ABSTRACT
Capsule: Duration and peak frequency of Wren Troglodytes troglodytes songs differ between Welsh islands (Skokholm, Flat Holm, and Sully Island) and a mainland site, suggesting random or cultural divergence.
Aims: The study tests for differences in bioacoustic traits in Wren song between four breeding sites.
Methods: Songs from breeding male Wrens were recorded from three Welsh islands and a representative location on the local mainland. Each sonogram was measured for six variables from the whole song and two distinct phrases from the beginning and middle. The variables were measured manually using an open source acoustic tool and compared between sites using generalized linear mixed-effects models.
Results: There was significant variation in song variables between island sites and the mainland. Skokholm Wren songs were significantly longer in duration than all other sites by up to 66%. Peak frequency was significantly different across all sites with Sully Island having the highest peak frequency and Skokholm the lowest.
Conclusion: The differences in song duration and frequency do not directly suggest a trend associated with selective pressures such as habitat or noise, but instead may be explained by non-selective drivers such as cultural drift or local dialect formation.
Bird song plays a prominent role in species recognition, mate selection, and territory defence (Lynch Citation1996, Martin & Martin Citation2001, Slabbekoorn & Smith Citation2002). The evolution of song structure is likely to be driven by a combination of mechanisms including vocal physiology (Podos & Nowicki Citation2004), sexual selection (Price Citation1998, Macdougall-Shackleton Citation1997), novel song learning (Slabbekoorn & Smith Citation2002), and environmental factors that affect signal transmission (Boncoraglio & Saino Citation2007). Bird morphology has a strong influence on vocal characteristics, for example, body size is negatively correlated with song frequency, and bill size is positively correlated with song duration (Derryberry et al. Citation2018). Singing effort may also have an important role in attracting mates and achieving breeding success. Male quality may be assessed by prospective females on the basis of song complexity, amount of singing, and amplitude (Eriksson & Wallin Citation1986, Searcy & Johnson Citation1996, Ballentine et al. Citation2004, Nowicki & Searcy Citation2005). Background noise, such as traffic can affect song characteristics in individuals (Brumm & Naguib Citation2009) and has the potential to drive changes in the song characteristics of local populations (Luther & Baptista Citation2010). For example, a study of White-crowned Sparrows Zonotrichia leucophrys showed that songs in urban areas had higher minimum frequencies than those in rural environments seemingly in response to greater ambient noise (Luther & Baptista Citation2010). Additionally, breeding Nightingales Luscinia megarhynchos had higher vocal amplitudes in noisier territories compared with those in quieter settings (Brumm Citation2004).
Although vocal signals are usually learned in songbirds (Kroodsma Citation2004), they can vary in their acoustic characteristics (e.g. duration or syllable types) within populations (Catchpole & Slater Citation2003). Physical separation of populations through habitat fragmentation or island colonization can disrupt the cultural exchange of songs between local populations, leading to the development of localized ‘acoustical niches’ or local dialects (Baker et al. Citation2003, Laiolo & Tella Citation2005, Baker et al. Citation2006, Catchpole & Slater Citation2003). Divergence of song structure between populations may also occur due to cultural drift, where random elements of song characteristics are reproduced or lost through successive generations (Wilkins et al. Citation2013). This has been shown to occur more frequently in small or isolated populations, like those on small islands (Lynch Citation1996). Island populations often show divergent song types, which are often more simple or completely novel, compared to mainland counterparts (Baker et al. Citation2003, Citation2006). For example, Black-capped Chickadees Poecile atricapillus showed substantial differences between islands and mainland population in the number of tones and repertories, structural diversity and dialects (Kroodsma et al. Citation1999), and Blue Tits Cyanistes caeruleus differed in the tempo, frequency, syntax, and trill of their songs between Mediterranean populations and the island of Corsica (Doutrelant et al. Citation1998).
