ABSTRACT
‘Peeks’, or keeping an eye open, in wintering Common Pochard Aythya ferina, while in a sleeping posture, were relatively brief, typically (66% of observations) lasting less than one second. However, in 37% of individuals some peeks lasted 10 s or more. Contrary to our prediction of lower anti-predatory vigilance rates in larger groups, there was a positive relationship between peek duration and the numbers of neighbours in a flock.
Sleep is a behavioural state typically thought to be critical in physical and neural recovery, and has been documented in most animal taxa where studied (Lesku et al. Citation2009). It is characterized by changes in the electrical activity of the brain, as well as by various behavioural criteria, such as reduced responsiveness to stimuli, immobility that may be rapidly reversed, eye closure, specific resting sites and characteristic sleeping postures (Siegel Citation2008, Lesku et al. Citation2009). However, behaviour and posture are not always suitable indicators of sleep, as sleeping animals may give impression of wakefulness, while those that are awake may look like they are asleep (Aulsebrook et al. Citation2016, Rattenborg et al. Citation2017). Moreover, some birds and aquatic mammals exhibit unihemispheric sleep, whereby one cerebral hemisphere sleeps whereas the other one is awake, and the eye opposite to the awake hemisphere is open (Rattenborg et al. Citation1999a, Lima et al. Citation2005).
Unihemispheric sleep is documented in various avian taxa (Rattenborg et al. Citation2000, Citation2016). In this form of sleep, the avian brain displays interhemispheric electroencephalography (EEG) asymmetry, usually accompanied with asymmetric eye closure (Rattenborg et al. Citation2000, Mascetti Citation2016). In addition, in many birds vigilant sleep is recorded, i.e. sleep interrupted with short eye openings, so-called peeks, used to scan the environment for predators and/or competitors (Lendrem Citation1983, Gauthier-Clerc et al. Citation1998). While peeking may indicate unihemispheric sleep, it may not be justified to assume that peeking birds are in unihemispheric sleep as, due to lateral eye position on the avian head, typically it is not possible to observe both eyes simultaneously under field conditions. However, regardless of our inability to distinguish between these two states, the function of both unihemispheric sleep and peeks is likely to monitor the environment for potential threats (Rattenborg et al. Citation1999b, Beauchamp Citation2009). As such, we predicted that peeks would be shorter when sleeping in larger groups that provide better protection from approaching threats in group-living organisms (Beauchamp Citation2008).
Despite much interest in avian vigilant sleep, there is still no adequate description of peeks in terms of duration. Lendrem (Citation1983) assumed ‘negligible duration’ of peeks, whereas Lendrem (Citation1984) described peeks less than 1 s in duration as ‘short’. Similarly, some authors defined peeks as brief periods of eye opening (Gauthier-Clerc et al. Citation1994, Citation1998), while others did not make any assumptions with respect to peek duration (Beauchamp Citation2009, Stuber et al. Citation2014); in turn, Gauthier-Clerc et al. (Citation2002) considered ducks awake, while still in sleeping posture, if peeks lasted longer than 1 s.
In this study, we examined the distribution of the duration of peeks in wintering Common Pochard Aythya ferina (hereafter Pochard) while in a sleeping posture. Prolonged periods of eye opening may indicate wakefulness or lower-quality rest relative to higher-quality forms of sleep, i.e. bihemispheric sleep, when both eyes are closed (Rattenborg et al. Citation1999a, Rattenborg & Amlaner Citation2009). Specifically, our goals were to examine: (1) the percentage of time ducks in the sleeping posture spend with one eye open, (2) the mean and maximum duration of peeks, and (3) the effects of flock size and distance-to-neighbour on peek duration.
