ABSTRACT
Capsule
Sooty Falcon Falco concolor movement patterns were strongly dependent on the phase of their annual cycle, and were clearly discernible in terms of the average hourly travel speeds of falcons during those phases.
Aims
To better understand the movements of Sooty Falcons on migration, during the winter and as non-breeding individuals.
Methods
Eleven Sooty Falcons were tracked via satellite, and their movements were analysed.
Results
Movement patterns were strongly dependent on the phases of the annual cycle. Falcons exhibited limited movement (i.e. shortest step-lengths and smallest areas) when on the wintering and breeding grounds. Migration to and from the wintering grounds in Madagascar was characterized by high movement rates (long step-lengths), sometimes over 50 km/h. Some Sooty Falcons stopped on the African mainland coast prior to crossing the Mozambique Channel. Movement patterns during winter were similar to those exhibited by falcons while summering, except that the movements of breeding birds were spatially more restricted.
Conclusions
Our results highlight the need for conservation approaches appropriate to the different phases of the annual cycle, including the importance of areas immediately around island breeding sites, summering and coastal staging areas, and the need to support prey abundance along the migration route and on wintering grounds.
Movement is a characteristic of animal life, but the pattern of movement often varies across taxa, individuals, time and space (Hansson & Åkesson Citation2014). Migration is perhaps the most widely appreciated type of animal movement because it is often spectacular, easily observed for some species at some locations, and spatially and temporally predictable (Alerstam Citation1990, Wilcove & Wikelski Citation2008, Newton Citation2010). While local movement patterns often are motivated by needs such as securing food, avoiding predators and finding mates, long distance migration is thought to have evolved as a mechanism for avoiding periodic declines in local resource (food) availability or otherwise unfavourable environmental conditions (Berthold Citation2001, Newton Citation2010). Because migration often involves travelling very long distances and crossing potentially hostile landscapes, the movement patterns during migration are expected to differ from those seen within the wintering or summering areas. However, both long and short distance movement patterns are affected by factors like species morphology, weather, habitat or landscape characteristics, social and reproductive status, and human disturbance (Alerstam Citation1990, Bildstein Citation2006, Newton Citation2008, Karelus et al. Citation2017). Describing movement patterns is key to understanding how animals utilize space and resources such as habitat components and prey (Cody Citation1985, Karelus et al. Citation2017, Citation2019, Criffield et al. Citation2018) or, for birds, how they select geographical features to enable efficient flight (Kerlinger Citation1989, Bildstein Citation2006). A knowledge of the movement ecology of a species is also important for its conservation, as it enables wildlife managers to determine space and resource needs, and the locations of animals relative to the distribution of threats, thereby providing evidence for the formulation of location- or spatial scale-specific conservation measures (Allen & Singh Citation2016).
Relative to other periods of their lives, the ecology of birds after leaving the natal site and before recruitment is the most poorly known. This includes juveniles, immatures and adult non-breeding ‘floaters’. For many migratory avian species we know more about their ecology during the breeding season than at other times of the year because nesting birds are often easier to survey and monitor, and reproductive success is an important demographic parameter sought by researchers and conservation biologists. The paucity of data during the non-breeding season is particularly acute in species that migrate because they are typically difficult to observe over extended periods while on migration, and their non-breeding grounds may be unknown, remote or otherwise difficult to access (Newton Citation2008, Faaborg et al. Citation2010). Additionally, the ecology of floating birds and their role in population dynamics are difficult to study because they can be challenging to observe and catch for marking (Smith Citation1978, Hunt Citation1998, Newton & Rothery Citation2001). However, conservation of a migrating species necessitates consideration of the entire ecological network, including breeding and non-breeding grounds and stopover areas (Faaborg et al. Citation2010, Allen & Singh Citation2016), and data gaps in any part of that network can undermine conservation efforts. Although gathering movement data has been difficult or technically constrained in the past, miniaturization of telemetry technology in recent years has enabled rather precise tracking of even relatively small birds over long distances and in remote areas (Meyburg et al. Citation2011, Javed et al. Citation2012, Willemoes et al. Citation2014). Satellite telemetry data have enabled researchers to uncover many facets of the ecology of migratory birds for which our knowledge had been limited almost exclusively to their breeding grounds (Meyburg et al. Citation2011, Kassara et al. Citation2017).
The Sooty Falcon Falco concolor is a small raptor of conservation concern that breeds in the Middle East and northeast Africa, and is currently classified as ‘vulnerable’ (BirdLife International Citation2020). Sooty Falcons have an unusual breeding strategy (shared only with the Eleonora’s Falcon, Falco eleonorae) in that they nest during the high boreal summer (July–October; hereafter ‘summer’), enabling them to feed their chicks on small birds migrating south during autumn. The autumn migration of Sooty Falcons generally starts in October or November and almost all individuals spend the boreal winter (hereafter ‘winter’) in Madagascar, where their diet appears to comprise mostly of insects, especially dragonflies (Odonata; Walter Citation1979a, Citationb, Zefania Citation2001). Although the ecology of Sooty Falcons during the breeding season has received some scientific attention (Walter Citation1979b, Frumkin & Pinshow Citation1983, Kavanaugh & King Citation2008, McGrady et al. Citation2016, Citation2017), very little is known about their ecology and behaviour during migration or while on the wintering grounds; we also know little about the ecology of non-breeders at any time of the year. Our knowledge of Sooty Falcon ecology during the non-breeding season comes mostly from anecdotal observations (Walter Citation1979a, Citationb), and a single survey at 44 sites in Madagascar (Zefania Citation2001). To our knowledge, the only published account of Sooty Falcon movement comes from the tracking of a single individual (Javed et al. Citation2012).
Here, we provide the first quantification of Sooty Falcon movement patterns across the annual cycle of the species. We expected that Sooty Falcon movement would differ between phases (summer, winter, spring northward migration and autumn southward migration) of the annual cycle, and that summer movements would differ between breeders and non-breeders. Specifically, we examined the hypotheses that: (1) the movement pattern of breeders and juveniles would be restricted by the breeding process, and mostly centred on the nesting site during the breeding season; (2) non-breeders would visit breeding grounds in the summer, but would move around more widely because their movements would not be restricted by nest locations; (3) during migration, Sooty Falcons would travel with greater average speed, but would also take advantage of feeding opportunities en route; (4) migration routes used by breeders would be more direct than those taken by juveniles and non-breeders because they were generally more experienced and were more motivated to reach breeding areas than non-breeders; and (5) while on the non-breeding grounds, birds would wander more widely, as a response to the ephemeral occurrence patterns of their insect prey.