One of the more widely studied bird songs is that of the Wren Troglodytes troglodytes, whose song is structurally complex, comprising a mixture of melodious and repeated tones, trills, clicks and buzzes, which can be highly variable across its Eurasian range (Armstrong Citation1955, Cramp Citation1988). There is evidence of local dialects with distinct shared phrases within local populations as well as some general geographical trends such as song duration and mean frequency decreasing with latitude (Chappuis, Citation1969, Cramp Citation1988). Within the British Isles, there are four recognized endemic subspecies zetlandicus, fridariensis, hebridensis, and hirtensis, that are found on islands and are vocally distinct from the indigenus and troglodytes races on the British mainland. The island subspecies tend to be more variable, with a slower ‘less urgent’ delivery and often have a more pronounced ‘buzz’ (continuous modulation) segment (Cramp Citation1988). The complexity of song structure in Wrens makes a quantitative comparison of song elements between individuals challenging, and difficult to compare between studies due to the incompatible verbal descriptions of phrases. Perhaps because of this challenge, few studies have examined bioacoustic variation in Wrens from other islands in the British Isles, despite the potential for divergence driven by separation and variable environmental conditions.
In this study, we test for differences in basic bioacoustic traits (duration and peak frequency) of Wren songs between the local mainland and three Welsh islands. If the driver of bioacoustic variation is selective, based on differences in habitat and noise, we expect to find differences between the mainland and the three islands. Alternatively, if the variation is driven by non-selective pressures such as cultural drift and local dialect, then we might expect random variation between any of the sites.
Methods
Study sites
The study compares songs from breeding male Wrens from three Welsh islands (Sully Island, Flat Holm, and Skokholm; ) and sites from the local British mainland in Cardiff. Sully Island (or Ynys Sili in Welsh; 51°23ʹ44ʺN 3°11ʹ58ʺW) is a 5.86 ha tidal island situated 400 m off the South Wales coast in the Severn Estuary. Flat Holm (Yns Echni; 51°22ʹ41ʺN 3°07ʹ18ʺW) is 32 ha and situated 4.5 km from the South Wales coast in the Severn Estuary. Skokholm (Ynys Sgogwm; 51°41ʹ56ʺN 5°16ʹ40ʺW) is 106 ha island and located 2.82 km from the Pembrokeshire coast.
Figure 1. Location of Welsh islands Sully Island, Flat Holm and Skokholm, and the local mainland study site in Cardiff.
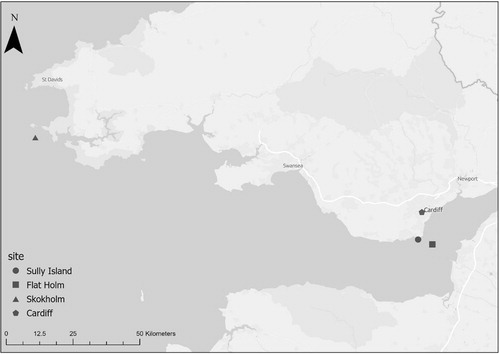
All three islands have broadly similar vegetation comprising areas of rough maritime grassland, bracken Pteridium sp. and scrub (Goodman & Gillham Citation1954, Ross-Smith et al. Citation2013, Thompson Citation2008). Flat Holm scrub cover is significantly greater than Skokholm. The mainland bioacoustics data came from sites around Cardiff (Wales, 51°29ʹ12ʺN 3°10ʹ39ʺW) including urban areas, public parks, and semi-natural habitats.
Bioacoustics
Sound recordings were made during the breeding season at male territories: in June 2017 on Sully Island and the mainland (Cardiff), in July 2017 on Skokholm, and in August 2017 on Flat Holm. Territories were identified by the presence of singing males. As Wrens avoid song overlap with neighbours (Yang & Slabbekoorn, Citation2014), any song heard immediately after another (within two seconds) from an adjacent area was considered to be a separate territory. All song recordings were made using a Tascam DR-40 linear pulse code modulation digital recorder equipped with a Movo VXR40 condenser shotgun microphone and headphones, pointed directly towards the singing individual or leaving it under the specific location at a distance of 1–10 m. We recorded 24 songs from 10 males on Flat Holm, 18 songs from six males on Sully Island, and 29 songs from seven males on Skokholm. For the mainland (Cardiff), 19 songs were recorded from eight males.
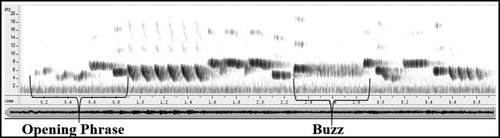
Each sonogram was measured for six variables including two generic measurements of song: total duration (seconds) and peak frequency (hertz) which are commonly used in bird song analysis (Baker et al. Citation2003, Brumm Citation2004, Bermúdez-Cuamatzin et al. Citation2009, Sangster & Luksenburg Citation2015). Two phrases of the song were singled out on the basis of their presence in all recordings, their structural distinctiveness, and their position within the song (either at the beginning or in the middle). The first we define as the ‘Opening Phrase’, which is synonymous with the ‘Type A segment’ as described by Cramp (Citation1988), which precedes the first repeated uniform phrase (). The measurement of the Opening Phrase includes its duration (seconds) and peak frequency (hertz). The second phrase we define as the ‘Buzz’ (Type C; Cramp Citation1988), which is a continuous unit of rapid tones, in which the duration and peak frequency were measured.
