ABSTRACT
Capsule: Wintering male Bull-headed Shrikes Lanius buchepalus preferred vegetable fields with perch sites to search for and detect terrestrial prey, and males occupying territories with large areas of vegetable fields acquired more prey and cached more food.
Aims: To better understand effects of habitat use on food acquisition and food caching of the Bull-headed Shrike, we investigated relationships between habitat quality (measured through foraging-site selection and foraging success) and food caching during the non-breeding season.
Methods: We monitored 66 territorial male shrikes during the non-breeding season from 2014 to 2016, and collected data on foraging-site selection, foraging success, and food caching.
Results: Our field observations showed that male shrikes preferred to forage over vegetable fields and that males occupying territories incorporating large areas of that habitat were able to acquire more food items and store more food caches in their territories during the nonbreeding season.
Conclusion: We suggest that for male Bull-headed Shrikes, a winter-breeding food-storing passerine, the quality of habitat in the nonbreeding season has the potential to affect their subsequent fitness.
Food caching is a well-known behaviour in animals that inhabit environments where food availability fluctuates seasonally (Vander Wall Citation1990). Food hoarders often rely on food caches stored in autumn for survival during winter (Jansson et al. Citation1981, Halonen et al. Citation2007), moreover some species support their reproduction from food caches (Koenig & Mumme Citation1987, Stacey & Ligon Citation1987, Korpimäki Citation1989, Esely & Bollinger Citation2002, Antczak et al. Citation2005). A subset of food hoarders commence breeding in winter when food availability is very low (e.g. Wolverine Gulo gulo, Persson Citation2005; American Red Squirrel Tamiasciurus hudsonicus, Fletcher et al. Citation2013; Gray Jay Perisoreus canadensis, Sechley et al. Citation2014). Winter-breeding food caching species are considered to be more heavily dependent on stored food than food caching species that breed in warmer periods, and the great variation in their individual fitness can be strongly influenced by the conditions they experience in the nonbreeding season, leading to carryover effects (Harrison et al. Citation2011). However, to date, the mechanisms driving carryover effects on reproduction in winter-breeding food caching species have only been identified in a limited number of species (Persson Citation2005, Fletcher et al. Citation2013, Sechley et al. Citation2014).
The basis for the carryover effects on reproduction in winter-breeding food hoarders has mainly come from studies of females (Persson Citation2005, Fletcher et al. Citation2013, Sechley et al. Citation2014). This is because females typically allocate more energy resources to reproduction (egg production, gestation, or lactation). Little is known about the carryover effect on male reproductive performance in winter-breeding food caching species (Soulsbury Citation2019). Males typically invest in acquiring mating opportunities (Andersson Citation1994). Male secondary sexual traits serve to attract potential mates and/or repel rivals, and the energetic costs are usually very high (Searcy & Nowicki Citation2005). We therefore hypothesized that if carryover effects on male reproductive performance occurred in winter-breeding food caching species, (i) the expression of male condition-dependent sexual traits and their mating success would be highly dependent on food caches stored during the nonbreeding season, and (ii) food hoarding would be influenced by nonbreeding season environmental variables.
True shrikes of the family Laniidae commonly impale prey on sharp objects in their territories for later consumption (Lefranc & Worfolk Citation1997). Although both male and female shrikes exhibit such behaviour, males often cache more frequently (Applegate Citation1977, Keynan & Yosef Citation2010, Antczak et al. Citation2012), and the food caching of males is considered to be related to sexual selection (Yosef & Pinshow Citation1989, Citation1994, Keynan & Yosef Citation2010, Antczak et al. Citation2005, Citation2012, Morelli et al. Citation2013, Citation2015, Nishida & Takagi Citation2019). The Bull-headed Shrike Lanius bucephalus is a common winter-breeding passerine occurring in Japan. During the nonbreeding season from summer to autumn, territorial male Bull-headed Shrikes store, by impaling on thorns, twigs and barbed wire, a wide variety of prey such as insects, arthropods, earthworms, reptiles, frogs and fish; they then retrieve most of their food caches just before winter-breeding commences (Nishida & Takagi Citation2019). During the breeding period, male shrikes sing until they mate. Female Bull-headed Shrikes prefer males singing at a high tempo, a trait which reliably reflects the nutritional condition of males (Nishida & Takagi Citation2018, Citation2019). Observation showed that male shrikes that retrieved more food caches before breeding improved their body condition and sang at a higher tempo (Nishida & Takagi Citation2019). In addition, field experiments showed that when food caches were artificially removed from male territories, the males sang at slower tempos and failed to mate; conversely, when their food caches were augmented, males sang at higher tempos and mated earlier (Nishida & Takagi Citation2019). The food caches of male Bull-headed Shrikes thus provide supplementary nutrition improving their song quality and mating success. In order to understand the mechanistic links between a non-breeding season process and the subsequent mating success of male food hoarding Bull-headed Shrikes, we studied the environmental factors that influenced their food acquisition and food caching during the non-breeding season.