Methods
Fieldwork
We fitted 9.5 g solar-powered Argos Platform Transmitter Terminals (PTT) to 10 Sooty Falcons (6 juveniles, 4 adults) on the breeding grounds in Oman (approximately 23.6°N, 58.5°E, McGrady et al. Citation2016), and one to an adult on the non-breeding grounds in Madagascar (approximately 18.8°S, 47.5°E; ). In Oman, adults were captured when incubating eggs, using nooses and dummy eggs (Gosler Citation2004); juveniles were fitted with transmitters just before fledging. The individual fitted with a transmitter in Madagascar was captured using elevated mist nets. PTTs were fitted as a backpack, using Teflon® ribbon with an integrated degradable link (Meyburg & Fuller Citation2007). PTTs were programmed to record a location every hour, but the amount of energy produced by the solar panels could affect the frequency of location data.
Table 1. Summary of transmitters deployed on Sooty Falcons. J = juvenile; A = adult; M = male, F = female; U = sex unknown.
Data analyses
The Argos system estimates the location of the PTT and assigns a Location Class (LC: 3, 2, 1, 0, A, B, Z) to each location estimate, which describes its likely accuracy. We used only the more accurate locations (LC 1–3) in our analyses. Nominal accuracy of LC3, LC2 and LC1 location estimates is <250 m, <500 m and <1500 m, respectively (http://www.argos-system.org/manual/3-location/34_location_classes.htm). We filtered the data using the framework described by Fleming et al. (Citation2020), and also applied speed-distance filters (100 km/h, https://www.movebank.org/cms/movebank-content/general-data-filters) to the data to exclude implausible locations.
We classified each location into one of the following seasonal categories: (1) late breeding (i.e. incomplete breeding season of adult birds and juveniles marked on the breeding grounds), (2) autumn migration, (3) autumn stopover, (4) autumn coastal staging, (5) wintering, (6) spring migration, (7) spring stopover, and (8) summering (nominal breeding season, whether birds bred or not). The autumn coastal staging was when birds stopped on the coast of the African mainland (Mozambique and southern Tanzania) for several days before crossing to their wintering grounds in Madagascar. We distinguished the coastal staging from autumn stopovers because birds may have been waiting for favourable weather conditions to make the water crossing (Bildstein Citation2006, McGrady et al. Citation2006), rather than being driven primarily by the need to re-fuel, as was presumed at other stopovers and, therefore, they may have used the areas differently. We used net squared displacement (NSD) to determine the date ranges for each of these categories (Börger & Fryxell Citation2012, Singh et al. Citation2016) for each bird (online Appendix S1, Figure S1). We then visually inspected the trajectories of birds at each of the dates at the beginning and end of each category based on the NSD plot and verified that those dates accurately encompassed the phase of migration and seasonal categories. We considered departure on autumn migration to be the first location when falcons moved away from known breeding areas (i.e. Oman and Eritrea), and did not return. Likewise, we considered the start of spring migration to be the first location when falcons moved in a concerted manner away from their wintering areas, heading in the direction of summering areas. We considered birds to be wintering as soon as they arrived on Madagascar. Because of gaps in the data, actual arrival and departure dates at summering and wintering grounds could vary by ±2 days. We used ‘summering’ to describe birds (one juvenile and one adult) that wandered during the nominal breeding season, and apparently did not acquire a breeding site.
We estimated 95% utilization distributions using kernel density estimation (KDE; Worton Citation1989) with bivariate normal kernels for individuals during each season (excluding migration) in which at least 10 locations were available. We used the average reference bandwidth for all birds in each seasonal category. To calculate the average reference bandwidth, we first ran KDEs for each seasonal category for each bird using the reference bandwidth, then averaged the results of individual reference bandwidth for each seasonal category. Because wintering grounds were on the island of Madagascar, and the KDEs included areas of sea which we presumed the birds did not use to any great extent (no locations were over water during wintering on Madagascar), we clipped those KDEs to the coastline. To investigate the movements made by the birds, we calculated hourly step-lengths (distance between successive locations obtained within approximately 1 h of each other); we did not include steps that were created from two successive locations taken greater than approximately 1 h apart, as in the cases when scheduled fixes were missed for some reason (e.g. poor view of satellites, low power, poor signal quality, noise at the transmitter frequency, etc.) or when fixes were excluded due to apparent inaccuracy.
For all periods except migration, we extracted land cover information for each location. We obtained land cover data from the European Space Agency (ESA) GlobCover 2009 Project © ESA 2010 and Université catholique de Louvain (http://due.esrin.esa.int/page_globcover.php) at 300 m resolution. We combined the land cover types in the raster into six broad land cover types based on the similarity of cover and structure, and determined the proportions of locations across land cover types during each seasonal category. Due to gaps in the tracking data and the large areas over which the birds ranged, we did not perform formal habitat selection analyses.
All analyses were performed in the R computing environment (version 3.4.4; R Core Team Citation2018). We estimated utilization distributions with the adehabitatHR package (Calenge Citation2006), clipped non-breeding ranges to Madagascar using the package rgeos (Bivand & Rundel Citation2016), calculated net squared displacement (NSD) and step-lengths using the package adehabitatLT (Calenge Citation2006), and extracted land cover types at locations with the package raster (Hijmans Citation2015). Sooty Falcons are trans-equatorial migrants. We use seasonal terminology (i.e. spring, summer, autumn, winter) corresponding to the location of their breeding grounds in the northern hemisphere.
Results
Overall, 11 Sooty Falcons were tracked between October 2013 and February 2015 (; ). Each bird was tracked through a mean (± se) of 110 ± 28 days (minimum: 44 days, maximum: 304 days, median: 64 days), and had a mean of 54 ± 15 days when at least one location was collected (minimum: 18, maximum: 166, median: 24). Data collection rates varied considerably between individuals and across phases of the annual cycle (Appendix S1).
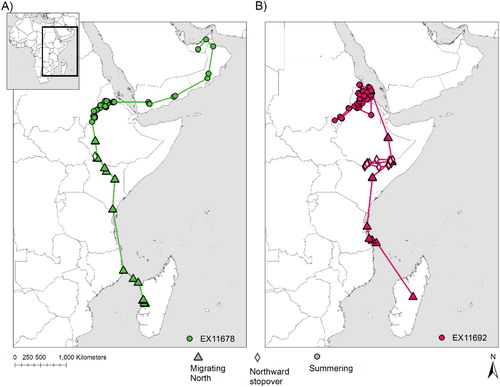
Figure 1. Locations from (A) juvenile and (B) adult Sooty Falcons as they migrated from their natal/breeding sites in Oman to wintering grounds, with stopovers and coastal staging. Not all birds were tracked for the entire migration due to mortality or tag/harness failure (see for details). Lines between locations do not imply straight line flight, but instead indicate the sequence in which locations were recorded.