All recordings were processed using the opensource tool WaveSurfer version 1.8.8 (Royal Institute of Technology Stockholm, Sweden). The duration of all variables was measured manually. The frequency (Hz) was calculated using a Hamming and a Fast Fourier Transformation (FFT; this transforms a signal from a ‘time domain’ into ‘frequency domain’ which provides a single summarized value for frequency from several possible values) following procedures in Francis et al. (Citation2011).
Data analysis
All songs were attributed to a site, including ‘Mainland’, ‘Sully’, ‘Flat Holm’, and ‘Skokholm’. The effect of site for each of the six measurements was analysed using general linear mixed-effects models (GLMM) with a ‘Gaussian’ error distribution and ‘identity’ link function, with ‘individual identity’ as a random effect to account for repeated measurements from the same individuals. All analyses were performed using the statistical software R Version 3.6.1 (R Development Core Team Citation2019) and the package ‘lme4’ (Bates et al. Citation2015). Data exploration and model validation procedures followed Zuur et al. (Citation2007) visually inspecting the model residuals for normality and homoscedasticity. Means are presented with ±SE.
Results
Total song
Mean song duration was significantly different between sites (F3,28.03 = 19.63, P < 0.0001; (a)) with the largest difference between Skokholm and Flat Holm (2.18 ± 0.29 s). This equates to 66.26% longer. There was no significant difference in song duration between Sully Island and Mainland ((a)). Song peak frequency was significantly different between Sully Island and Skokholm (F3, 19.4 = 3.58, P = 0.0327; (b); (b)). Sully Island songs were 1345.5 ± 414.1 Hz higher than the Skokholm mean value.
Figure 3. (a) Song duration and (b) song peak frequency differences between sites. Circles indicate mean values with standard error bars.
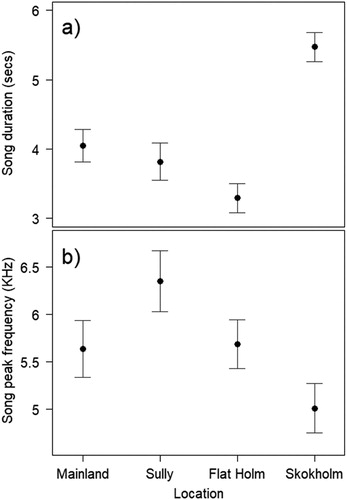
Table 1. Analysis of song variables measured on Wren songs from Flat Holm, Skokholm, Sully and Mainland. The intercept represents the Mainland. Estimates are from generalized linear mixed models.
Opening Phrase
The mean Opening Phrase duration was 1.32 ± 0.08 s, which was 176% longer in Skokholm than all other sites (F3,32.884 = 55.64, P < 0.0001; (a)), with the largest difference with the mainland (+1.61 ± 0.155 s; (a)). There were no significant differences between the other sites. The mean Opening Phrase peak frequency was 5502.00 ± 99.11 Hz, which was similar between Mainland and Flat Holm, but significantly different with Sully Island and Skokholm (F3,94 = 4.50, P = 0.0053; (b), (b)).
Figure 4. (a) Opening Phrase duration and (b) frequency differences between sites. Circles indicate mean values with standard error bars.
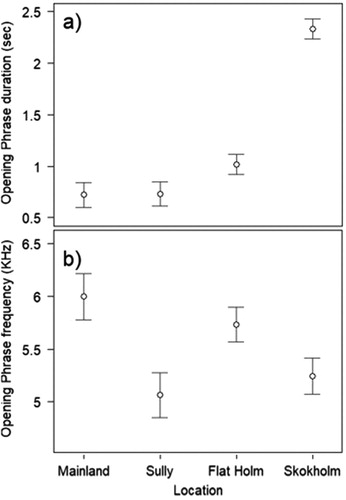
Table 2. Analysis of Opening Phrase and Buzz variables measured on wren songs from Flat Holm, Skokholm, Sully and Mainland. The intercept represents the mainland. Estimates are from generalized linear mixed models.