The Laniidae generally require open hunting grounds with perch sites from which they can search for and detect terrestrial insects and small vertebrates (Yosef Citation1993, Citation2004, Yosef & Grubb Citation1994, Morelli et al. Citation2016). Having acquired sufficient food to meet their hunger, they then store many food caches in their territories (Wemmer Citation1969, Valera et al. Citation2001, Keynan & Yosef Citation2010). In the shrikes, habitat quality may thus affect food acquisition and food caching by territory owners during the nonbreeding breeding season (habitat-mediated carryover effects hypothesis: Sechley et al. Citation2014). It is known that open agricultural land is an important foraging habitat for Bull-headed Shrikes (Matsui & Takagi Citation2017, Hamao et al. Citation2018, Nishida & Takagi Citation2018). According to the habitat-mediated carryover effects hypothesis, we predict that male Bull-headed Shrikes occupying high-quality habitats will acquire more prey items and cache food more frequently during the nonbreeding season, than males occupying low-quality habitats.
Methods
Study site and species
The Bull-headed Shrike is a socially monogamous passerine species occurring throughout Japan (Ornithological Society of Japan Citation2012). Their main prey consists of insects (Ogawa Citation1977). Most populations of Bull-headed Shrike are migratory (Lefranc & Worfolk Citation1997). Both sexes of this species arrive in our study area in southern Osaka, Japan (34°28ʹN 135°35ʹE) during September and October. Males establish wintering territories in areas of agricultural fields, whereas females establish theirs in residential areas and forest (Nishida & Takagi Citation2018). Males and females overwinter in their own territories until the end of January. Individual territories hardly overlap. Male shrikes use their wintering territories also for breeding (February to May), but females leave their winter territories to breed in the territories of favoured males (Nishida & Takagi Citation2019). From February onwards males sing in their wintering territories and females prefer those singing at a high tempo as reproductive partners (Nishida & Takagi Citation2018, Citation2019). Mated pairs then build open cup nests in shrubs, bushes, or bamboo clumps in the males’ territories. Females lay four to six eggs and incubate their clutches for about two weeks. Both parents feed their nestlings in the nests and fledglings in the vicinity of the nests (Nishida & Takagi Citation2018). The Bull-headed Shrike population in our study area is considered to migrate to the north or northwest after breeding (Lefranc & Worfolk Citation1997), returning to our study area during September and October. As the return rate of the Bull-headed Shrike is usually low (Takagi Citation2003a), new populations are formed almost every year and territories are rarely re-occupied by the same individuals in subsequent years.
Territory mapping
We mapped the winter territories of 66 male Bull-headed Shrikes in the morning (0600 to 1000) from October 2014 to January 2015 (nmale = 15), from October 2015 to January 2016 (nmale = 26), and from October 2016 to January 2017 (nmale = 25). Since birds are usually more active in sunny or cloudy weather than in rain, and because it is easy to acquire foraging data, surveys were conducted only on sunny or cloudy days. Although newly arrived Bull-headed Shrikes show direct aggressive behaviour between individuals for acquisition of territory, their aggression decreases after they have established their territories. For territory mapping, we randomly chose one male among individuals showing little aggression with their neighbours (i.e. those whose territories were considered to be established), tracked him for about four hours using binoculars (10.5 × 44), and recorded the perch sites that he used on a map. A minimum convex polygon of the perch sites used was defined as his territory. Male Bull-headed Shrike territories do not overlap and the position of the territories did not change during the nonbreeding and breeding seasons (Nishida & Takagi, Citation2018). We were, therefore, able to use male territories as identification markers, and ensure that data (male foraging behaviour, food caches, etc.) were accurately assigned to individual birds. In our study area, farmers cultivate rice Oryza sativa subsp. Japonica, vegetables such as Japanese Radish Raphanus sativus var. longipinnatus, Taro Colocasia esculenta and Napa Cabbage Brassica rapa var. pekinensis, and fruits such as oranges Citrus unshiu and figs Ficus carica (Japan Ministry of Agriculture, Forestry and Fisheries Citation2015). We classified the habitat types in territories as: vegetable field, rice field, orchard, forest, abandoned farmland, road, building or pond. Aerial photographs and Quantum GIS 2.10.1 software (www.qgis.org) were used to calculate each male’s territory size and the area of each habitat type it contained.
