 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Capsule: The use of mist nets placed at more than 9 m high and small GPS tags fitted with a pelvic harness, and equipped to download remotely, allows the capture and tracking of breeding White-backed Woodpeckers Dendrocopos leucotos.
Aims: To describe and test a method for capturing, ringing and GPS-marking medium-sized woodpeckers.
Methods: Birds were captured using an overlapping mist net system located between two poles with a minimum height of 9.25 m and a maximum of 12.95 m, previously designed for catching bats. Once captured, the birds were ringed and fitted them with a remote download GPS tag, weighing 3.4 g.
Results: Twenty individuals (17 adults and 3 juveniles) from 14 territories were captured during the 28 sampling days. All marked breeders continued with chick provisioning and in 12 of the territories were successful in fledging young. We found no significant difference in the number of fledglings between nests where the breeders were captured and tagged with devices (1.71 ± 0.19 se) and nests where the breeders were not captured (1.71 ± 0.29 se). The GPS tags allowed us to obtain a mean of 102.6 (±15.91 se) locations for each bird during a tracking period averaging 57.8 (±10.4 se) days. Despite the steep slope and the high forest canopy in the hábitat, 77.09% of GPS locations were accurate to within 20 m.
Conclusion: Our results showed that this method allows us to obtain important information about the habitat use of this species during the breeding period without any apparent effect on reproductive success.
With an estimated population of 78–95 breeding pairs (Campión & Senosiain Citation2003), the White-backed Woodpecker, Dendrocopos leucotos, is one of the rarest breeding bird species in the Iberian Peninsula. Its worldwide distribution covers a wide range from the Pyrenees to Japan and from Turkey to Finland (Grangé & Vuilleumier Citation2009). Within this vast area, there are numerous small local populations, some of them composed of a very small number of individuals and with a high degree of isolation (Winkler et al. Citation1995). This peculiar distribution has given rise to 12 subspecies (Matveyev & Vasic Citation1973, Haffer Citation1989), with differences in patterns of colouration, song and behaviour (Matveyev Citation1966, Gorman Citation2014).
The Pyrenees population belongs to the subspecies Dendrocopos leucotos lilfordi (Sharpe & Dresser Citation1871) and is located in mature beech forest with abundant dead wood (Fernández et al. Citation1994, Grangé Citation2009), usually in steeply sloped areas, subjected to very high rainfall (Cárcamo et al. Citation2019) and lacking tracks or roads (Gobierno de Navarra Citation2020). Given these difficult habitat conditions, no individuals of this species had been captured and marked in the Iberian Peninsula until the current study (Aranzadi Society of Sciences and SEO/BirdLife Ringing Schemes pers. com) and there had been no studies of habitat use.
In an attempt to fill this knowledge gap, a program for capturing, tagging and tracking was planned, which would allow the collection of accurate information about habitat use and behaviour of this rare woodpecker. Hence, our first aim was to describe a method for capturing, tagging and tracking of breeding White-backed Woodpeckers.
Methods
Study area and nest monitoring
The study was developed in the northwest of Navarra, in the Spanish Pyrenees, in an area with dense mature Beech forest Fagus sylvatica. These forests are included in the Special Conservation Areas (SCA) of Natura 2000 (Roncesvalles-Selva de Irati ES0000126, Alduides ES2200019 and Belate ES2200018).
The study was carried out during the springs of 2017, 2018 and 2019 with a search for nests starting in March (Grangé Citation2015). Every year, the known territories and surrounding areas with suitable habitat were checked using listening points, with and without using a digital playback (Grangé Citation2015, De Santis et al. Citation2007). When the presence of White-backed Woodpeckers was verified, the area was checked for new nests as indicated by holes with new wood chips at the base of the nesting tree. Nests were checked each week from a safe distance (50–75 m) using binoculars or a telescope until the first chick feeding was detected, indicating that eggs were hatched (Grangé Citation2015). Capturing the woodpeckers was carried out a week after the first chick feeding, when feeding rates are at their highest (Grangé Citation2015), so increasing the capture possibilities during the movements from the nest to the foraging sites. After capturing the adults, the nests continued to be monitored every two days until all chicks fledged. The number of fledglings was considered as a productivity value (Grangé Citation2015, Cárcamo et al. Citation2019) and was estimated by direct observation when the young appeared at the entrance of the nest to be fed, and during the first days after fledging, when young remained around the nest calling to their parents (Grangé Citation2015, Cárcamo et al. Citation2019). Sexual dimorphism of the juveniles facilitated the determination of the number of fledgings in the cases where only one young at a time was observed (Grangé Citation2015).