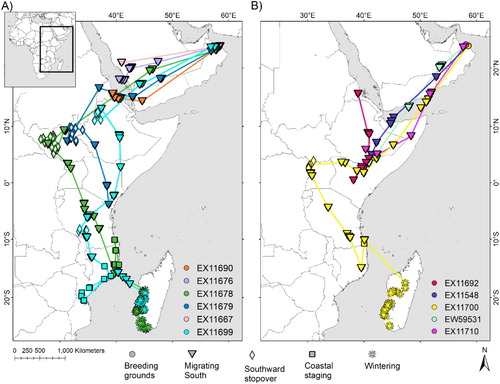
Figure 2. Locations from (A) juvenile and (B) adult Sooty Falcons at their wintering grounds and while on migration to and from the wintering grounds. Lines between locations do not imply straight line flight, but instead indicate the sequence in which locations were recorded.
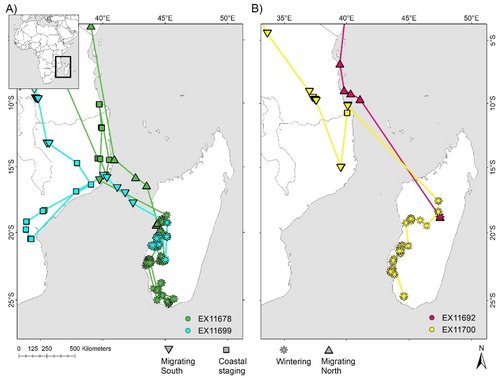
Figure 3. Locations from (A) juvenile and (B) adult Sooty Falcons from start of northward movement at the end of winter through to arrival in summering areas, with stopovers identified. Only two individuals (EX11678 and EX11692) were tracked for their entire spring migration; neither appeared to have bred in the year they were tracked (2014). Lines between locations do not imply straight line flight, but instead indicate the sequence in which locations were recorded.
Sooty Falcons had the smallest utilization distributions while at the breeding grounds and ranged over much larger areas during other seasons (). Averaged across all seasonal categories, birds moved with a mean hourly step-length of 2.71 ± 0.25 km (n = 806 step-lengths, minimum: 0.00 km, maximum: 78.56 km). Birds moved with similar hourly step-lengths while at the breeding and wintering grounds, and these step-lengths were shorter than those in other seasonal categories. Hourly step-lengths were the longest when falcons were migrating, followed by those while birds were summering, at stopovers or while coastal staging (). The proportion (by seasonal category) of locations in each of the six land cover categories is shown in .
Figure 4. The average hourly step-lengths (straight-line distance between two successive hourly locations) for individual Sooty Falcons during each phase of their annual cycle. Error bars represent standard errors.
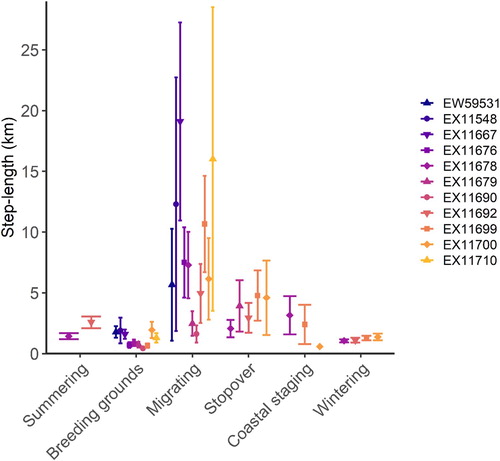
Figure 5. The proportion of locations of individual tracked Sooty Falcons in each of the six land cover types per phase of their annual cycle (i.e. number of locations during each phase in a land cover type divided by the total number of locations in that phase).
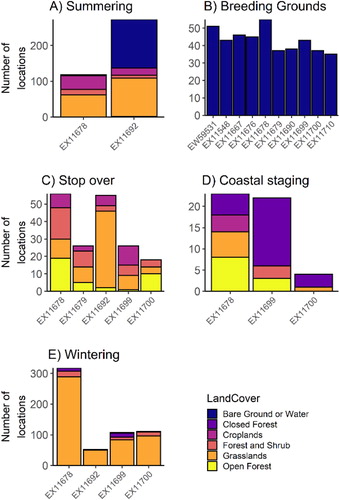
Table 2. Estimated range sizes from 95% kernel density estimator (95% KDE) for each Sooty Falcon while A) summering, B) at breeding grounds, C) at stopovers, D) while in coastal staging, and E) while at wintering grounds in Madagascar. The average among all birds within a season is shown as well as the bandwidth (m) used for each season.
Summering and breeding season
Sooty Falcons fledged in Oman ranged over a mean (± se) area of 23.32 ± 2.28 km2 prior to their first migration; breeding adults tagged in Oman ranged over a mean area of 37.31 ± 5.23 km2 (). Sooty Falcon locations while on the breeding grounds in Oman were almost exclusively over bare ground/open water ().
Two non-juvenile, apparently non-breeding, Sooty Falcons were tracked during the summer. The adult falcon that was captured in Madagascar (EX11692) ranged over 244107 km2 in Eritrea and Ethiopia in summer 2014 (, ). Although that individual spent a significant amount of time around the Dahlak archipelago (approximately 16.49°N 40.137°E), where Sooty Falcons are known to breed, its occasional movements to distant inland locations in Ethiopia suggest that it did not breed (). Another falcon (EX11678, in its second calendar year) that was tracked during summer 2014, ranged over a very large area (NE Ethiopia: 19 May – 18 July, Oman via Yemen: 20–25 July, UAE/Oman/Saudi Arabia border: 27 July – early August; , ). It was found dead at a remote desert location near Yibal, Oman (23.19°N 56.03°E), far from any known Sooty Falcon breeding site; its signal suggested it died on 11 August 2014. These two birds had larger utilization distributions and moved with generally longer average hourly step-lengths while summering than did breeding or recently fledged birds. However, birds at the breeding grounds generally moved with similar mean hourly step-lengths to those on their wintering grounds (), despite their average ranges at breeding grounds being generally smaller than those on the wintering grounds (). Tracking locations of the two summering individuals were mostly over grassland and bare ground/open water ().
Autumn migration
and summarize southward migration of Sooty Falcons. The birds left Oman on autumn migration from late-October to mid-November. There was low variability between dates of departure for adults or juveniles. The individual that summered in Eritrea/Ethiopia (EX11692) started autumn migration on about 29 October 2014.