The study was conducted on the Danube River, in Belgrade, Serbia, from December 2018 to March 2019. The study location was a public city area frequented by visitors and maintenance staff. Typically, several hundreds of Pochards winter in that section of the river (Šćiban et al. Citation2012); the approximate river surface area where we were able to observe ducks was 5 ha. In general, the observations lasted two hours and took place in the morning hours, or shorter if the birds flew away. During each visit, from a couple of vantage points, we counted all Pochards afloat within the area. To examine peek duration, we recorded focal individuals with a high-definition digital camera, (Panasonic HDC-TM60), with an optical zoom 35x, mounted on a tripod at approximately 1.5 m above the ground, at a distance 50–100 m. Only birds in the sleeping posture, i.e. with the bill under scapulars (Amlaner & Ball Citation1983) were recorded. Due to the biased lateral orientation of birds floating on the moving river, we were only able to record consistently their right eye. To reduce the possibility of observing the same individual twice, subsequent individuals within the same observation session were recorded from different portions of the flock. In addition, we did not record more than 10% of the individuals constituting a flock, and no more than 10% of the total number of individuals counted on that day. To examine the effect of flock size on peeking in birds, at the beginning of each focal observation we recorded the flock size. We defined a flock as all Pochards in approximately a 20 m radius from the focal bird, with adjacent birds no more than 10 m apart.
One of us (SK) viewed the video recordings (30fps, 1920 × 1080 pixels) on a desktop personal computer using VLC Media Player, in ¼ slow-motion, when onset of each opening/closure of eye was recorded. Time was recorded in ms (but presented here as s with 3 decimals shown). Hereafter, any period of eye opening, regardless of its duration, is referred to as a ‘peek’. In addition, for the majority of videos we were able to examine the nearest neighbour distance, the total number of nearby neighbours, and the neighbours’ behaviours. Every ten seconds, starting from the first video frame, we recorded the distance to the nearest neighbour (in bird length units: one bird is approximately 30 cm), neighbour density (the number of neighbours up to three body lengths, about 1 m from the focal individual), and the behavioural status of neighbours defined as ‘in a sleeping posture’, or ‘not in a sleeping posture’; the latter was applied for swimming, preening and stretching.
Prior to data analyses, we generated frequency histograms. To assess differences between focal males and females, with respect to the percentage of time birds spent with one eye open, we used the Mann–Whitney U-test. Differences in the duration of peeks were analysed using a mixed effect model, with sex being treated as a fixed factor, whereas identities of focal individuals were treated as a random effect. To transform the distribution of peek durations into a more Gaussian (normal) distribution, data were log10 transformed before analysis. To examine the effect of neighbour numbers and distance to neighbour on peek duration, we also used a mixed effect model. Prior to analysis, we checked for collinearity between the explanatory variables using Pearson correlation coefficients (Zuur et al. Citation2009). The fixed factors in the model were the nearest neighbour distance, expressed as the mean of the distances to neighbours at the beginning and end of a 10 s bin, the number of nearby neighbours, expressed as the mean of the number of ducks recorded around the focal individual at the beginning and end of a 10 s bin, the interaction between these two variables, the mean number of nearby neighbours in the sleeping posture, as there is increasing evidence that flocking birds may copy the behaviours of their neighbours (Beauchamp Citation2011), and the total flock size. Using a random number generator, for each focal individual, we randomly chose one 10 s bin that was included in the analysis. Validation of models was conducted using diagnostic plots (a histogram of residuals, Q–Q plot, and plotted residuals versus fitted values) and dispersion statistics (Zuur et al. Citation2009). All statistical analyses were carried out using R v3.6.1 (R Core Team Citation2019).