Capturing methods
Since trees in study area were quite tall, with the average height of nesting trees around 20 m (Cárcamo et al. Citation2019), and considering that woodpeckers move through the forest canopy, it was necessary to place mist nets at great heights. We used a system of aluminium poles of 45 mm external diameter and 1.8 m length, previously designed for catching bats (https://batmanagement.com/products/triple-high-mist-net-pole-system), which were assembled to reach a minimum height of 9.25 m and a maximum height of 12.95 m. Over these poles a pulley system was mounted, allowing the nets to be raised and lowered in order to extract the bird once it was captured. The nets used were 12 m long and 2.80 m high, with a mesh of 19 mm and four shelves. Three nets placed one on top of the other were used to get a 8.40 m high and 12 m long net. At the beginning of the project, a digital call-back with male, female and chicks alert vocalizations (de Santis et al. Citation2007) and an artificial decoy simulating an adult male White-backed Woodpecker were used (http://replica-animal.com/es/replicas-realizadas/aves-posadas/77-pico-dorsiblanco-dendrocopos-leucotos). They seemed to show little or no effect on the response of the species and considering possible attraction of predators, we opted to stop using of them. Finally, as was mentioned above, we decided to concentrate capture efforts on the chick rearing phase, when both adults were provisioning chicks, by placing the nets crossing the trajectories of movements from the nest to foraging sites. Visual contact was always maintained with the mist net, in order to extract the birds as soon as they were captured. The capture of breeders was attempted at all nests found, however, there were seven nests (three in 2017, two in 2018 and two in 2019) in which height or local conditions made the net too low, making it impossible to catch any birds. These nests were used as the comparison group since their breeders were not captured.
Marking procedure
All captured birds were fitted with a numbered metal ring (size C) of the Aranzadi Ringing Scheme and three coloured rings in individual combinations, so that a remote reading code consisting of two colours on each leg was obtained. Moreover, these individuals were fitted with a global positioning system (GPS) tracking device. Due to the small size of the species, with adults weighing around 100 g and chicks 80 g, the use of conventional satellite GPS transmitters was not possible. Furthermore, the local terrain and absence of tracks made the use of terrestrial very high frequency (VHF) transmitters considerably more difficult. For these reasons, we used a new model of remote download GPS transmitter manufactured by Biotrack (PinPoint GPS-VHF-75). The transmitter was attached with a pelvic harness according to the methods of Rappole & Tipton (Citation1991). The harness was made of transparent silicone thread with a thickness of 0.8 mm in 2017 and 0.6 mm in 2018 and 2019 (Strong & Stretchy Crystal Elastic Beading Cord). The harness was pre-tied with a 70–80 mm leg-loop span following Naef-Daenzer (Citation2007) and adjusted to be a good fit to the bird in the field. Once the transmitter was placed over the synsacrum, we applied non-reactive, non-toxic n-butyl cyanoacrylate adhesive (Vetbond 3M) over the knot to prevent loss. The total weight of the transmitter and harness was 3.6 g, so less than 5% of the bird's body mass (Vukovich & Kilgo Citation2009, Snijders et al. Citation2017).
In addition, for each individual, the body mass and the length of the folded wing, tail and tarsus were recorded following Baker (Citation1993). Fat deposits and the presence of an incubation patch were also noted (Kaiser Citation1993, Svensson Citation1992). Standardized photographs of the head, wing and rump were taken for future studies of colouring pattern and moult. Finally, for each individual, two tertial feathers and/or rump feathers were collected for use in subsequent genetic analysis. The total time spent on each bird’s processing never exceeded 20 min.
Tracking protocol
Transmitters were configured to record five locations per day on non-consecutive days. This information was stored by the device and downloaded once a month. In order to download the data, the specimen was first located using VHF tracking with a conventional Yagi antenna. To reduce battery costs, the VHF location and discharge option was activated for only 4 h every 30 days.