Sooty Falcons migrated between breeding and non-breeding areas via an inland route. However, adults migrated south along a more eastern and direct route than that used by the tracked juveniles (). Tracking data from four falcons indicated at least one stopover during their autumn migrations (, ). Mean (± se) area of stopover sites was 147272.26 ± 26042.01 km2 (). Mean (± se) hourly step-length at autumn stopovers was 3.12 km ± 0.80 km (n = 49 step-lengths). Stopover kernels of individuals did not overlap with one another showing that tracked birds used different areas to stopover.
All three autumn-migrating falcons that made it that far exhibited what we defined as coastal staging at the Mozambique/Tanzania coast prior to crossing the ocean to Madagascar. Only two of these birds (EX11678 and EX11699) provided enough data to estimate a KDE while staging: EX11678 moved within a KDE area of 181099.24 km2 during 7.5 days of coastal staging and EX11699 moved within an area of 232203.84 km2 during 9.75 days of coastal staging. Mean (± se) hourly step-length during coastal staging for all three birds was similar to that during stopovers at 2.7 ± 1.1 km (n = 21). About 50% of locations classified as coastal staging areas were over closed forest; the rest were over open forest, grasslands, cropland and forest/shrub (). Visual examination of maps of wind direction and speed (Global Forecast System: https://www.ncdc.noaa.gov/data-access/model-data/model-datasets/global-forcast-system-gfs) did not suggest that falcons left the African mainland in response to particularly favourable tailwinds.
Sooty Falcons from Oman took 36–58 days (mean = 49.6, sd = 11.9, n = 3, ) from departure to arrival on Madagascar; they arrived during December and early January. Mean (± se) hourly step-length for all birds while migrating in autumn was 6.8 ± 1.2 km (n = 129 step-lengths). Four successive hourly locations were collected for EX11699 over the open sea as it crossed to Madagascar; mean flight speed during that time was 42.95 km/h, the maximum was 78.6 km/h.
Wintering season
Of the Sooty Falcons captured in Oman, three (EX11678, EX11699 and EX11700) provided location data during wintering. All spent the boreal winter in southern and southwestern Madagascar, where they ranged over large areas. In contrast, EX11692, which was captured and tagged in Madagascar, had a small non-breeding home range at the Antananarivo airport (, ). The mean (± se) hourly step-length for this bird during winter was 1.1 ± 0.3 km (n = 26 step-lengths). The mean hourly step-length for the other three birds with wintering data was 1.2 ± 0.1 km (n = 285 step-lengths). Locations of falcons while on Madagascar were mostly over grassland ().
Spring migration
Two falcons were tracked on their spring migration (, ), an immature reared in Oman (EX11678) and the adult (EX11692) captured during the winter in Madagascar. They initiated migration in 2014 on 1 May and 21 March, respectively (). The adult falcon flew 149.85 km between the three successive hourly locations that were obtained while the bird was over open water (mean speed = 49.91 km/h). Some of the crossing was made at night. The bird made landfall about 130 km south of Dar es Salaam, Tanzania. The immature falcon crossed the water between Madagascar and Mozambique on 3 May 2014; three successive hourly locations were obtained while the bird was over open water. In that time, the bird flew 342.79 km, a mean speed of 48.97 km/h. This bird likely made landfall near Nacala, Mozambique (14.5°S 40.7°E).
As with the autumn migration, once over the African mainland, birds used an inland route. Mean (± se) hourly step-length was 6.3 ± 2.6 km on spring migration. The adult falcon made a stopover while heading north, in the border area of Ethiopia, Kenya and Somalia. The mean (± se) hourly step-length at stopovers during the spring migration was 2.9 ± 1.2 km (n = 30 step-lengths). Only EX11692 appeared to have completed its spring migration, taking 43 days to migrate between Madagascar and one of the most northern islands of the Dahlak Archipelago, Eritrea. EX11678 was also tracked to areas within the breeding distribution, although it was never located near known breeding locations. At the time of its death, EX11678 was still moving northward, and seems to have been a floating non-breeder.
Discussion
Our overarching expectation that Sooty Falcon movement patterns were strongly dependent on the phase of their annual cycle was met, and those patterns were clearly discernible in terms of the average hourly travel speeds of falcons during those phases. Relevant to our specific hypotheses, falcons exhibited limited movement (i.e. shortest step-lengths) when on wintering and breeding grounds (hypothesis 1), and non-breeders visited breeding areas during the summer, but ranged more widely, sometimes dwelling away from the breeding sites (hypothesis 2). Migrations to and from the wintering grounds were characterized by high hourly travel speeds (long step-lengths), with birds sometimes travelling over 50 km/h (hypothesis 3), though falcons also made stopovers, including staging along the African mainland coast before crossing the sea to Madagascar. Hypothesis 4, that breeders would take more direct routes between summering and wintering areas, was also supported, albeit with a small sample size. Also, with one exception (EX11692), Sooty Falcons ranged over large areas on their wintering grounds (supporting hypothesis 5), although we lacked data to link this to prey availability. These results, although based on a fairly small sample size, concur with limited information that existed regarding Sooty Falcon movements during the non-breeding months (Walter Citation1979b, Javed et al. Citation2012), but provide many additional details regarding their movement patterns.
Summertime movements
Breeding and newly fledged Sooty Falcons in Oman had small home ranges. This reflects the facts that breeding adults are tied to a nest site, and that prey availability near the nest would be increasing as the migration of small birds gathers momentum in late summer. In contrast, summertime ranges of non-breeding falcons were orders of magnitude larger because they were not tied to a nest site, and because they might have been prospecting for breeding possibilities amongst dispersed nesting areas. Ranging behaviour is known to vary with age and breeding status in a number of raptor species (Newton Citation1979, Haworth et al. Citation2006, Tanferna et al. Citation2013), and Eleonora’s Falcons that become breeders are known to range far from breeding colonies during the pre-breeding period (Mellone et al. Citation2013b).
Migration
Tracked Sooty Falcons departed from breeding areas in October and November, presumably because food resources declined as the migration of the birds upon which the falcons fed waned (Newton Citation2008), although we had no data on prey abundance. Departure dates were similar across years. The individual that summered in Eritrea-Ethiopia (EX11692), an adult and apparent non-breeder, was the earliest to depart a breeding site, but it moved only to a non-breeding area on mainland Eritrea and did not commence migration until later. Presumably it made this move because its non-breeder status meant it was not tied to a brood of growing nestlings that needed to be fed. Such pre-migration movements have been recorded for Eleonora’s Falcon (Mellone et al. Citation2012b), but in that case it was a falcon that had successfully reared offspring.
