the colour polymorphism in this species. For example, pairs of light males and dark females, were predicted to produce a morph ratio of 4.35 light to 1 dark offspring but actually produced 1 light to 1.49 dark, suggesting the phenomenon of transmission ratio distortion (TRD), which occurs when some alleles of the parents are transmitted to the offspring with a higher or lower frequency than expected by Mendelian theory (Bosch et al. Citation2019).
Since the last decade of the twentieth century, Booted Eagle populations of the eastern Palearctic have staged a strong expansion, occupying a large area previously uninhabited, comprising the northern half of Kazakhstan and areas of southern Western Siberia in the Russian Federation, among the longitudes 50°E to 90°E and latitudes 45°N to 55°N. These populations, mainly of the dark morph, have had high productivity with a breeding success of 90% (Karyakin Citation2007). The role of polymorphism in this expansion is worthy of further study but polymorphic birds, such as the Booted Eagle, may have some advantages in a world where environments and climates are changing (Galeotti et al. Citation2003, Amar et al. Citation2014).
Supplemental Material
Download MS Word (107.5 KB)Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
ORCID
Josep Bosch http://orcid.org/0000-0002-2776-6378
References
- Amar, A., Koeslag, A. & Curtis, O. 2013. Genetic plumage polymorphism in a newly colonised Black Sparrowhawk population: classification, temporal stability and inheritancepatterns. J. Zool. 289: 60–67. doi: 10.1111/j.1469-7998.2012.00963.x
- Amar, A., Koeslag, A., Malan, G., Brown, M. & Wreford, E. 2014. Clinal variation in the morph ratio of Black Sparrowhawks Accipiter melanoleucus in South Africa and its correlation with environmental variables. Ibis 156: 627–638. doi: 10.1111/ibi.12157
- Amar, A., Reynolds, C., Van Velden, J. & Briggs, C.W. 2019. Clinal variation in morph frequency in Swainson’s hawk across North America: no support for Gloger’s ecogeographical rule. Biol. J. Linn. Soc. DOI:10.1093/biolinnean/blz037/5479425.
- Baiges, C. 2014. Més de 10 anys de seguiment de l’àguila calçada al Parc natural dels Ports. Cingles, 4: 7–11 (in Catalan).
- Bosch, J. 2003. Fenología y parámetros reproductivos del aguililla calzada Hieraaetus pennatus en Cataluña central (España). Ardeola, 50: 181–189 (in Spanish).
- Bosch, J. 2011. Población y ecología reproductora de la aguililla calzada en Cataluña. In Zuberogoitia, I. & Martínez, J.E. (eds) Ecology and Conservation of European Forest-Dwelling Raptors, 87–92. Bilbao: Diputación Foral de Bizkaia.
- Bosch, J, Martínez, J.E., Calvo, J.F., Zuberogoitia, I. & Jiménez-Franco, M.V. 2015. Does rainfall affect the productivity of the Booted Eagle (Aquila pennata) during the breeding period in Mediterranean environments? J. Ornithol. 156:1–8. doi: 10.1007/s10336-014-1112-2
- Bosch, J., Calvo, J.F., Bermejo, A. & De la Puente, J. 2016. Factors influencing the movements during the breeding season of a female booted eagle (Aquila pennata) tagged by satellite in central Catalonia (Sapin). Slovak Raptor J. 10: 81–94. doi: 10.1515/srj-2016-0004
- Bosch, J., Mestre, J., Baiges, C., Martínez, J.E., Calvo, J.F. & Jiménez-Franco, M.V. 2019. Colour plumage polymorphism in the Booted Eagle: inheritance pattern and temporal stability of the morph frequencies. J. Zool. DOI:10.1111/jzo.12666.