Since canopy cover and/or terrain roughness can cause errors in GPS locations (Frair et al. Citation2004), we only considered locations with horizontal dilution of precisión (HDOP) lower than 5. This is equal to a precision of 20 m according to the manufacturer's specifications and our previous field test.
Productivity and statistical analysis
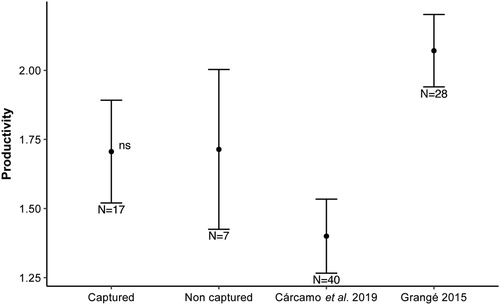
We built a Poisson generalized linear model (GLM), using the R package MASS (Venables & Ripley Citation2002), to analyse if productivity was affected by the capture and GPS marking of the breeders. We compared the productivity (number of fledglings) of 17 nests, in which we had captured and tagged breeders, with productivity of 7 nests in which we were not able to capture any adults. Besides this, we compared our results with those found in two previous studies: one study from the same population (Cárcamo et al. Citation2019) and another from a nearby population in the western French Pyrenees (Grangé Citation2015). Both previous studies estimated fledgling numbers using the same methods as used here. We performed a Tukey post-hoc analysis, using the R package lsmeans (Russell Citation2016), to determine significant differences between the three studies compared. The analyses were done in R 3.6.1 environment (R Core Team Citation2019) and we used the package ggplot2 in R (Wickham Citation2016) to produce the figure.
Results
With this method, during the springs of 2017, 2018 and 2019 with a total of 28 sampling days, spending an average of 4.8 h per day, it was possible to capture 20 individual White-backed Woodpeckers; 6 in 2017, 8 in 2018 and 6 in 2019. At two nests we caught both breeders, in a further two nests we caught a breeder and a juvenile and, in another nest, we caught only a juvenile (this last one was not considered for analysis). According to the typical mouth pattern described by Winkler et al. (Citation1995), only two of the breeders were second year birds (Euring code 5), with all others being after-second-year birds (Euring code 6).
All marked breeders continued to raise chicks. Between 10 and 45 min after being released, they returned to feed their offspring, with no apparent interference of the tag while going in and out of their nest hole. All the nests except two culminated successfully in the raising of chicks. These two failed nests were predated by a European Pine Marten Martes martes more than a week after the marking and without any apparent relationship to the capture. The result of both the GLM and the Tukey post-hoc analysis showed no significant difference in number of fledglings between nests where the breeders were captured and tagged with devices (mean ± se = 1.71 ± 0.19) and nests where the breeders were not captured (mean ± se = 1.71 ± 0.29) (Z-ratio = −0.14, P = 1.0), as well as no significant differences in productivity between this and previous studies in which birds were not caught (Z-ratio = 0.86, P = 0.82 in the case of Cárcamo et al. Citation2019 and Z-ratio = 0.85, P = 0.83 from Grangé Citation2015; ). Note that the nest where two breeders were captured and marked with GPS was successful and had two fledglings.
Figure 1. Comparison of productivity (mean number of fledglings ± se) between the nests where breeders were captured (this study), the nests in the same area without capturing breeders (data extract from Cárcamo et al. Citation2019) and productivity of nests in a nearby area in the French Pyrenees without capturing breeders (data extract from Grangé Citation2015). There were no significant differences between the groups (P > 0.05).
One male and one female marked in different territories in 2017 were still carrying their transmitters in 2018, and were observed breeding without problems related to the tag. In addition, the male was recaptured and assessed by a veterinarian as showing no injuries associated with the harness; it showed a higher body mass and a higher score in fat deposits tan in the previous year (102.5 g and 0.5 fat deposit in 2017 and 104.3 g and 1.5 fat deposit in 2018).