Most migratory bird species, including raptors, feed while on migration and may stop at locations along their migration route where food is plentiful (Newton Citation2008). The migrating falcons made stopovers, most likely to feed, and may have encountered concentrations of prey like swarming termites (Rhinotermitidae) or quelea colonies. Some Sooty Falcon stopover locations were in areas also used by migrating Eleonora’s Falcons for stopovers, especially savannah areas of Ethiopia and South Sudan (Gschweng et al. Citation2008, Hadjikyriakou et al. Citation2020b). However, details of stopover behaviour could not be discerned due to limited tracking data, and indeed some stopovers may not have been recorded due to data gaps. During stopovers Sooty Falcons used a variety of land covers and their step-lengths suggest that the birds still moved about at these sites, presumably reflecting the fact that Sooty Falcons are aerial hunters of birds and insects that may themselves have been migrating (Anderson Citation2009). Our limited data suggest a fly-and-forage strategy like that of Eleonora’s Falcon’s (Mellone et al. Citation2013a, Hadjikyriakou et al. Citation2020b).
The autumn migration route was inland from the East African coast and was not the shortest path between breeding areas and Madagascar. This was true for all ages but most conspicuously so for juveniles (for which we had a larger sample). We presume that the indirect migration route may result from falcons following a path that also offers hunting possibilities (see above), as has been suggested for other small migratory falcon species (Newton Citation1986, Strandberg et al. Citation2008, Anderson Citation2009, López-López et al. Citation2010, Hadjikyriakou et al. Citation2020b). We have no observations of feeding by tracked Sooty Falcons while on migration, and no data on the prey they hunt. However, many species that feed on small vertebrates when breeding, shift to feeding on large insects when migrating and wintering (Bildstein Citation2006). A compilation of data by Ristow (Citation2004) for the closely related Eleonora’s Falcon shows that insects become relatively more important than birds in their diet during the winter months spent in Africa. Additionally, reports from Madagascar note the importance of insects in the diet of Sooty Falcons (Walter Citation1979a, Citationb, Zefania Citation2001), and they feed on airborne termites and mole crickets (Gryllotalpidae) in East Africa during spring migration (S. Thomsett, pers. comm.). Indeed, even on the breeding grounds, insects are consumed and may be an important component of Sooty Falcon diet during the early breeding season when abundance of avian prey may be low (McGrady et al. Citation2017).
Our tracking suggested that, like Eleonora’s Falcons (Mellone et al. Citation2013a, Hadjikyriakou et al. Citation2020b), Sooty Falcons exhibit a loop migration, although we tracked only a single individual across its complete annual cycle. Adult falcons, whether breeders or non-breeders, appeared to migrate more directly between summering and wintering grounds than young birds, but data gaps and small samples precluded conclusive results. More direct migration by adults may reflect their migratory experience, better hunting skills, and an enhanced drive during their spring migration to arrive early at breeding areas so as to access preferred nest sites or mates (Newton Citation1979, Citation2008).
Like Eleonora’s Falcons (Gschweng et al. Citation2008, Kassara et al. Citation2012), some Sooty Falcons dwelt for some time on the African mainland before crossing the Mozambique Channel. We speculated that the staging that occurred on the Mozambique and Tanzania coasts in autumn, and the timing of crossing in spring, might be the result of falcons waiting for favourable (tail) wind conditions before crossing the sea (Mellone et al. Citation2011, Citation2015, Nourani et al. Citation2018). However, visual examination of large-scale weather data did not provide evidence for this; winds appeared light and variable in direction at times of observed crossings. Additionally, even if falcons waited for advantageous weather conditions to make the flight across the sea, they may, at the same time, have encountered good foraging opportunities, and the benefit of those may have been as important, if not more important, than the good weather for crossing. The similarity in step-length at coastal staging areas and other stopover sites () supports this possibility. On the other hand, the fact that maximum speeds during crossing of Mozambique Channel could be almost twice the average speeds during migration over land (e.g. for EX11699) suggests that wind conditions could affect when falcons chose to cross the channel. Some Sooty Falcons made at least a portion of the crossing of the Mozambique Channel at night; similar observations have been reported for Eleonora’s Falcons (Gschweng et al. Citation2012), and migrating Amur Falcons Falco amurensis fly at night when crossing between India and Africa (Dixon et al. Citation2011, http://www.satellitetracking.eu/inds/showmap/?check_308=308). Mean flight speeds of Sooty Falcons crossing the Mozambique Channel in autumn (42.95 km/h) fell within the range of those reported for Eleonora’s Falcon (range: 31.8–62.1 km/h, mean: 54.2 km/h; López-López et al. Citation2010). Small sample sizes and absence of detailed data on weather conditions preclude more in-depth analyses. In any case, for decades, occasional sightings have been made of Sooty Falcons on the African mainland during the nominal wintering period. It should be noted that coastal Mozambique, Tanzania and South Africa are considered part of the winter range of Sooty Falcon (del Hoyo et al. Citation1994, BirdLife International Citation2020), and thus must present hunting opportunities for falcons that ultimately winter on Madagascar.
We recorded night-time migration by Sooty Falcons, as did Javed et al. (Citation2012), with the most obvious instances being during the sea crossing between the African mainland and Madagascar. In reference to those, however, the crossing distance (minimum 430 km) and the recorded migration speeds (41–79 km/h) meant that at least some night-time migration was required, no matter when the crossing was initiated. Good weather conditions could motivate night-time flight during migration. However, as mentioned above, and in contrast to Javed et al. (Citation2012), we did not find evidence for this in the falcons we tracked.
Unlike the overland migratory routes of closely related Eurasian Hobby Falco subbuteo (Strandberg et al. Citation2008, Meyburg et al. Citation2011) and Eleonora’s Falcon (Gschweng et al. Citation2008), those of Sooty Falcons did not include crossings of very large terrestrial ecological barriers (i.e. Sahara Desert). Instead, they travelled through a mosaic of habitats that in most cases probably provided at least some hunting opportunities. Hunting opportunities encountered during migration and the spatial relationship of the Arabian Peninsula and African land masses may serve to funnel individual Sooty Falcons along geographically similar migration routes, the habitat mosaics and ephemeral nature of their prey may also result in variation in individual migration paths. This may account for the individual variation in route and pace of migration, as well as observations of Sooty Falcons rather far west in Africa (M. Gschweng, unpubl. data, http://sootyfalconoman.blogspot.com/; Buij Citation2011). Because we tracked relatively few birds our ability to perform statistical tests and make population level inferences regarding migration patterns was limited.