Observations of male foraging behaviour
During the territory mapping surveys (nmale = 66), we observed male foraging behaviour from a distance of about 20 m. As the Bull-headed Shrike is a mainly insectivorous passerine (Ogawa Citation1977), we defined attacks by males on prey such as insects as foraging attempts, and did not include attacks on other birds as foraging attempts. We classified the foraging of males as foraging towards prey in the air or on the ground, and then classified the habitat types for ground foraging as vegetable field, rice field, orchard, forest, abandoned farmland, road, building or pond. The results of foraging attempts were classified as ‘successful’, when males were seen to carry prey, as ‘unsuccessful’ when they carried no prey, or as ‘not determined’ when we were unable to confirm the result of the foraging attempt because the males were lost to view. The success rate of attempts was calculated as the percentage of successful attempts of the total of successful and unsuccessful attempts observed. We recorded the perches males used just before foraging as tree, pole, power line, roof and ground, and estimated the height of each perch site above the ground (0 m, >0 and ≤1.5 m, >1.5 and ≤3 m, >3 and ≤6 m, >6 and ≤12 m, and >12 m).
Survey of food caches in male territories
We defined the food caches of the Bull-headed Shrikes as prey impaled on sharp objects in their territories. As the Bull-headed Shrikes use many food-storage sites in their territories and store several food items at each food-storage site, we surveyed potential food-storage sites (e.g. trees and barbed wire) in territories of 17 males once a month from October 2015 to January 2016 (nmale = 9) and from October 2016 to January 2017 (nmale = 8), to count the total number of food caches in each territory. In order to accurately count the number of food caches, we carefully examined the territory of one individual per day. For this reason, we chose 17 males at random (from the 66 males whose territories had been mapped and whose foraging behaviour had been studied) and conducted surveys of their food caches. Each cache found was given a numbered label (10 cm from each cache) and recorded on a map, to reduce miscounts of food caches.
Male morphological traits and age
To measure morphological traits of the 66 male Bull-headed Shrikes whose foraging behaviour was observed, we tried to capture all of them. However, we were able to catch only 10 males during their breeding season with mist nets as other studies proceeded in parallel. We measured morphological traits (tarsus length, tail length, and, natural wing length) to the nearest 0.01 mm with digital callipers.
Male age was identified as ‘yearling’ (i.e. born within the previous year) or ‘adult’ (i.e. older than yearling), based on the presence of buff-tipped upper greater primary coverts in adults (Yamagishi Citation1982), or from the colour rings attached when previously caught. We were thus able to determine the ages of 10 individuals captured, as well as 23 individuals captured previously. We were, therefore, able to use morphological data for 10 males, and age data for 33 males in our analysis.
Weather conditions
We used minimum daily temperature and average daily wind intensity (m/s) as recorded by the Japan Meteorological Agency at a site located about 15 km from our research site.
Statistical analysis
To investigate whether male Bull-headed Shrikes preferred to forage in specific habitats, we performed habitat selection analysis by comparing the number of foraging attempts observed and expected in each habitat. When the number of attempts observed was greater than the number of attempts expected, the shrikes were considered to have preferred to forage in the habitat, and when the number of attempts observed was fewer than the number of attempts expected, they were considered to avoid the habitat. The number of attempts expected in each habitat was calculated as the multiplication of the total number of foraging attempts observed (during four-hours surveys of male foraging) and the ratio of the area of each habitat (that was calculated as the area of each type of habitat divided by the total area of male territories). The statistical test for differences between the attempts observed and expected in each habitat was performed using Manly’s standardized selection ratio method. In the analysis, resource selectivity index w of each habitat was calculated as the number of foraging attempts observed divided by the number of attempts expected in the habitat (Manly’s standardized selection ratio method, design type III function; Manly et al. Citation2007). The index, w, is 1 when male shrikes use the habitat at random. If 95% confidence intervals of index w did not include 1, then males were considered to significantly prefer (w > 1) or avoid (w < 1) the habitats.
To examine which factors were associated with the success rate of male foraging, we constructed generalized linear mixed models (GLMMs) with a Bernoulli distribution and a logit link function, including foraging attempt (successful: 1; unsuccessful: 0) as the response variable. Since foraging attempts include repeated samples from the same individual, we included individual identity as a random factor in the GLMMs. We considered the following seven categories as potential factors affecting male foraging success: (1) foraging habitat type, (2) perch type, (3) perch height, (4) weather conditions (daily minimum temperature and daily average wind intensity), (5) male morphological traits (length of wing, tail and tarsus), (6) age and (7) year, date and time (the year and date of each field observation and the time of the observation of each foraging attempt). We constructed seven models with one of these categories as the explanatory variable and analysed each model. Because the sample size for male morphological traits was small (nmale = 10), we were unable to analyse a model with all morphological traits. We thus constructed three separate models with one morphological trait as an explanatory variable and analysed each model.