In the spring of 2019, it was verified that individuals marked in 2018 with a 0.6 mm silicone thread no longer carried their transmitters. The thinner thread was able to hold the transmitter for the entire follow-up period, but broke up before the start of the next reproductive season. After the loss of transmitters, coloured rings allowed the identification of individuals by technicians of the HABIOS project, by foresters and by amateur ornithologists. Through colour ring resightings, it was possible to determine that 9 of the 12 adults captured in 2017 and 2018 were alive a year after their capture, which implies a minimum survival rate for adults of 75% ().
Table 1. Survival data of marked adult White-backed Woodpeckers in 2017 and 2018. F = female; M = male.
The proportion of the precise locations (HDOP < 5) was 77.09% (±1.67 se), allowing us to obtain a mean of 102.6 (±15.91 se) exact locations per bird for a mean of 57.8 (±10.4 se) days, and to characterize the territories of 14 pairs. It was not possible to download data from three breeders. One disappeared after the predation of its nest by a Pine Marten, another lost its GPS device and in the third case, it was not possible to find the bird 30 days after marking, probably due to a an unconfirmed predation event.
Discussion
The capture of forest birds that move through the highest parts of the trees is challenging (Stokes et al. Citation2000), especially if they are not responsive to the use of playbacks, as is the case of the White-backed Woodpecker in the Pyrenees. For this reason, it was necessary to resort to mist nets placed at great heights with the help of poles and pulleys (Humphrey et al. Citation1968, Stokes et al. Citation2000). In our case, nets placed between 9.25 and 12.95 m were effective, and resulted in the capture of 17 individual White-backed Woodpeckers from 24 nests. The main limitation of this technique was the maximum height that could be reached with the nets, since it was very difficult to install nets above 12 m. This meant that the capture technique was not useful for all nests of the White-backed Woodpecker, which can constructs it nests up to 20 m high (Cárcamo et al. Citation2019). This limitation could be resolved if it were possible to attract the birds using a decoy or a playback, as described for other woodpecker species (York et al. Citation1998), however, in our case, the birds showed no apparent response to the use of sounds or decoys. This, together with the possible problems associated with the use of playbacks or decoys (Harris & Haskell Citation2013), makes it inadvisable to use these tools to capture White-Backed Woodpeckers, at least in the Pyrenees.
The impact of capture and marking on the birds, seemed to be reasonably low, since all adults continued with chick provisioning immediately after tagging. Furthermore, the tags did not interfere with movement and out of the nest hole. Neither did tagging showed any significant impact on breeding performance, as marked birds produced a very similar number of fledglings to unmarked birds. Moreover, reproductive success was also similar to that described in previous studies performed in our study area (Cárcamo et al. Citation2019) and on the northwest Pyrenees (Grangé Citation2015), using the same assessment protocols.
The survival of adult birds also did not appear to be influenced by tagging, since the 75% relocation of marked birds found in our work is similar to the 77–89% fond in previous studies with less invasive protocols (Pasinelli Citation2006).
Our results showed that the two types of threads used to make the pelvic harnesses were able to hold the transmitter during at least the following two months. In addition, examination of the male recaptured after wearing the 0.8 mm thick harness for a year, confirmed that this type of material did not cause apparent skin injuries, nor did it reduce its body mass or fat deposits. Although this was only one bird, the result is consistent with previous studies in which pelvic harnesses, made of similar elastic silicone thread, were used to hold different bird tracking devices (Robles et al. Citation2007, Lislevand & Hahn Citation2013). However, since the current battery life of these GPS tags is limited to a few months, it is not necessary to maintain the harness for a full year. Therefore, we recommend the use of the 0.6 mm thread, due to its ability to hold the device during the monitoring period and fall off before the next breeding period. The GPS transmitters used have provided a large number of precise locations with much less effort than would be needed in radio surveying with VHF transmitters (Ettwein et al. Citation2019). In addition, the storage and remote download options for data significantly reduce interference with birds as it is not necessary to capture them to obtain the information.