- Bourgeois, Y.X.C., Delahaie, B., Gautier, M., Lhuillier, E., Malé1, P-J. G., Bertrand, J.A.M., Cornuault, J., Wakamatsu, K., Bouchez, O., Mould, C., Bruxaux, J., Holota1, H., Milá, B. & Thébaud, C. 2017. A novel locus on chromosome 1 underlies the evolution of a melanic plumage polymorphism in a wild songbird. R. Soc. Open Sci. 4: 160805. doi: 10.1098/rsos.160805
- Briggs, C.W., Woodbridge, B. & Collopy, M.W. 2010. Inheritance patterns of plumage morph in Swainson’s Hawks. J. Raptor Res. 44: 232–235. doi: 10.3356/JRR-09-83.1
- Brown, L. & Amadon, D. 1968. Eagles, Hawks & Falcons of the World. London: Hamlyn.
- Carlon, J. 1996. Response of Booted eagles to human disturbance. Br. Birds 89: 267–274.
- Casado, E., Suárez-Seoane, S., Lamelin, J. & Ferrer, M. 2008. The regulation of brood reduction in Booted Eagles Hieraaetus pennatus through habitat heterogeneity. Ibis 150: 788–798. doi: 10.1111/j.1474-919X.2008.00862.x
- Chakarov, N., Boerner, M. & Krüger, O. 2008. Fitness in common buzzards at the cross-point of opposite melanin-parasite interactions. Funct. Ecol. 22: 1062–1069. doi: 10.1111/j.1365-2435.2008.01460.x
- Chakarov, N., Boerner, M. & Krüger, O. 2011. Biological consequences of plumage polymorphism in common buzzard. In Zuberogoitia, I. & Martínez, J.E. (eds) Ecology and Conservation of European Forest-Dwelling Raptors, 234–241. Bilbao: Diputación Foral de Bizkaia.
- Chang, V., Lejeune, J. & Cheng, K.M. 2010. The pattern of inheritance of melanin-based plumage color variants in the Gyrfalcon (Falco rusticolus). J. Raptor Res. 44: 224–232. doi: 10.3356/JRR-09-61.1
- Clark, W.S. 1999. A Field Guide to the Raptors of Europe, the Middle East and North Africa. Oxford: Oxford University Press.
- Clark, W.S. & Davies, R. 2018. African Raptors. London: Helm.
- Cramp, S. & Simmons, K.E.L. 1980. Handbook of the Birds of Europe, the Middle East and North Africa, Vol. II. Oxford: Oxford University Press.
- Delhey, K., Smith, J. & Peters, A. 2013. Colour-variable birds have broader ranges, wider niches and are less likely to be threatened. J. Evol. Biol. 26:1559–1568. doi: 10.1111/jeb.12157
- Díaz, J. 2006. El águila calzada y su conservación en la Comunidad de Madrid. Madrid: Comunidad de Madrid/Obra Social. Fundación “La Caixa”/FICAS ( in Spanish).
- Díaz, J. & Cebollada, F. 2011. Monitoring and conservation of booted eagle (Aquila pennata) in the Sierra de Guadarrama (Central Spain). In Zuberogoitia, I. & Martínez, J. E. (eds) Ecology and Conservation of European Forest-Dwelling Raptors, 70–80. Bilbao: Diputación Foral de Bizkaia.
- Ferguson-Lees, J. & Christie, D. 2001. Raptors of the World. London: Christopher Helm.
- Forsman, D. 2016. Flight Identification of Raptors of Europe, North Africa and the Middle East. London: Helm.
- Forsman, A. & Hagman, M. 2009. Association of coloration mode with population declines and endangerment in Australian frogs. Conserv. Biol. 23: 1535–1543. doi: 10.1111/j.1523-1739.2009.01244.x
- Fowlie, M.K. & Krüger, O. 2003. The evolution of plumage polymorphism in birds of prey and owls: the apostatic selection hypothesis revisited. J. Evol. Biol. 16: 577–583. doi: 10.1046/j.1420-9101.2003.00564.x
- Furness, R.W.1987. The Skuas. Calton: T & A D Poyser.