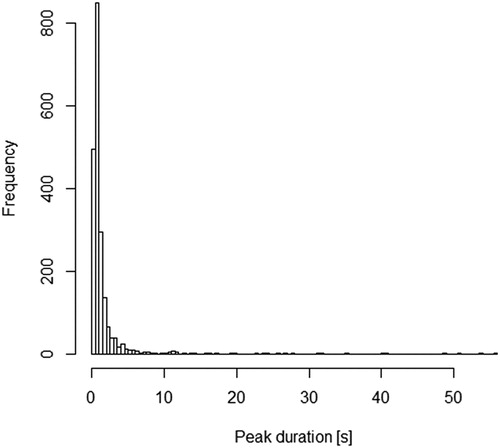
We observed 81 individual Pochard (37 males and 44 females). The mean duration of focal observations was 64.346 s (ranging from 29.421 to 185.488 s). On average, the birds spent 62% of their time with the right eye open (range 21–100%); there was no significant difference in the percentages between males and females (W = 989, P = 0.105). The mean duration of peeks was 1.532 s ± 0.080 (SE), range 0.007–55.883 s. There was no significant difference in the duration of peeks among males and females (F = 5.950 × 10−5, P = 0.994). In the mixed effects model we used, the only significant predictor of the peek duration was the number of nearby neighbours, yielding a positive association between these variables (P = 0.036) (, ).
Figure 1. Peek duration for apparently sleeping Common Pochards in relation to the number of nearby neighbours.
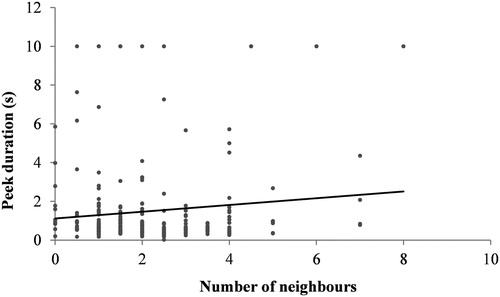
Table 1. Estimated regression parameters for predictors included in a mixed effect model with peek duration as the dependent variable and individual duck identity as a random effect.
Out of 2050 peeks we measured, 66% lasted 0–1 s (N = 1344), 21% lasted 1–2 s (432), 5% 2–3 s (102), 3% 3–4 s (56), 2% 4–5 s (36), 1% 5–6 s (18), < 1% 6–7 s (8), < 1% 7–8 s (8), < 1% 8–9 s (2), < 1% 9–10 s (1), and 2% lasting ≥ 10 s (43) (). In 59% (49) of individuals we recorded peeks lasting over 5 s, whereas in 37% (31) of individuals peeks lasted over 10 s. Three individuals kept their eye open during the entire observation period (durations: 48.955, 50.102 and 54.000 s).
Peeks are brief periods of eye opening allowing birds to inspect their environment while in sleep (Lendrem Citation1983). This study showed that the majority of peeks in Pochard were indeed relatively brief, lasting less than 1 s, and nearly 90% of all peeks lasted less than 2 s. However, in many focal individuals short peeks were interrupted with prolonged periods of eye opening, lasting five or ten seconds and more (maximum duration 56 s). Although there is no neurological information on how long peeks should last to qualify ducks as ‘awake’, in any study where all subjects in a sleeping posture were considered asleep, clearly this state would be overestimated for the time budgets and activity patterns of ducks.
Peeking in sleeping birds may play a similar role as scanning or sentinelling behaviour in awake individuals – in this way, animals may acquire information on predators and competitors in their surroundings (Lenderm Citation1983, Beauchamp Citation2009). In sexually dimorphic ducks, higher levels of vigilance in males can be attributed to a higher predation risk due to brighter colours (Gauthier-Clerc et al. Citation1998), or mate guarding (Guillemain et al. Citation2003), whereas higher levels of vigilance in females may be a consequence of their care for ducklings (Gauthier-Clerc et al. Citation2002). We did not detect differences in the peek duration between the sexes, most likely as consortships were still not forming or established outside the breeding period.
Typically, antipredator vigilance rates decrease with group size as animals may allocate less time to antipredator behaviour because of dilution effect, collective detection of predators or collective defense, whereas social vigilance, i.e. monitoring of conspecifics, may increase with group size (Beauchamp Citation2014). In support of the anti-predatory hypothesis, a study on the Barbary Dove Streptopelia risoria showed that duration of peeks increased in the presence of a predator and decreased with flock size, allowing birds to spend more time with their eyes closed (Lendrem Citation1983). Similarly, the proportion of time Mallards Anas platyrhynchos spent with an eye open while in a sleep posture increased at the edge of the group, where birds were more likely to be exposed to a predatory attack (Rattenborg et al. Citation1999b).