To test the habitat-mediated carryover effect hypothesis on food caching, we investigated whether male Bull-headed Shrikes occupying high-quality habitats cached food during the nonbreeding season. We constructed the GLM with a negative binomial distribution and a log link function, including the total number of food caches stored during the nonbreeding season as the response variable, the degree of habitat quality (i.e. area of each habitat type in each male’s territory) as the explanatory variable, and the survey years as the covariate variable. As the return rate of the Bull-headed Shrike is usually low (Takagi Citation2003a) and new populations are formed almost every year, the total number of food caches stored in male territories during the nonbreeding season does not have the structure of repeated observation within an individual. We did not, therefore, need to construct the GLMM including individual identity as a random effect.
All statistical analyses were performed using the statistical software R version 3.1.0 software (R Foundation for Statistical Computing, Vienna, Austria), and packages ‘adehabitatHS’, ‘lme4’, ‘lmerTest’, ‘pscl’, ‘phia’, and ‘multcomp’. The significance level for all statistical analyses was set at P < 0.05, and P values were obtained using Bonferroni correction. In our analyses, we used only data for which the characteristics of foraging accounted for more than 80% of the total observations of foraging attempts, so as to avoid the extreme declines of statistical power caused by multiple adjustments of P values. Unless otherwise stated, values are given as means and SD.
Ethical note
All field observations adhered to the Association for the Study of Animal Behaviour guidelines for the treatment of animals in behavioural research and were conducted in compliance with the Regulations on Animal Experiments of Osaka City University. No permits were needed from the Japanese government because our study was based on field observations of the Bull-headed Shrike.
Results
Descriptions of male foraging behaviour
We recorded a total of 397 foraging attempts by 66 male Bull-headed Shrikes during a total of 110 h of observations. Of the foraging attempts observed, 119 were successful, 197 were unsuccessful and 81 were not determined. The foraging success rate of each male was 41.9 ± 32.8%. Males hunted either terrestrial prey (98.3% of all foraging attempts observed) or flying insects (1.7%).
Males used a variety of perches just before hunting. Perch types mainly used were trees (46.9%), poles (31.0%), and roofs (9.0%; online Table S1). Perch height varied from 0 m to more than 12 m, with the majority of > 0 and ≤1.5 m (43.8%), >1.5 and ≤3 m (35.5%), and >3 and ≤6 m (11.1%; Table S2).
We observed males foraging in seven habitat types – vegetable fields, rice fields, abandoned farmlands, orchards, buildings, roads, and forests – but they used mainly vegetable fields (52.3%) and rice fields (33.9%; Table S3).
As the main foraging strategy (hunting terrestrial prey), the main perch types (trees, poles, roofs), perch heights (>0 and ≤1.5 m, >1.5 and ≤3 m, and >3 and ≤6 m), and foraging habitats (vegetable fields and rice fields) accounted for more than 80% of all observations of foraging attempts (Tables S1–S3), we used the ground foraging strategy, the three perch types, the three perch heights, and the two foraging habitats of male foraging in the following analysis, and the rest of the observations were excluded from the analyses.
Habitat selection by foraging males
Territory mapping surveys showed that male territories had a mean area of 7.028 ± 5.351 m2 (nmale = 66). The mean area of vegetable fields and rice fields in each territory was 23.9 ± 17.4% and 33.5 ± 20.6% respectively (). Habitat selection analysis showed that male Bull-headed Shrikes did not prefer to use rice fields (Manly’s standardized selection ratio method; nmale = 66, wi = 0.943, 95%CI = [0.572, 1.314]), but significantly preferred to use vegetable fields (nmale = 66, wi = 2.031, 95%CI = [1.302, 2.760]; ).
Table 1. Foraging habitat selection analysis comparing the proportion (±SD) of area of each habitat and the percentage of foraging attempts in each habitat by male Bull-headed Shrikes. The preference index is indicated by w, see Methods for details.
Factors affecting male foraging success
Mean foraging success on vegetable fields was 43.6 ± 37.2% and on rice fields 34.2 ± 39.7%. Males hunted prey more successfully from vegetable fields than from rice fields (reference category: interceptrice field; interceptvegetable field = 0.678, SE = 0.297, z = 2.282, P = 0.023). The success rate of hunting from trees, poles, and roofs was 45.6 ± 39.3%, 35.2 ± 36.7%, 30.6 ± 36.1%, respectively. No correlations were found between the success rate and perch types used just before foraging (Table S4). The success rate of perch height of > 0 and ≤1.5 m, >1.5 and ≤3.0 m, and > 3 and ≤6 m was 35.0 ± 36.5%, 50.1 ± 38.1%, and 43.5 ± 45.1%, respectively. Similarly, there were no differences between success rate and perch height (Table S5). The success rate of adult and yearling males was 39.8 ± 31.8% and 37.2 ± 26.6%. No relationships were found between success rate and male age (Table S6), and male morphological traits (Table S7). Weather conditions did not affect foraging success (Table S8). In addition, foraging success was not influenced by the year and date of each field observation and the time of the observation of each foraging attempt (Table S9).