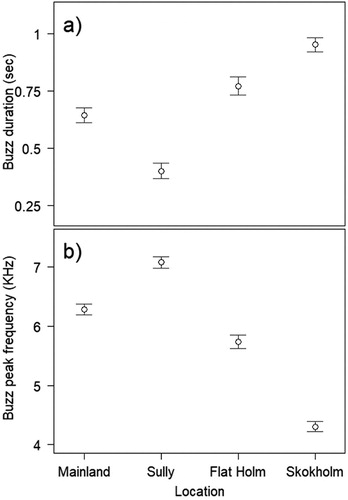
Buzz
The mean Buzz duration was 0.70 ± 0.03 s and was significantly different between all sites (F3,21.06 = 51.02, P < 0.0001; (c), (a)), with the longest duration on Skokholm (0.95 ± 0.04 s) and shortest on Sully Island (0.40 ± 0.05 s). The mean Buzz peak frequency was 5707.00 ± 138.3 Hz, which was significantly different between sites (F3,18.98 = 163.97, P < 0.0001; (d), (b)) with the lowest frequency on Skokholm (4304.07 ± 127.57 Hz) and highest on Sully Island (7078.9 ± 132.93 Hz).
Discussion
This study tested song differentiation of Wren populations of three Welsh islands and representatives from the local mainland. In most cases, the bioacoustic variables compared showed significant differences between sites. In all cases, the mainland values were located in the centre of the range, with islands on either side, both higher and lower. This pattern initially suggests random divergence rather than a trend responding to selective pressures such as habitat differences.
Song duration was significantly different between most sites, most notably between Skokholm, the mainland and Flat Holm (in order of decreasing song duration). If the observed variation was driven by a selective pressure, such as ambient noise or presence of vertical barriers, we should expect Wrens from the mainland to sing shorter songs, while island Wrens, less hindered by interrupting noises, sing for longer. It has been observed that songs are shorter and faster in cities in comparison to nearby forests (Slabbekoorn & de Boer-Visser Citation2006). House Finches Carpodacus mexicanus too, sing shorter songs with fewer notes at noisier locations (Fernández-Juricic et al. Citation2005, Bermúdez-Cuamatzin et al. Citation2011). Shorter songs may be less disrupted by echoes and clashing sound waves bouncing off surfaces (Bradbury & Vehrencamp Citation1998). The longest songs on Skokholm supports this idea, assuming the island is less noisy than the mainland, however, we would expect the other island songs to show a similar trend given the similarity of their habitats. The songs from Flat Holm were shorter than the mainland, which does not support this explanation, however, they share their breeding habitat with up to 8000 breeding Lesser Black-backed Gulls Larus fuscus (Ross-Smith et al. Citation2013). It is, therefore, possible that in this acoustically challenging situation, the impact of ambient noise is far greater than on the mainland and only a short song will carry through any brief intervals in the chaotic soundscape of gull calls. Song transmission is not only affected by natural sounds like waterfalls (Derryberry et al. Citation2016) or abiotic sounds such as road traffic (Brumm Citation2004), but also by the noise generated from other animals (Krause Citation1992). Whether this situation in Flat Holm is a functional response to the ambient noise could be tested by comparing songs when gulls are breeding to the period when gulls are absent (i.e. outside the breeding season).
It follows that with longer songs, there will be longer individual components of those songs. This is true of the Skokholm Wrens, which had significantly longer Opening Phrases and Buzz segments than did Wrens at the other sites. The songs of the endemic Scottish island subspecies are described as having a ‘less hurried’ delivery with pronounced Buzz segments compared with the mainland subspecies (Cramp Citation1988), which appears to be consistent in this case. Sully is the island with the shortest physical separation to the mainland (400 m) and so there is a greater potential for new birds to arrive and maintain homogeneity with the mainland population. Evidence to support this can be seen in the song duration, where no significant differences with the mainland were found. Respectively, Flat Holm, with the greater separation to the mainland (4.5 km) and Skokholm, sitting in between (2.82 km), had Wrens which sang significantly shorter and longer songs. These two populations seem to be taking different drifting trajectories in their song duration. These islands have no connectivity between them as they are separated more than 150 km. It is possible that differences in songs arise through random loss or change, consistent with cultural drift or dialect formation. It is possible to measure cultural drift when new and isolated populations are established in reintroduction programmes (Parker & Laurence Citation2008). A well-documented example is in the translocation of Saddlebacks Philesturnus rufusater in the north of New Zealand which showed that in less than 50 years the song evolved in two distinctive lineages (Parker et al. Citation2012).