One of the problems that may have appeared in our monitoring is the loss of location precision due to the topography of the habitat and the canopy cover, as described in the first studies carried out with GPS tags (Frair et al. Citation2004). This situation could induce errors in hábitat-use analysis because the fixes lost could be concentrated in the closed canopy forests or rough areas that could be under-represented in GPS data (Frair et al. Citation2004). However, the proportion of precise locations in our study was very high (over 75%), despite working in a dense forest with steep slopes, suggesting that current technology allows monitoring even in this kind of habitat. Although the short working life of GPS tags does not currently allow the collection of information about the survival or causes of death of the birds, as might have been possible using VHF tracking (Robles et al. Citation2007, Kilgo & Vukovich Citation2012), the complementary use of coloured rings does provide longer term monitoring options to provide at least annual survival data (Väli & Bergmanis Citation2017).
Acknowledgements
We especially thank Gorka Gorospe, Gonzalo Dean, Alfonso Senosiain and Susana Cárcamo, who have collaborated in the present work by locating and monitoring nests, as well as the Environmental Guards of the Demarcations of Aezkoa and Ultzama, especially to Xabier Azpiroz and Javier Zuazu. The Government of Navarra provided the permits for the work. Two anonymous reviewers provided very valuable comments that helped us to improve an earlier version of this paper. We thank Mark den Toom for his helpful advice and English revision. This study contributes to the project POCTEFA HABIOS (EFA 079/15) funded by the ‘Fondo Europeo de Desarrollo Regional' (Feder). https://www.habios.eu/el-proyecto/
Additional information
Funding
References
- Baker, K. 1993. Identification Guide to European Non-Passerines: BTO Guide 24. British Trust for Ornithology, Thetford.
- Campión, D. & Senosiain, A. 2003. Pico dorsiblanco (Dendrocopos lucotos). In Martí, R. & del Moral, J. C. (eds) Atlas de las Aves Reproductoras de España, 360–361. Ministerio de Medio Ambiente, Dirección General de Conservación de la Naturaleza; Sociedad Española de Ornitología (SEO/BirdLife), Madrid.
- Cárcamo, S., Elosegi, M.M., Senosiain, A. & Arizaga, J. 2019. Nest site selection and reproduction parameters in the White-backed Woodpecker Dendrocopos leucotos lilfordi Sharpe & Dresser, 1871 in Navarre. Munibe Cienc. Nat. 67: 31–44.
- De Santis, E., Imperio, S., Fabrizi, E., Campanella, G., Savo, E. & Cecere, J.G. 2007. Use of playback technique for population monitoring of White-backed Woodpecker Dendrocopos leucotos lilfordi in Central Appennines (Italy – Monti Simbruini Natural Regional Park). European Bird Census Council Meeting, Chiavenna (Italy).
- Ettwein, A., Lanz, M. & Pasinelli, G. 2019. Variation in home range size of the white-backed woodpecker. 8th International Woodpecker Conference, Poland.
- Fernández, C., Azkona, P. & Lorente, L. 1994. Corología y caracterización del hábitat del pico dorsiblanco (Dendrocopos leucotos) en el Pirineo occidental. Ardeola 41: 135–140.
- Frair, J.L., Nielsen, S.E., Merrill, E.H., Lele, S.R., Boyce, M., Minro, R.H., Stenhouse, G.B. & Beter, H.L. 2004. Removing GPS collar bias in habitat selection studies. J. Appl. Ecol. 41: 201–212. doi: 10.1111/j.0021-8901.2004.00902.x
- Gobierno de Navarra. 2020. Pistas forestales de Navarra. https://gobiernoabierto.navarra.es/es/open-data/datos/pistas-forestales-principales-ejes-shp.
- Gorman, G. 2014. Woodpeckers of the World. The Complete Guide. A&C Black, London.
- Grangé, J.L. 2009. Caractéristiques des arbres de nid chez le Pic à dos blanc Dendrocopos leucotos lilfordi dans les Pyrénées occidentales françaises. Le Casseur d’os 9: 92–110.
- Grangé, J.L. 2015. Breeding biology of the Lilford Woodpecker Dendrocopos leucotos lilfordi in the Western Pyrenees (SW France). Denisia 36: 99–111.
- Grangé, J.L. & Vuilleumier, F. 2009. Le Pic à dos blanc Dendrocopos leucotos: deux scénarios pour expliquer l’histoire de son peuplement dans le sud de l’Europe et analyse des rapports taxonomiques entre les sous-espèces lilfordi et leucotos. Nos Oiseaux 56: 195–222.