Habitat-mediated carryover effect on male food caches
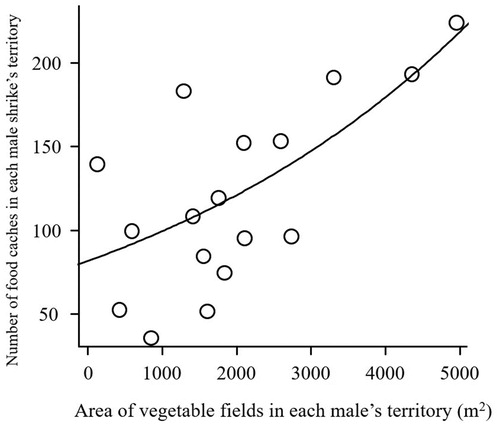
We surveyed 17 male territories during the nonbreeding season (from October to January), and found a total of 2099 food caches. There was no relationship between the number of caches and the area of rice fields in a male’s territory (nmale = 17, slopearea of rice field = −30.98, SE = 32.02, z = −0.967, P = 0.333). However, males occupying territories with large areas of vegetable fields cached more food during the nonbreeding season (nmale = 17, slopearea of vegetable field = 244.6, SE = 91.29, z = 2.680, P = 0.007; ).
Discussion
We found that male Bull-headed Shrikes preferred to use vegetable fields as foraging habitat, and males that occupied territories with large areas of vegetable fields were able to acquire more food items and cache more food in their territories during the nonbreeding season. It is known that male Bull-headed Shrikes retrieve most of their stored food just before the breeding season commences, and males that consume many food caches improve their song quality, and in turn increase their mating success (Nishida & Takagi Citation2018, Citation2019). Therefore, we suggest that in the male Bull-headed Shrike, habitat quality can affect the expression of sexual traits several months later through food caching. This is the first study supporting the habitat-mediated carryover effect in the context of sexual selection in a winter-breeding, food caching species.
Foraging of male Bull-headed Shrikes
We found that Bull-headed Shrike males used trees and poles as perch sites to search for terrestrial prey and hunted terrestrial prey on vegetable fields and rice fields (Tables S1–S3). This is a typical foraging strategy in the Laniidae (Yosef Citation1993, Citation2004, Yosef & Grubb Citation1994, Morelli et al. Citation2016). Habitat selection analysis showed that male Bull-headed Shrikes preferred to use vegetable fields as foraging habitat (), and males occupying territories with large areas of vegetable fields were able to acquire more food items. In an earlier study, we presented evidence that the paternal provisioning rate to nestlings was positively related to the area of vegetable fields within the males’ territories (Nishida & Takagi Citation2018). It seems that vegetable fields are an important foraging habitat for the population of Bull-headed Shrikes that we studied.
The Bull-headed Shrike generally requires an open hunting substrate to search for and detect prey on the ground (Matsui & Takagi Citation2017, Hamao et al. Citation2018, Nishida & Takagi Citation2018). In our study area, farmers cultivate vegetables all-year-round (Japan Ministry of Agriculture, Forestry, and Fisheries Citation2015), and they regularly remove the undergrowth from their vegetable fields. In addition, farmers use many poles to support the stems and branches of their vegetables (personal observation). These vegetable fields seem to provide ideal open hunting grounds with many perch sites where Bull-headed Shrikes can search for and detect terrestrial prey.
Male Bull-headed Shrikes also used rice fields as an important foraging habitat (Table S3), but they did not significantly prefer to use rice fields in relation to their area (). Takagi (Citation2003a), studying a different population of Bull-headed Shrikes in Hokkaido, found that they also did not prefer to use rice fields. Our field observations showed that foraging success in rice fields is lower than in vegetable fields, and the area of rice fields was not related to the number of successful hunts by males. This suggests that rice fields are not a useful habitat for foraging Bull-headed Shrikes. The rice field substrate consists of clay (Amano Citation1985) so that rainwater tends to pool on the ground. The main prey of Bull-headed Shrikes is insects (Ogawa Citation1977) that tend to prefer to live on dry substrates. In addition, rice fields offer few perch sites because rice farmers remove poles and trees from their rice fields as they interfere with mechanized rice-harvesting. For this reason, rice fields are an unsuitable habitat for foraging Bull-headed Shrikes. Finally, there appear to be fewer food resources in rice fields rendering them unsuitable for foraging. However, as we did not survey and measure the actual amount of prey in each habitat in male territories, this remains an issue for future study.