- Galeotti, P. & Rubolini, D. 2004. The niche variation hypothesis and the evolution of colour polymorphism in birds: comparative study of owls, nightjars and raptors. Biol. J. Linn. Soc. 82: 37–248. doi: 10.1111/j.1095-8312.2004.00355.x
- Galeotti, P., Rubolini, D., Dunn, P.O. & Fasola, M. 2003. Colour polymorphism in birds: causes and functions. J. Evol. Biol. 16: 635–646. doi: 10.1046/j.1420-9101.2003.00569.x
- García-Dios, I.S. 2006. Dieta del aguililla calzada Hieraaetus pennatus en el sur de Ávila: importancia de los paseriformes. Ardeola 53: 39–54 (in Spanish).
- García-Dios, I.S. 2017. El Águila Calzada. Almenara, Spain: Tundra Ediciones. ( in Spanish).
- Génsbøl, B. 2008. Birds of Prey. London: Collins.
- Gloger, C.L. 1833. Das Abändern der Vögel durch Einfluss des Klimas. Breslau: August Schulz (in German).
- Hamilton, W.J. & Heppner, F. 1967. Radiant solar energy and the function of black homeotherm pigmentation: an hypothesis. Science 155: 196. doi: 10.1126/science.155.3759.196
- Huxley, J.S. 1955. Morphism in birds. Acta Int. Ornithol.Congr. 6: 309–328.
- Iribarren, J.J. 1975. Biología del águila calzada (Hieraaetus pennatus) durante el periodo de nidificación en Navarra. Ardeola 21: 305–320 (in Spanish).
- Iribarren, J.J. & Rodríguez Arbeloa, A. 1988. Sobre la biología del águila calzada Hieraaetus pennatus (Gmelin, 1788) en Navarra. Publicaciones de biología de la Universidad de Navarra. Série zoológica. Año 1988, no 17, 1–17 (in Spanish).
- Jacquin, L., Lenouvel, P., Haussy, C., Ducatez, S. & Gasparini, J. 2011. Melanin-based coloration is related to parasite intensity and cellular immune response in an urban free living bird: the feral Pigeon Columba livia. J. Avian Biol. 42: 11–15. doi: 10.1111/j.1600-048X.2010.05120.x
- Karyakin, I.V. 2007.The Booted Eagle in the Volga region, Ural and Siberia, Russia. Raptors Conservation 9: 27–62.
- Lei, B., Amar, A., Koeslag, A., Gous, T.A. & Tate, G.J. 2013. Differential haemoparasite intensity between Black Sparrowhawk (Accipiter melanoleucus) morphs suggests an adaptive function for polymorphism. Plos One 8: e81607. doi: 10.1371/journal.pone.0081607
- López-López, P., De la Puente, J., Mellone, U., Bermejo, A. & Urios, V. 2016. Spatial ecology and habitat use of booted eagles (Aquila pennata) during the breeding season: implications for conservation. J. Ornithol. 157: 981–993. doi: 10.1007/s10336-016-1357-z
- MacColl, A.D.C., Stevenson, I.R. & Richardson, D.S. 2013. Melanocortin-1-receptor (MC1R) variation is not associated with parasite burden in a neotropical bird, the Bananaquit (Coereba flaveola). Biol. J. Linn. Soc. 104: 882–888. doi: 10.1111/bij.12009
- Martínez, J.E., Pagán, I., Palazón, J.A. & Calvo, J.F. 2007. Habitat use of booted eagles (Hieraaetus pennatus) in a special conservation area: implications for conservation. Biodivers. Conserv. 16: 3481–3488. doi: 10.1007/s10531-006-9053-6
- Martínez, J.E., Calvo, J.F., Jiménez-Franco, M.V., Zuberogoitia, I. & López-López, P. 2016. Colour morph does not predict brood size in the Booted Eagle. Ornis Fenn. 93: 130–136.