Wintertime movements
On Madagascar, tracked falcons used areas previously identified as part of their main wintering range: mostly in the south and southwestern parts of the country, and at the Ivato International Airport, near Antananarivo in the central part of the country (Walter Citation1979a, Zefania Citation2001, Javed et al. Citation2012). None of the tracked birds ranged into eastern or northern parts of the country, consistent with the information that Sooty Falcons are less common in those areas (Langrand Citation1990, Zefania Citation2001, R. Thorstrom & L.-A. Réné de Roland, unpubl. data). South and southwest Madagascar are the driest parts of the country, although the months when falcons are present comprise the rainy season. Southern Madagascar, however, is not uniformly dry; more eastern areas are wetter and cyclones can occur along the south coast (as in January 2014, which may have affected birds we tracked). Most areas over which falcons moved were mosaics of dry woodland, bushland and grass, interspersed in places with agricultural land. They also ranged over xeric succulent forests near Morandava. In contrast, the falcon tracked by Javed et al. (Citation2012) seemed to prefer agricultural areas. Our findings on Sooty Falcon habitat use during winter need to be considered in light of imprecision or inaccuracy of freely available vegetation/land cover data, something that could compromise their validity (Hadjikyriakou et al. Citation2020a).
Both Sooty and Eleonora’s Falcons winter almost exclusively in Madagascar, but their distributions are largely non-overlapping, with Sooty Falcons being more common in the drier regions of the south and southwest (Walter Citation1979a, Zefania Citation2001), and Eleonora’s Falcons being found more commonly in the humid northeast (Zefania Citation2001, Gschweng et al. Citation2012, Mellone et al. Citation2012a, Kassara et al. Citation2017). Indeed, on both wintering/non-breeding and summering/breeding grounds Sooty Falcons occur in environments that are generally more arid than those used by Eleonora’s Falcon. Both Eleonora’s and Sooty Falcon appear to feed primarily on insects while in Madagascar and make use of habitat mosaics that provide hunting opportunities and perhaps enhance prey abundance through edge effects (Gschweng et al. Citation2012, Mellone et al. Citation2012a).
The Sooty Falcons that wintered in southern Madagascar wandered over large areas and ranged in a manner similar to that of the bird tracked by Javed et al. (Citation2012). Although we have no data on the availability of potential prey, this rather nomadic existence likely allows falcons to take best advantage of sometimes ephemeral concentrations of large insects (e.g. locusts, Acrididae) that may erupt during sporadic rain events. Indeed, a locust plague caused substantial damage to crops in Madagascar in 2014, a year when we tracked two (EX11699, EX11700) falcons wintering on Madagascar (http://www.fao.org/news/story/en/item/251877/icode/). A willingness to wander also allows falcons to mitigate the effects of bad weather, like cyclones, that might negatively affect prey numbers and hunting success. Despite the apparent differences between the habitats used by falcons in this study and those used by the bird tracked by Javed et al. (Citation2012), we speculate that falcon movements most likely reflected the availability of arthropod prey.
Raptors tend to be more nomadic on their non-breeding grounds than they are on their breeding grounds (Bildstein Citation2006), in large part because they are not tied to a nest site or dependent offspring. However, species that could move over large areas may have small home ranges in good quality habitats with plentiful food. In contrast to the birds tracked in southern Madagascar (Javed et al. Citation2012; M. McGrady, unpubl. data), the bird that was tracked at the airport had a small home range, indicating that the airport had a surfeit of food, a likelihood reinforced by the presence of many Sooty and some Eleonora’s Falcons roosting in nearby trees (Walter Citation1979a, M. McGrady, unpubl. data). Because there may be differences in space-use among wintering Sooty Falcons, data from individuals pursuing different strategies are needed if we are to effectively manage and conserve the species.
No Sooty Falcons were tracked in Madagascar for more than one winter, and it is not known to what extent they show fidelity to non-breeding areas. Predictable concentrations of wintering Sooty Falcons at Morandava and the Antanarivo Airport (Walter Citation1979a, Zefania Citation2001) suggest the possibility that some individuals might return annually to such areas. Although data from Sooty Falcons were sparse (due to the loss of some transmitters, and gaps in data from birds that did make it to Madagascar), they suggest winter ranging behaviours that correspond with those of the closely related Eleonora’s Falcon, which show a significant overlap of ranges between years (i.e. fidelity, Kassara et al. Citation2017), and between specific monthly periods in separate years as they changed habitat use during the course of the winter (Hadjikyriakou et al. Citation2020a).
Madagascar is known for its biodiversity and high rate of endemism across a variety of taxa, and a number of habitats, animal and plant species are under severe conservation threat (Ganzhorn et al. Citation2001). For the most part, the tracked Sooty Falcons did not frequent the habitats identified as being under more general conservation threat (Goodman & Patterson Citation1997, Conservation International Citation2014), but ranged over dry bushland. However, some birds spent time north of Morandava in threatened succulent woodlands, where Walter (Citation1979a), Zefania (Citation2001), and we (unpubl. data) observed many Sooty Falcons. Because insects are an important food resource on the wintering grounds, which are not necessarily associated with endangered habitats, conservation measures for this species need not focus on habitats per se, rather on conditions that support healthy insect populations. Intensification of agriculture, increased use of insecticides, reduction of wet areas, and locust control programmes are factors that might reduce insect numbers and diversity, and thus impact Sooty Falcons in Madagascar. Additionally, climate change could impact on Sooty Falcons and their prey if southern areas of Madagascar become even more arid or if frequency or intensity of cyclones in the region increases (Kassara et al. Citation2017). Villagers in the Morandava area are known to collect and eat Sooty Falcons that have been soaked and grounded by cyclonic events (L.-A. Réné de Roland unpubl, data). However, the nomadic movement behaviour of the Sooty Falcons may allow them the flexibility to mitigate changes in food availability that may occur due to climate change and anthropogenic factors.
Transmitter effects
We note that some falcons tracked by us perished, or transmitters failed or were dropped, mostly during their migration from breeding areas to Madagascar. In only one case were we able to determine the fate of the bird/transmitter. The relatively high apparent mortality during migration (assuming the worst that all lost tags were mortalities), especially amongst juveniles, is in line with what has been found for other raptors (Klaassen et al Citation2014). However, the transmitters we used were near the top end of the rule-of-thumb recommendation of 4–5% of body mass (Kenward Citation2001). Hadjikyriakou et al. (Citation2020a) report no noticeable effect of transmitters on Eleonora’s Falcons, some of which were about 5% of the mass of the marked bird. However, as a matter of caution and in agreement with Javed et al. (Citation2012), we suggest that any future tracking of Sooty Falcons use lighter transmitters, which are now available.