The foraging success of animals is generally influenced not only by the quality of habitat but also by age relating to the learning process of foraging and morphological traits relating to flight manoeuvrability (Marchetti & Price Citation1989). For example, in a population of Bull-headed Shrikes breeding in Hokkaido, adult males provide high-quality food to their offspring (Takagi Citation2003b), suggesting that older individuals have greater foraging skills. If male Bull-headed Shrikes occupying territories with large areas of vegetable fields were adults and/or had traits that improve foraging efficiency, then the relationship between successful hunting and habitat quality could be a pseudo-correlation in our study. Weather conditions are also closely related to foraging behaviour (e.g. Red-backed Shrike Lanius collurio, Tryjanowski et al. Citation2003; Bull-headed Shrike, Takagi Citation2001). However, our detailed analysis showed that male foraging success was not related to male age or morphological traits, or the types and heights of perches used just before hunting, or the weather conditions on the date of our observations. Our previous studies have shown that the area of vegetable fields in male territories was not correlated with the age or morphological traits of male Bull-headed Shrikes (Nishida & Takagi Citation2018). We suggest therefore that habitat quality directly affects the foraging and hunting success of male Bull-headed Shrikes.
Food caching by male Bull-headed Shrikes
Our field observations showed that male Bull-headed Shrikes occupying territories with large areas of vegetable fields were able to hunt more prey and cached more food within their nonbreeding territories (). Shrikes generally cache more food after acquiring sufficient food to meet their hunger (Wemmer Citation1969, Valera et al. Citation2001, Keynan & Yosef Citation2010). For example, there was a positive relationship between the quantity of mealworms experimentally provided to Southern Grey Shrikes Lanius meridionalis and the frequency at which they cached them (Keynan & Yosef Citation2010). Hunger and the amount of prey acquired during the nonbreeding season were therefore considered to be proximate factors inducing food caching in Bull-headed Shrikes. Male Bull-headed Shrikes retrieve most of their food caches just before commencing winter-breeding (Nishida & Takagi Citation2019). Male shrikes with more food caches sing at a higher tempo and are preferred by females (Nishida & Takagi Citation2018, Citation2019). In field experiments, removing food caches from male territories led to male shrikes singing at a slower tempo and failing to mate; conversely augmenting their food caches led to males singing at a faster tempo and mating early (Nishida & Takagi Citation2019). These experiments indicated that food caches provided supplementary nutrition and improved the song quality and mating success of male Bull-headed Shrikes. In our present study, we found that the number of food items stored before the winter-breeding season commenced was strongly dependent on the quality of the nonbreeding territory and food acquisition during the nonbreeding season. In addition, our earlier study showed that males overwintering in high-quality territories (i.e. territories with large areas of vegetable fields) sang at a higher tempo during the breeding season (Nishida & Takagi Citation2018). This is the first study supporting the habitat-mediated carryover effect in the context of sexual selection in a winter-breeding food caching species.
However, we need to note a pseudo-correlation between territory quality and food caching in male Bull-headed Shrikes. We were not able to control other potential confounding factors (such as male age, morphological traits, and weather conditions) that had the potential to affect food caching. Therefore, in order to demonstrate habitat-mediated carryover effects on food caching more clearly in this species, it will be necessary to conduct experimental manipulations such as male territory transposition experiments, then investigate the relationship between food caching and territory quality.
Although we focused on the number of food caches stored by male Bull-headed Shrikes, environmental factors during the nonbreeding season may affect the quality of their food caches. For example, a study of the Gray Jay (Sechley et al. Citation2014) showed that weather conditions (e.g. temperature) during the period when food items were stored can reduce the quality of food caches and adversely affect subsequent reproductive performance (Sutton et al. Citation2019). As Bull-headed Shrikes store perishable food such as insects (Karasawa Citation1976, Nishida & Takagi Citation2019), environmental factors, (e.g. rain, temperature, and UV radiation) might strongly degrade the quality of their food in the nonbreeding season and then their reproductive performance in the following breeding season (Sutton et al. Citation2016). Attention should also be paid to the impacts of agricultural herbicides or pesticides on Bull-headed Shrikes. Insects preferred by shrikes sometimes cause crop damage. Farmers might use herbicides or pesticides frequently in years with high insect infestations, which may lead to ecological traps. In the future, we should assess the detrimental impact of environmental conditions during the non-breeding season associated with human activities (e.g. climate change and use of pesticides) on the fitness of the Bull-headed Shrikes that store perishable food.
Acknowledgments
We are greatly indebted to Professor Masanori Kohda, Associate Professor Satoshi Awata, Dr Shun Satoh, Mr Taiga Saeki, and Mr Daisuke Aoki for their valuable comments on and suggestions relating to this study. We are grateful to farmers in the study area for kindly supporting our field research, and to Mr Kota Nomoto for valuable information about shrike caches. We also thank Dr Mark Brazil, Scientific Editing Services, for assistance in the preparation of the final draft of the manuscript. Y. N. and M. T. designed the study. Y. N. collected data and performed analyses. Y. N. and M. T. wrote the manuscript. We declare we have no competing interests.