- Moroz, V.A. & Vetrov, V.V. 2013. About breeding of the Booted Eagle in the Lugansk district, Ukraine. Raptors Consevation 27: 197–207.
- Mundy, N.I. 2005. A window on the genetics of evolution: MC1R and plumage colouration in birds. Proc. R. Soc. B. 272: 1633–1640. doi: 10.1098/rspb.2005.3107
- Newton, I. 1979. Population Ecology of Raptors. London: Poyser.
- Palomino, D. & Carrascal, L.M. 2007. Habitat associations of a raptor community in a mosaic landscape of central Spain under urban development. Landsc. Urban Plann. 83: 268–274. doi: 10.1016/j.landurbplan.2007.04.011
- Paulson, D.R. 1973. Predator polymorphism and apostatic selection. Evolution 27: 269–277. doi: 10.1111/j.1558-5646.1973.tb00672.x
- Pizzatto, L. & Dubey, S. 2012. Colour-polymorphic snake species are older. Biol. J. Linn. Soc. 107: 210–218. doi: 10.1111/j.1095-8312.2012.01936.x
- Preston, C.R. 1980. Differential Perch Site selection by Color morphs of the Red-Tailed Hawk (Buteo jamaicensis). The Auk 97: 782–789.
- Prieta, J. 2007. Aves de Extremadura, Vol. 3 (2001–2003). Mérida: Digital version. ADENEX. ( in Spanish)
- R Development Core Team. 2018. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Rohwer, S. & Paulson, D.R. 1987. The avoidance-image hypothesis and color polymorphism in Buteo hawks. Orn. Scand. 18: 285–290. doi: 10.2307/3676897
- Roulin, A. 2003. Geographic variation in sexual dimorphism in the barn owl (Tyto alba): a role for direct selection or genetic correlation? J. Avian Biol. 34: 251–258. doi: 10.1034/j.1600-048X.2003.03022.x
- Roulin, A. & Wink, M. 2004. Predator-prey relationships and the evolution of colour polymorphism: a comparative analysis in diurnal raptors. Biol. J. Linn. Soc. 81: 565–578. doi: 10.1111/j.1095-8312.2004.00308.x
- Sánchez-Zapata, J.A. & Calvo, J.F. 1999. Raptor distribution in relation to landscape composition in semi-arid Mediterranean habitats. J. Appl. Ecol. 36: 254–262. doi: 10.1046/j.1365-2664.1999.00396.x
- Schmutz, S.M. & Schmutz, J.K. 1981. Inheritance of color phases of Ferruginous Hawks. Condor 83: 187–189. doi: 10.2307/1367430
- Steyn, P. & Grobler, J.H. 1981. Breeding biology of the Booted Eagle in South Africa. Ostrich 52: 108–118. doi: 10.1080/00306525.1981.9633593
- Tate, G.J. & Amar, A. 2017. Morph specific foraging behavior by a polymorphic raptor under variable light conditions. Sci. Rep. 7: 9161. DOI: 10.1038/s41598-017-07829-x.
- Tate, G.J., Bishop, J.M. & Amar, A. 2016. Differential foraging success across a light level spectrum explains the maintenance and spatial structure of colour morphs in a polymorphic bird. Ecol. Lett. 19: 687–694. doi: 10.1111/ele.12606
- Viada, C. & De Pablo, F. 2009. Cens d’àguila calçada Hieraaetus pennatus a Balears al 2009 i estat de conservació. AnuariOrnitològic de les Balears 24: 1–15 (in Catalan).
- Walsberg, G.E. 1983. Coat color and solar heat gain in animals. Bioscience 33: 88–91. doi: 10.2307/1309169
- Wunderle, J.M. 1981. An analysis of a morph ratio-cline in the Bananaquit (Coereba flaveola) in Grenada, West Indies. Evolution 35: 333–344.