Conclusions
Our study and that of Javed et al. (Citation2012) have provided valuable data on several aspects of Sooty Falcon migration, wintering, and the summering of non-breeders, but they are based on tracking of a fairly small number of individuals, and lean heavily on making comparisons with Eleonora’s Falcon, for which more birds have been tracked (Gschweng et al. Citation2008, Mellone et al. Citation2012a, Kassara et al. Citation2017, Hadjikyriakou et al. Citation2020a, Citationb). This highlights the fact that our knowledge is far from complete, and that better tracking data from a larger number of individuals are needed, along with detailed information on habitat and weather conditions. The effects of age and sex are also aspects that need to be examined (Mellone et al. Citation2013a, Citation2015), when more data are collected. Additionally, information is required on prey type and availability during migration, on the non-breeding grounds and in the summer. Amongst other things, it will only be possible to untangle the relative influence of foraging opportunities and weather on timing of the sea crossing, and explain the movement of Sooty Falcons on the non-breeding grounds if more and better data are collected. Encouragingly, the growing pool of research on Eleonora’s Falcon movement (Kassara et al. Citation2017, Hadjikyriakou et al. Citation2020a, Citationb) shows what might be achieved for Sooty Falcons by using improved tracking technology on larger samples.
Sooty Falcons are vulnerable to disturbance and habitat loss at breeding sites, and the presumed reduction in songbird numbers in autumn (Gallo Orsi et al. Citation2014). Although not currently threatened, their unusual ecology, rather limited and fragmented breeding distribution and restricted wintering distribution all suggest that Sooty Falcons could be vulnerable to climate change, and inevitable changes in land-use patterns across their annual distribution (Gallo Orsi et al. Citation2014, BirdLife International Citation2020), a vulnerability that Eleonora’s Falcons could also face (Kassara et al. Citation2017). Additionally, Sooty Falcons apparently face a high risk of mortality while away from breeding areas (McGrady et al. Citation2016, this study), but we know little about the causes. Conservation of the global population of Sooty Falcons necessitates protection of the entire ecological network, including summering/breeding and wintering/non-breeding grounds, migration routes and coastal staging sites. Understanding where, when and how Sooty Falcons face high risk of mortality will be important in maintaining population persistence.
Acknowledgements
We thank the Environmental Authority in Oman (formerly the Ministry for Environment and Climate Affairs) and Royal Oman Police for permission to conduct our research. Our work could not have been accomplished without the logistical support of the officers and sailors of the Royal Yachts of Royal Court Affairs. In Madagascar the manager of Ivato International Airport and Moise aided our capture of Sooty Falcons there. We are grateful to I. R. Hartley, two anonymous reviewers and an anonymous Associate Editor for many helpful comments and suggestions. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Alerstam, T. 1990. Bird Migration. Cambridge University Press, Cambridge.
- Allen, A.M. & Singh, N.J. 2016. Linking movement ecology with wildlife management and conservation. Front. Ecol. Evol. 3: 1–13.
- Anderson, R.C. 2009. Do dragonflies migrate across the western Indian Ocean? J. Trop. Ecol. 25: 347–358.
- Berthold, P. 2001. Bird Migration: a general survey. Oxford University Press, Oxford.
- Bildstein, K.L. 2006. Migrating Raptors of the World – Their Ecology and Conservation. Cornell University Press, Ithaca.
- BirdLife International. 2020. Species factsheet: Falco concolor. Downloaded from http://www.birdlife.org on 30 September 2020.
- Bivand, R. & Rundel, C. 2016. rgeos: interface to Geometry Engine – Open Source (GEOS). R package version 0.3-20. https://CRAN.R-project.org/package=rgeos.
- Börger, L. & Fryxell, J.M. 2012. Quantifying individual differences in dispersal using net squared displacement. In Clobert, J., Baguette, M., Benton, T.G. & Bullock, J.M. (eds) Dispersal and Spatial Evolutionary Ecology, 223–230. Oxford University Press, Oxford.
- Buij, R. 2011. Sightings of Sooty Falcon Falco concolor in the far north of the Cameroon. Bull. Afr. Bird Club 18: 211–214.
- Calenge, C. 2006. The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Model 197: 516–519.
- Cody, M.L. 1985. Habitat Selection in Birds. Academic Press, New York.
- Conservation International. 2014. Ecosystem profile. Madagascar and Indian Ocean Islands. Report to Critical Ecosystem Partnership Fund.
- Criffield, M., van de Kerk, M., Leone, E., Cunningham, M.W., Lotz, M., Oli, M.K. & Onorato, D.P. 2018. Assessing impacts of intrinsic and extrinsic factors on Florida Panther movements. J. Mammal. 99: 702–712.
- del Hoyo, J., Elliot, A. & Sargatal, J. 1994. Handbook of Birds of the World. Vol. 2. New World Vultures to Guineafowl. Lynx Edicions, Barcelona.
- Dixon, A., Batbayar, N. & Purev-Ochir, G. 2011. Autumn migration of an Amur Falcon Falco amurensis from Mongolia to the Indian Ocean tracked via satellite. Forktail 27: 86–89.
- Faaborg, J., et al. 2010. Conserving migratory land birds in the New World: do we know enough? Ecol. Appl. 20: 398–418.
- Fleming, C.H., et al. 2020. A comprehensive framework for handling location error in animal tracking data. bioRxiv preprint. doi:10.1101/2020.06.12.130195.
- Frumkin, R. & Pinshow, B. 1983. Notes on the breeding ecology and distribution of the Sooty Falcon Falco concolor in Israel. Ibis 125: 251–259.
- Gallo Orsi, U., Williams, N.P., Javed, S. & McGrady, M. 2014. International Single Species Action Plan for the Sooty Falcon Falco concolor. CMS Raptors MoU (Draft), Abu Dhabi.
- Ganzhorn, J.U., Lowry, P.P., Schatz, G.E. & Sommer, S. 2001. The biodiversity of Madagascar: one of the world’s hottest hotspots on its way out. Oryx 35: 346–348.
- Gschweng, M., Kalko, E.K., Querner, U., Fiedler, W. & Berthold, P. 2008. All across Africa: highly individual migration routes of Eleonora’s Falcon. Proc. R. Soc. B 275: 2887–2896.
- Gschweng, M., Kalko, E.K., Berthold, P., Fiedler, W. & Fahr, J. 2012. Multi-temporal distribution modelling with satellite tracking data: predicting responses of a long-distance migrant to changing environmental conditions. J. App. Ecol. 49: 803–813.
- Goodman, S.M. & Patterson, B.D. 1997. Natural Change and Human Impact in Madagascar. Smithsonian Institution Press, Washington, DC.
- Gosler, A. 2004. Birds in the hand. In Sutherland, W.J., Newton, I. & Green, R.E (eds) Bird Ecology and Conservation: a handbook of techniques, 85–118. Oxford University Press, Oxford.