Songs are often delivered at higher frequencies and at greater amplitudes in noise-polluted habitats to modulate above the lower frequency anthropogenic noise (Cardoso & Atwell Citation2011). The higher frequency is suggested to be a physiological adjustment to singing louder but can occur independently. Independence of these variables was observed in our study where Skokholm had the lowest song frequency. While both Flat Holm and Skokholm contain gull colonies, the size, density and the overlap between these and suitable breeding habitat for Wrens is far lower on Skokholm than on Flat Holm (Gonzalo-Tarodo pers. obs, see also Brown & Eagle Citation2019, Ross-Smith et al. Citation2013). While our study is limited by a lack of contemporaneous measures of ambient noise, these do appear lower, at least to human ears, within the breeding territories on Skokholm and may enable a lower frequency song to be delivered. However, Flat Holm Wrens breed mainly within the gull colony which is restricted to the southern 16 ha of the island (Ross-Smith et al. Citation2013), compared to the 35–50 ha of available habitat on Skokholm. The quieter but briefer songs of Wrens on Flat Holm may be a behavioural response to allow them to deliver a song to near neighbours or potential mates during brief periods of lower ambient noise in the gull colony. No gulls breed on Sully, but likewise there is very limited habitat, and thus fewer competitors.
The island races of Wren in Britain have shown significant differences in body size, feather tone, and song patterns before genetic studies confirmed the differences (Cramp Citation1988). The location of our sites are found in the unclear limit between the races Troglodytes troglodytes troglodytes and Troglodytes troglodytes indigenus, with Skokholm birds facing more towards indigenus and Sully, Flat Holm and mainland to troglodytes. With this uncertain limit between races we could be experiencing a mixture of genetic content between the two and this could add to the difficulty in forming clear conclusions from this study. Further phylogenetic studies are needed to uncover this hypothesis.
Although the sample sizes used in this study are small, they reflect the low number of breeding Wrens on each of the islands. Despite the small sample sizes, significant differences are still apparent, providing novel insight to song divergence in relatively local populations. The timing of recording among the sites could have influenced the results of this study. However, given the territoriality that male Wrens present throughout the year (Armstrong Citation1955, Cramp Citation1988), we are confident that the differences between the recorded songs from June to August were not due to seasonal differences. Further work to build on this study could encompass more of the many islands in the British Isles, as well as within island variation in proximity to, and consideration of, sources of ambient noise. Beyond cultural dialectic divergence, further work to investigate differences could include phylogenetic distances, gut microbial communities, and parasite loads.
Acknowledgements
Thanks to Matt Brown, Gisselle Eagle, Richard Brown and Jack Powell, the island wardens, for access, data and fieldwork assistance.
References
- Armstrong, E. A. 1955. The Wren. Collins, London, UK.
- Baker, M.C., Baker, M.S. & Baker, E.M. 2003. Rapid evolution of a novel song and an increase in repertoire size in an island population of an Australian songbird. Ibis 145: 465–471. doi: 10.1046/j.1474-919X.2003.00190.x
- Baker, M.C., Baker, M.S. & Tilghman, L.M. 2006. Differing effects of isolation on evolution of bird songs: examples from an island-mainland comparison of three species. Biol. J. Linn. Soc. Lond. 89: 331–342. doi: 10.1111/j.1095-8312.2006.00677.x
- Ballentine, B., Hyman, J. & Nowicki, S. 2004. Vocal performance influences female response to male bird song: an experimental test. Behav. Eco. 15: 163–168. doi: 10.1093/beheco/arg090
- Bates, D., Mächler, M., Bolker, B. & Walker, S. 2015. “Fitting Linear Mixed-Effects Models Using lme4.” J. Stat. Softw. 67: 1–48.
- Bermúdez-Cuamatzin, E., Ríos-Chelén, A.A., Gil, D. & Garcia, C.M. 2009. Strategies of song adaptation to urban noise in the house finch: syllable pitch plasticity or differential syllable use? Behaviour 146: 1269–1286. doi: 10.1163/156853909X423104
- Bermúdez-Cuamatzin, E., Ríos-Chelén, A.A., Gil, D. & Garcia, C.M. 2011. Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol. 7: 36–38.