In our study, the overall flock size did not have an effect on peek duration, possibly due to ongoing changes of the flock size through our observation periods, as all ducks we observed were swimming or carried by the water current. However, we did detect a significantly positive relationship between peek duration and the number of nearby neighbours measured at short time intervals. Because of limitation of the acuity of their visual system, most birds, including ducks, can more easily receive information on their environment from their nearby neighbours (Caves et al. Citation2018). In this case, it seems that ducks were not affected just by one, the nearest neighbour, but rather by the group of individuals surrounding them. Although the predation risk in urban environments, such as our study site, is expected to be relaxed for many avian species (Faeth et al. Citation2005), the public city area where we worked is frequently visited by White-tailed Sea-eagles Haliaeetus albicilla (Simić & Puzović Citation1998). However, during our recording sessions, we never observed eagles, and the positive relationship between peek durations and the numbers of nearby neighbours is consistent with ducks probably increasing social rather than antipredatory vigilance. Accordingly, we frequently observed collisions between birds in close proximity, so ducks at our site may have used peeks to monitor their neighbours’ locations to prevent unwanted contact during sleeping.
Acknowledgements
We thank the referee and two anonymous reviewers for comments on the manuscript.
Additional information
Funding
References
- Amlaner, Jr., C.J. & Ball, N.J. 1983. A synthesis of sleep in wild birds. Behaviour 87: 85–119. doi: 10.1163/156853983X00138
- Aulsebrook, A.E., Jones, T.M., Rattenborg, N.C., Roth II, T.C. & Lesku, J.A. 2016. Sleep ecophysiology: integrating neuroscience and ecology. Trends Ecol. Evol. 31: 590–599. doi: 10.1016/j.tree.2016.05.004
- Beauchamp, G. 2008. What is the magnitude of the group-size effect on vigilance? Behav. Ecol. 19: 1361–1368. doi: 10.1093/beheco/arn096
- Beauchamp, G. 2009. Sleeping gulls monitor the vigilance behaviour of their neighbours. Biol. Lett. 5: 9–11. doi: 10.1098/rsbl.2008.0490
- Beauchamp, G. 2011. Collective waves of sleep in gulls (Larus spp.). Ethology 117: 326–331. doi: 10.1111/j.1439-0310.2011.01875.x
- Beauchamp, G. 2014. Social Predation: how group living benefits predators and prey. Academic Press, London.
- Caves, E.M., Brandley, N.C. & Johnsen, S. 2018. Visual acuity and the evolution of signals. Trends Ecol. Evol. 33: 358–372. doi: 10.1016/j.tree.2018.03.001
- Faeth, S.H., Warren, P.S., Shochat, E. & Marussich, W.A. 2005. Trophic dynamics in urban communities. BioScience 55: 399–407. doi: 10.1641/0006-3568(2005)055[0399:TDIUC]2.0.CO;2
- Gauthier-Clerc, M., Tamisier, A. & Cezilly, F. 1994. Sleeping and vigilance in the white-faced whistling-duck. Wilson Bull. 106: 759–762.
- Gauthier-Clerc, M., Tamisier, A. & Cezilly, F. 1998. Sleep–vigilance trade-off in green-winged teals (Anas crecca crecca). Can. J. Zool. 76: 2214–2218. doi: 10.1139/z98-166
- Gauthier-Clerc, M., Tamisier, A. & Cézilly, F. 2002. Vigilance while sleeping in the breeding pochard Aythya ferina according to sex and age. Bird Study 49: 300–303. doi: 10.1080/00063650209461279
- Guillemain, M., Caldow, R.W., Hodder, K.H. & Goss-Custard, J.D. 2003. Increased vigilance of paired males in sexually dimorphic species: distinguishing between alternative explanations in wintering Eurasian wigeon. Behav. Ecol. 14: 724–729. doi: 10.1093/beheco/arg060
- Lendrem, D.W. 1983. Sleeping and vigilance in birds. I. Field observations of the mallard (Anas platyrhynchos). Anim. Behav. 31: 532–538. doi: 10.1016/S0003-3472(83)80076-1
- Lendrem, D.W. 1984. Sleeping and vigilance in birds, II. An experimental study of the Barbary Dove (Streptopelia risoria). Anim. Behav. 32: 243–248. doi: 10.1016/S0003-3472(84)80343-7
- Lesku, J.A., Martinez-Gonzalez, D. & Rattenborg, N.C. 2009. Phylogeny and ontogeny of sleep. In Stickgold, R. & Walker, M. (eds) The Neuroscience of Sleep, 61–69. Academic Press, Oxford.