- Haffer, J. 1989. Parapatrische Vogelarten der oalaarktischen Fauna. J. Ornithol.130: 475–512. doi: 10.1007/BF01918466
- Harris, J.B.C. & Haskell, D.G. 2013. Simulated birdwatchers’ playback affects the behavior of two tropical birds. PLoS One 8: e77902. doi: 10.1371/journal.pone.0077902
- Humphrey, P.S., Bridge, D. & Lovejoy, T.E. 1968. A technique for mist-netting in the forest canopy. Bird Band. 39: 43–50. doi: 10.2307/4511439
- Kaiser, A. 1993. A new multi-category classification of subcutaneous fat deposits of Songbirds. J. Field Ornithol. 64: 246–255.
- Kilgo, J.C. & Vukovich, M. 2012. Factors affecting breeding season survival of red-headed woodpeckers in South Carolina. J. Wildl. Manag. 76: 328–335. doi: 10.1002/jwmg.282
- Lislevand, T. & Hahn, S. 2013. Effects of geolocator deployment by using flexible leg-loop harnesses in a small wader. WSGB 120: 108–113.
- Matveyev, S.D. 1966. Some results of the revision of systematic categories of birds of the Balkans peninsula. Arhiv Biol. Nauka 18: 207–214.
- Matveyev, S.D. & Vasic, V.F. 1973. Cataloge faune Jugoslaviae. IV/3. Ljubljana.
- Naef-Daenzer, B. 2007. An allometric function to fit leg-loop harnesses to terrestrial birds. J. Avian Biol. 38: 404–407. doi: 10.1111/j.2007.0908-8857.03863.x
- Pasinelli, G. 2006. Population biology of European woodpecker species: a review. Ann. Zool. Fenn. 43: 96–111.
- R Core Team. 2019. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.Rproject.org/.
- Rappole, J.H. & Tipton, A.R. 1991. New harness design for attachment of radio transmitters to small passerines. J. Field Ornithol. 62: 335–337.
- Robles, H., Ciudad, C., Vera, R. & Baglione, V. 2007. No effect of habitat fragmentation on post-fledging, first-year and adult survival in the middle spotted woodpecker. Ecography 30: 685–694. doi: 10.1111/j.2007.0906-7590.05179.x
- Russell, V.L. 2016. Least-squares means: the R Package lsmeans. J. Stat. Softw. 69: 1–33.
- Sharpe, R.B. & Dresser, H.E. 1871. LIV. – On two undescribed species of European birds. J. Nat. Hist. 8: 436–437.
- Snijders, L., Weme, L.E.N., de Goede, P., Savage, J.L., van Oers, K. & Naguib, M. 2017. Context-dependent effects of radio transmitter attachment on a small passerine. J. Avian Biol. 48: 650–659. doi: 10.1111/jav.01148
- Stokes, A.E., Schultz, B.B., Degraaf, R.M. & Griffin, C.R. 2000. Setting mist nets from platforms in the forest canopy. J. Field Ornithol. 71: 57–66. doi: 10.1648/0273-8570-71.1.57
- Svensson, L. 1992. Identification Guide to European Passerines. British Trust for Ornithology, Thetford.
- Väli, Ü & Bergmanis, U. 2017. Apparent survival rates of adult Lesser Spotted Eagle Clanga pomarina estimated by GPS-tracking, colour rings and wing-tags. Bird Study 64: 104–107. doi: 10.1080/00063657.2016.1271395
- Venables, W.N. & Ripley, B.D. 2002. Modern Applied Statistics with S, 4th edn. Springer, New York.
- Vukovich, M. & Kilgo, J.C. 2009. Effects of radio transmitters on the behavior of Red-headed Woodpeckers. J. Field Ornithol. 80: 308–313. doi: 10.1111/j.1557-9263.2009.00235.x
- Wickham, H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York.
- Winkler, H., Christie, D.A. & Nurney, D. 1995. A Guide to the Woodpeckers, Piculets and Wrynecks of the World. Pica, Sussex.
- York, D.L., Davis, J.E., Cummings, J.L. & Wilson, E.A. 1998. Pileated woodpecker capture using a mist net and taped call. N. Am. Bird Bander 23: 81–82.