- Boncoraglio, G. & Saino, N. 2007. Habitat structure and the evolution of bird song: a meta-analysis of the evidence for the acoustic adaptation hypothesis. Funct. Eco. 21: 134–142.
- Bradbury, J.W. & Vehrencamp, S.L. 1998. Principles of Animal Communication, 1st edn, 75–112. Sinauer Associates, USA.
- Brown, R. & Eagle, G. 2019. The Skokholm Annual Report 2018. Wildlife Trust of South and West Wales.
- Brumm, H. 2004. The impact of environmental noise on song amplitude in a territorial bird. J. Anim. Ecol. 73: 434–440. doi: 10.1111/j.0021-8790.2004.00814.x
- Brumm, H. & Naguib, M. 2009. Environmental acoustics and the evolution of bird song. Adv. Study Behav. 40: 1–33. doi: 10.1016/S0065-3454(09)40001-9
- Cardoso, G.C. & Atwell, J.W. 2011. On the relation between loudness and the increased song frequency of urban birds. Anim. Behav. 82: 831–836. doi: 10.1016/j.anbehav.2011.07.018
- Catchpole, C.K. & Slater, P.J. 2003. Bird Song: Biological Themes and Variations, 1st edn. University Press, Cambridge.
- Chappuis, C. 1969. Un cline vocal chez les oiseaux palearctiques: variation tonale des vocalizations, sous differentes latitudes. Alauda 37: 59–71.
- Cramp, S. (ed) 1988. Handbook of the Birds of Europe, the Middle East and North Africa. The Birds of the Western Palearctic. Oxford Univ. Press, Oxford.
- Derryberry, E.P., Danner, R.M., Danner, J.E., Derryberry, G.E., Phillips, J.N., Lipshutz, S.E., Gentry, K. & Luther, D.A. 2016. Patterns of song across natural and anthropogenic soundscapes suggest that white-crowned sparrows minimize acoustic masking and maximize signal content. PLoS One 11: e0154456 doi: 10.1371/journal.pone.0154456
- Derryberry, E.P., Seddon, N., Derryberry, G.E., Claramunt, S., Seeholzer, G.F., Brumfield, R.T. & Tobias, J.A. 2018. Ecological drivers of song evolution in birds: disentangling the effects of habitat and morphology. Ecol. Evol. 8: 1890–1905. doi: 10.1002/ece3.3760
- Doutrelant, C., Aubin, T., Hitier, S. & Lambrechts, M.M. 1998. Two distinct song populations of blue tit Parus caeruleus in the French Mediterranean. Bioacoustics 9: 1–16. doi: 10.1080/09524622.1998.9753376
- Eriksson, D. & Wallin, L. 1986. Male bird song attracts females – a field experiment. Behav. Ecol. Sociobiol. 19: 297–299. doi: 10.1007/BF00300645
- Francis, C.D., Ortega, C.P. & Cruz, A. 2011. Noise pollution filters bird communities based on vocal frequency. PLoS One 6: e27052. doi: 10.1371/journal.pone.0027052
- Fernández-Juricic, E., Poston, R., De Collibus, K., Morgan, T., Bastain, B., Martin, C., Jones, K. & Treminio, R. 2005. Microhabitat selection and singing behavior patterns of male house finches (Carpodacus mexicanus) in urban parks in a heavily urbanized landscape in the western US. Urban Habitats 3: 49–69.
- Goodman, G.T. & Gillham, M.E. 1954. Ecology of the Pembrokeshire islands: II. Skokholm, environment and vegetation. J. Ecol. 43: 296–327. doi: 10.2307/2256864
- Krause, B.L. 1992. The habitat niche hypothesis: a hidden symphony of animal sounds. Lit. Rev. 36: 40–45.
- Kroodsma, D.E. 2004. The diversity and plasticity of birdsong. In P. Marler & H. Slabbekoorn (Eds.), Nature’s Music: the science of birdsong, Vol. 4: 108–131. Elsevier.