References
- Amano, Y. 1985. Classification of cultivated soils in Japan. Jpn. Agric. Res. Q. 18: 275–283.
- Andersson, M. 1994. Sexual Selection. Princeton University Press, Princeton, NJ.
- Antczak, M., Hromada, M. & Tryjanowski, P. 2005. Spatio-temporal changes in Great Grey Shrike Lanius excubitor impaling behaviour: from food caching to communication signs. Ardea 93: 101–107.
- Antczak, M., Hromada, M. & Tryjanowski, P. 2012. Sex differences in impaling behaviour of Great Grey Shrike Lanius excubitor: do males have better impaling skills than females? Behav. Process. 91: 50–53. doi: 10.1016/j.beproc.2012.05.007
- Applegate, R.D. 1977. Possible ecological role of food caches of loggerhead shrike. Auk 94: 391–392.
- Esely, J.D. & Bollinger, E.K. 2002. Patterns of impaling in a migratory population of the Loggerhead Shrike. Prairie Nat. 35: 1–8.
- Fletcher, Q.E., Landry-Cuerrier, M., Boutin, S., McAdam, A.G., Speakman, J.R. & Humphries, M.M. 2013. Reproductive timing and reliance on hoarded capital resources by lactating red squirrels. Oecologia 173: 1203–1215. doi: 10.1007/s00442-013-2699-3
- Halonen, M., Mappes, T., Meri, T. & Suhonen, J. 2007. Influence of snow cover on food hoarding in Pygmy Owls Glaucidium passerinum. Ornis Fenn. 84: 105–111.
- Hamao, S., Yamamoto, Y., Torikai, H., Ijichi, T. & Yoshikawa, M. 2018. Breeding ecology of naturally colonizing Bull-headed Shrikes on Kikaijima Island. Strix: J. Field Ornithol. 34: 69–80.
- Harrison, X.A., Blount, J.D., Inger, R., Norris, D.R. & Bearhop, S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80: 4–18. doi: 10.1111/j.1365-2656.2010.01740.x
- Jansson, C., Ekman, J. & von Brömssen, A. 1981. Winter mortality and food supply in tits Parus spp. Oikos 37: 313–322. doi: 10.2307/3544122
- Japan Ministry of Agriculture, Forestry & Fisheries. 2015. Basic data of agriculture, forestry, and fisheries in Osaka Prefecture. http://www.machimura.maff.go.jp/machi/contents/27/216/index.html.
- Karasawa, K. 1976. Observations on the impalements made by Shrikes. Jpn. J. Ornithol. 25: 94–100. doi: 10.3838/jjo1915.25.94
- Keynan, O. & Yosef, R. 2010. Temporal changes and sexual differences of impaling behavior in southern Grey Shrike (Lanius meridionalis). Behav. Process. 85: 47–51. doi: 10.1016/j.beproc.2010.06.005
- Koenig, W.D. & Mumme, R.L. 1987. Population Ecology of the Cooperatively Breeding Acorn Woodpecker. Princeton University Press, Princeton, NJ.
- Korpimäki, E. 1989. Breeding performance of Tengmalm's Owl Aegolius funereus: effects of supplementary feeding in a peak vole year. Ibis 131: 51–56. doi: 10.1111/j.1474-919X.1989.tb02743.x
- Lefranc, N. & Worfolk, T. 1997. Shrikes: a guide to the shrikes of the world. A&C Black, London.
- Manly, B.F.L., McDonald, L., Thomas, D.L., McDonald, T.L. & Erickson, W.P. 2007. Resource Selection by Animals: statistical design and analysis for field studies. Springer Science and Business Media, London.
- Marchetti, K. & Price, T. 1989. Differences in the foraging of juvenile and adult birds: the importance of developmental constraints. Biol. Rev. 64: 51–70. doi: 10.1111/j.1469-185X.1989.tb00638.x
- Matsui, S. & Takagi, M. 2017. Habitat selection by the Bull-headed Shrike Lanius bucephalus on the Daito Islands at the southwestern limit of its breeding range. Ornithol. Sci. 16: 79–86. doi: 10.2326/osj.16.79
- Morelli, F., Bussière, R., Goławski, A., Tryjanowski, P. & Yosef, R. 2015. Saving the best for last: differential usage of impaled prey by red-backed shrike (Lanius collurio) during the breeding season. Behav. Process. 119: 6–13. doi: 10.1016/j.beproc.2015.07.006
- Morelli, F., Mróz, E., Pruscini, F., Santolini, R., Goławski, A. & Tryjanowski, P. 2016. Habitat structure, breeding stage and sex affect hunting success of breeding Red-backed Shrike (Lanius collurio). Ethol. Ecol. Evol. 28: 136–147.