- Lima, S.L., Rattenborg, N.C., Lesku, J.A. & Amlaner, C.J. 2005. Sleeping under the risk of predation. Anim. Behav. 70: 723–736. doi: 10.1016/j.anbehav.2005.01.008
- Mascetti, G.G. 2016. Unihemispheric sleep and asymmetrical sleep: behavioural, neurophysiological, and functional perspectives. Nat. Sci. Sleep 8: 221–238. doi: 10.2147/NSS.S71970
- R Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
- Rattenborg, N.C., Lima, S.L. & Amlaner, C.J. 1999a. Half-awake to the risk of predation. Nature 397: 397–398. doi: 10.1038/17037
- Rattenborg, N.C., Lima, S.L. & Amlaner, C.J. 1999b. Facultative control of avian unihemispheric sleep under the risk of predation. Behav. Brain Res. 105: 163–172. doi: 10.1016/S0166-4328(99)00070-4
- Rattenborg, N.C., Amlaner, C.J. & Lima, S.L. 2000. Behavioural, neurophysiological and evolutionary perspectives on unihemispheric sleep. Neurosci. Biobehav. Rev. 24: 817–842. doi: 10.1016/S0149-7634(00)00039-7
- Rattenborg, N.C. & Amlaner, C.J. 2009. A bird’s-eye view on the function of sleep. In McNamara, P., Barton, R.A. & Nunn, C.L. (eds) The Evolution of Sleep: phylogenetic and functional perspectives, 145–171. Cambridge University Press, Cambridge.
- Rattenborg, N.C., Voirin, B., Cruz, S.M., Tisdale, R., Dell’Omo, G., Lipp, H.P. & Vyssotski, A.L. 2016. Evidence that birds sleep in mid-flight. Nat. Commun. 7: 12468. doi: 10.1038/ncomms12468
- Rattenborg, N.C., de la Iglesia, H.O., Kempenaers, B., Lesku, J.A., Meerlo, P. & Scriba, M.F. 2017. Sleep research goes wild: new methods and approaches to investigate the ecology, evolution and functions of sleep. Philos. Trans. R Soc. Lond. B Biol. Sci. 372: 20160251. doi: 10.1098/rstb.2016.0251
- Šćiban, M., Đapić, D., Sekereš, O., Pantović, U., Janković, M., Rudić, B., Medenica, I., Radaković, M., Radišić, D., Stanković, D., Radišić, D., Agošton, A. & Gergelj, J. 2012. Results of international water bird census in Serbia. Ciconia 21: 121–128.
- Siegel, J.M. 2008. Do all animals sleep? Trends Neurosci. 31: 208–213. doi: 10.1016/j.tins.2008.02.001
- Simić, D. & Puzović, S. 1998. The White-tailed Eagle (Haliaeetus albicilla) in the Belgrade area. Ciconia 7: 58–70.
- Stuber, E.F., Grobis, M.M., Abbey-Lee, R., Kempenaers, B., Mueller, J.C. & Dingemanse, N.J. 2014. Perceived predation risk affects sleep behaviour in free-living great tits, Parus major. Anim. Behav. 98: 157–165. doi: 10.1016/j.anbehav.2014.10.010
- Zuur, A.F., Ieno, E.N., Walker, N.J., Saveliev, A.A. & Smith, G.M. 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York.