- Kroodsma, D.E., Byers, B.E., Halkin, S.L., Hill, C., Minis, D., Bolsinger, J.R., Dawson, J.A., Donelan, E., Farrington, J., Gill, F.B. & Houlihan, P. 1999. Geographic variation in black-capped chickadee songs and singing behavior. Auk. 116: 387–402. doi: 10.2307/4089373
- Laiolo, P. & Tella, J.L. 2005. Habitat fragmentation affects culture transmission: patterns of song matching in DuPont’s lark. J. Appl. Ecol. 42: 1183–1193. doi: 10.1111/j.1365-2664.2005.01093.x
- Luther, D. & Baptista, L. 2010. Urban noise and the cultural evolution of bird songs. Proc. Royal Soc. B 277: 469–473. doi: 10.1098/rspb.2009.1571
- Lynch, A. 1996. The population memetics of birdsong. In D. E. Kroodsma & E. H. Miller (Ed.), Ecology and Evolution of Acoustic Communication in Birds, 181–197. Cornell University Press.
- Macdougall-Shackleton, S.A. 1997. Sexual selection and the evolution of song repertoires. In V. Nolan, E. D. Ketterson, & C. F. Thompson (Eds.), Current Ornithology, 81–124. Springer, Boston, MA.
- Martin, P.R. & Martin, T.E. 2001. Behavioral interactions between coexisting species: song playback experiments with wood warblers. Ecol. 82: 207–218. doi: 10.1890/0012-9658(2001)082[0207:BIBCSS]2.0.CO;2
- Nowicki, S. & Searcy, W.A. 2005. Song and mate choice in birds: how the development of behavior helps us understand function. Auk. 122: 1–14. doi: 10.1093/auk/122.1.1
- Parker, K.A. & Laurence, J. 2008. Translocation of North Island saddleback Philesturnus rufusater from Tiritiri Matangi Island to Motuihe Island, New Zealand. Conserv. Evid. 5: 47–50.
- Parker, K.A., Anderson, M.J., Jenkins, P.F. & Brunton, D.H. 2012. The effects of translocation-induced isolation and fragmentation on the cultural evolution of bird song. Ecol. Lett. 15: 778–785. doi: 10.1111/j.1461-0248.2012.01797.x
- Podos, J. & Nowicki, S. 2004. Beaks, adaptation, and vocal evolution in Darwin’s finches. Bioscience 54: 501–510. doi: 10.1641/0006-3568(2004)054[0501:BAAVEI]2.0.CO;2
- Price, T. 1998. Sexual selection and natural selection in bird speciation. Philos. Trans. R. Soc. B 353: 251–260. doi: 10.1098/rstb.1998.0207
- R Core Team. 2019. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Ross-Smith, V.H., Conway, G.J., Facey, R.J., Bailey, B.H., Lipton, M., Whitfield, S.A. & Ferns, P.N. 2013. Population size, ecology and movements of gulls breeding on Flat Holm Island. Birds in Wales 10: 7–21.
- Sangster, G. & Luksenburg, J.A. 2015. Declining rates of species described per taxonomist: slowdown of progress or a side-effect of improved quality in taxonomy? Syst. Biol. 64: 144–151. doi: 10.1093/sysbio/syu069
- Searcy, W.A. & Johnson, L.S. 1996. Female attraction to male song in house wrens (Troglodytes aedon). Behaviour 133: 357–366. doi: 10.1163/156853996X00495
- Slabbekoorn, H. & den Boer-Visser, A. 2006. Cities change the songs of birds. Curr. Bio. 16: 2326–2331. doi: 10.1016/j.cub.2006.10.008
- Slabbekoorn, H. & Smith, T.B. 2002. Bird song, ecology and speciation. Phil. Trans. R. Soc. Lond. B 357: 493–503. doi: 10.1098/rstb.2001.1056
- Thompson, G.V.F. 2008. The Natural History of Skokholm Island. Trafford Publishing, Bloomingto, USA.
- Wilkins, M.R., Seddon, N. & Safran, A.J. 2013. Evolutionary divergence in acoustic signals: causes and consequences. Trends Ecol. Evol. 28: 156–166. doi: 10.1016/j.tree.2012.10.002
- Yang, X.J. & Slabbekoorn, H. 2014. Timing vocal behavior: lack of temporal overlap avoidance to fluctuating noise levels in singing Eurasian wrens. Behav. Process. 108: 131–137. doi: 10.1016/j.beproc.2014.10.002
- Zuur, A., Ieno, E.N. & Smith, G.M. 2007. Analyzing Ecological Data. Springer Science & Business Media, New York, USA.