- Morelli, F., Saltarelli, M., Pruscini, F. & Benedetti, Y. 2013. First description of red-backed shrike Lanius collurio food caching in Central Italy: prey’s type and spatial position into the larders. Avocetta 37: 27–34.
- Nishida, Y. & Takagi, M. 2018. Song performance is a condition-dependent dynamic trait honestly indicating the quality of paternal care in the bull-headed shrike. J. Avian Biol. 49: e01794. doi: 10.1111/jav.01794
- Nishida, Y. & Takagi, M. 2019. Male bull-headed shrikes use food caches to improve their condition-dependent song performance and pairing success. Anim. Behav. 152: 29–37. doi: 10.1016/j.anbehav.2019.04.002
- Ogawa, I. 1977. Pellet analysis of the Bull-headed Shrike Lanius bucephalus and the seasonal change of food habits. Jpn. J. Ornithol. 26: 63–75. doi: 10.3838/jjo1915.26.63
- Ornithological Society of Japan. 2012. Check-list of Japanese Birds, 7th edn. The Ornithological Society of Japan, Tokyo, Japan.
- Persson, J. 2005. Female wolverine (Gulo gulo) reproduction: reproductive costs and winter food availability. Can. J. Zool. 83: 1453–1459. doi: 10.1139/z05-143
- Searcy, W.A. & Nowicki, S. 2005. The Evolution of Animal Communication: reliability and deception in signalling systems. Princeton University Press, Princeton, NJ.
- Sechley, T.H., Strickland, D. & Norris, D.R. 2014. Causes and consequences of pre-laying weight gain in a food-caching bird that breeds in late winter. J. Avian Biol. 45: 85–93. doi: 10.1111/j.1600-048X.2013.00296.x
- Soulsbury, C.D. 2019. Income and capital breeding in males: energetic and physiological limitations on male mating strategies. J. Exp. Biol. 222: jeb184895. doi: 10.1242/jeb.184895
- Stacey, P.B. & Ligon, J.D. 1987. Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of cooperative breeding. Am. Nat. 130: 654–676. doi: 10.1086/284737
- Sutton, A.O., Strickland, D. & Norris, D.R. 2016. Food storage in a changing world: implications of climate change for food-caching species. Clim. Change Responses 3: 12. doi: 10.1186/s40665-016-0025-0
- Sutton, A.O., Strickland, D., Freeman, N.E., Newman, A.E. & Norris, D.R. 2019. Autumn freeze-thaw events carry over to depress late-winter reproductive performance in Canada jays. R. Soc. Open. Sci. 6: 181754. doi: 10.1098/rsos.181754
- Takagi, M. 2001. Some effects of inclement weather conditions on the survival and condition of bull-headed shrike nestlings. Ecol. Res. 16: 55–63. doi: 10.1046/j.1440-1703.2001.00371.x
- Takagi, M. 2003a. Philopatry and habitat selection in Bull-headed and Brown shrikes. J. Field Ornithol. 74: 45–53. doi: 10.1648/0273-8570-74.1.45
- Takagi, M. 2003b. Different effects of age on reproductive performance in relation to breeding stage in Bull-headed Shrikes. J. Ethol. 21: 9–14. doi: 10.1007/s10164-002-0068-5
- Tryjanowski, P., Karg, M.K. & Karg, J. 2003. Diet composition and prey choice by the red-backed shrike Lanius collurio in western Poland. Belg. J. Zool. 133: 157–162.
- Valera, F., Krištín, A. & Hoi, H. 2001. Why does the Lesser Grey Shrike (Lanius minor) seldom store food? Determinants of impaling in an uncommon storing species. Behaviour 138: 1421–1436. doi: 10.1163/156853901317367672
- Vander Wall, S.B. 1990. Food Hoarding in Animals. University of Chicago Press, Chicago, IL.
- Wemmer, C. 1969. Impaling behaviour of the Loggerhead Shrike, Lanius ludovicianus Linnaeus 1. Z. Tierpsychol. 26: 208–224. doi: 10.1111/j.1439-0310.1969.tb01946.x
- Yamagishi, S. 1982. Age determination in the Bull-headed Shrike Lanius bucephalus based on buff-tips of greater primary coverts. J. Yamashina Institute Ornithol. 14: 96–102.
- Yosef, R. 1993. Influence of observation posts on territory size of Northern Shrikes. Wilson Bull. 105: 180–183.
- Yosef, R. 2004. Perch-site use and inter- and intraspecific aggression of migratory Brown Shrikes (Lanius cristatus) in southern Taiwan. Biol. Lett. 41: 113–118.
- Yosef, R. & Grubb, T.C. 1994. Resource dependence and territory size in Loggerhead Shrikes (Lanius ludovicianus). Auk 111: 465–469. doi: 10.2307/4088611
- Yosef, R. & Pinshow, B. 1989. Cache size in shrikes influences female mate choice and reproductive success. Auk 106: 418e421.