- Hadjikyriakou, T.G., Kassara, C., De Roland, L.A.R., Giokas, S., Tsiopelas, N., Evangelidis, A., Thorstrom, R. & Kirschel, A.N. 2020a. Phenology, variation in habitat use, and daily activity patterns of Eleonora’s falcon overwintering in Madagascar. Landscape Ecol. 35: 159–172.
- Hadjikyriakou, T.G., Nwankwo, E.C., Virani, M.Z. & Kirschel, A.N. 2020b. Habitat availability influences migration speed, refuelling patterns and seasonal flyways of a fly-and-forage migrant. Movement Ecol. 8: 10. doi:10.1186/s40462-020-0190-4.
- Hansson, L.-A. & Åkesson, S. 2014. Animal Movement Across Scales. Oxford University Press, Oxford.
- Haworth, P.F., McGrady, M.J., Whitfield, D.P., Fielding, A.H. & McLeod, D.R.A. 2006. Ranging distance of resident Golden Eagles Aquila chrysaetos in western Scotland according to season and breeding status. Bird Study 53: 265–273.
- Hijmans, R.J. 2015. raster: Geographic data analysis and modelling. R package version 2.4-20. https://CRAN.R-project.org/package=raster.
- Hunt, W.G. 1998. Raptor floaters at Moffats’ equilibrium. Oikos 82: 191–197.
- Javed, S., Douglas, D.C., Khan, S., Shah, J.N. & Al Hammadi, A.A. 2012. First description of autumn migration of Sooty Falcon Falco concolor from the United Arab Emirates to Madagascar using satellite telemetry. Bird Conserv. Int. 22: 106–119.
- Karelus, D.L., McCown, J.W., Scheick, B.K., van de Kerk, M., Bolker, B.M. & Oli, M.K. 2017. Effects of environmental factors and landscape features on movement patterns of Florida Black Bears. J. Mammal. 98: 1463–1478.
- Karelus, D.L., McCown, J.W., Scheick, B.K., van de Kerk, M., Bolker, B.M. & Oli, M.K. 2019. Incorporating movement patterns to discern habitat selection: Black Bears as a case study. Wildl. Res. 46: 76–88.
- Kassara, C., Fric, J., Gschweng, M. & Sfenthourakis, S. 2012. Complementing the puzzle of Eleonora’s Falcon (Falco eleonorae) migration: new evidence from an eastern colony in the Aegean Sea. J. Ornithol. 153: 839–848.
- Kassara, C., Gangoso, L., Mellone, U., Piasevoli, G., Hadjikyriakou, T.G., Tsiopelas, N., Giokas, S., López-López, P., Urios, V., Figuerola, J. & Silva, R. 2017. Current and future suitability of wintering grounds for a long-distance migratory raptor. Sci. Rep. 7: 8798.
- Kavanaugh, B. & King, H. 2008. Observations from 1998–2006 on the breeding population of Sooty Falcons Falco concolor on the Hawar Islands, Kingdom of Bahrain. Sandgrouse 30: 70–76.
- Kenward, R.E. 2001. A Manual for Wildlife Radio Tagging. Academic Press, London.
- Kerlinger, P. 1989. Flight Strategies of Migrating Hawks. University of Chicago Press, London.
- Klaassen, R.H., Hake, M., Strandberg, R., Koks, B.J., Trierweiler, C., Exo, K.M., Bairlein, F. & Alerstam, T. 2014. When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J. Anim. Ecol. 83: 176–184.
- Langrand, O. 1990. Guide to the Birds of Madagascar. Yale University Press, London.
- López-López, P., Liminana, R., Mellone, U. & Urios, V. 2010. From the Mediterranean Sea to Madagascar: are there ecological barriers for the long-distance migrant Eleonora’s Falcon? Landscape Ecol. 25: 803–813.
- McGrady, M.J., Al Fazari, W., Al Jahdhami, M.H. & Oli, M.K. 2016. Survival of Sooty Falcons (Falco concolor) in Oman. J. Ornithol. 157: 427–437.
- McGrady, M.J., Al Fazari, W.A., Al Jahdhami, M., Nicoll, M.A.C. & Oli, M.K. 2017. Sooty Falcon Falco concolor reproduction and population dynamics on the islands in the Sea of Oman. Ibis 159: 828–840.
- McGrady, M.J., Young, G.S. & Seegar, W.S. 2006. Migration of a Peregrine Falcon Falco peregrinus over water in the vicinity of a hurricane. Ringing Migr. 23: 80–84.
- Mellone, U., López-López, P., Limiñana, R. & Urios, V. 2011. Weather conditions promote route flexibility during open ocean crossing in a long-distance migratory raptor. Int. J. Biometeorol. 55: 463–468.
- Mellone, U., López-López, P., Limiñana, R. & Urios, V. 2012a. Wintering habitats of Eleonora’s Falcons Falco eleonorae in Madagascar. Bird Study 59: 29–36.
- Mellone, U., Urios, V., Rguibi-Idrisi, H., Limiñana, R., Benhoussa, A. & López-López, P. 2012b. Ranging behaviour of Eleonora’s Falcons Falco eleonorae during chick-rearing. Acta Ornithol. 47: 195–198.
- Mellone, U., López-López, P., Limiñana, R., Piasevoli, G. & Urios, V. 2013a. The trans-equatorial loop migration system of Eleonora’s Falcon: differences in migration patterns between age classes, regions and seasons. J. Avian Biol. 44: 417–426.
- Mellone, U., Lopez-Lopez, P., Liminana, R. & Urios, V. 2013b. Summer pre-breeding movements of Eleonora’s Falcon Falco eleonorae revealed by satellite telemetry: implications for conservation. Bird Conserv. Int. 23: 487–494.
- Mellone, U., Limiñana, R., López-López, P. & Urios, V. 2015. Regional and age-dependent differences in the effect of wind on the migratory routes of Eleonora’s Falcon. Curr. Zool. 61: 428–434.
- Meyburg, B.-U. & Fuller, M.R. 2007. Satellite tracking. In Bird, D.M. & Bildstein, K.L. (eds) Raptor Research and Management Techniques Manual, 242–248. Hancock House, Surrey, Canada.
- Meyburg, B.-U., Howey, P.W., Meyburg, C. & Fiuczynski, K.-D. 2011. Two complete migration cycles of an adult Hobby tracked by satellite. Br. Birds 104: 2–15.
- Newton, I. 1979. The Population Ecology of Raptors. T. & A.D. Poyser, Birkhamstead.
- Newton, I. 1986. The Sparrowhawk. T. & A.D. Poyser, Birkhamstead.
- Newton, I. 2008. The Migration Ecology of Birds. Academic Press, London.
- Newton, I. 2010. Bird Migration. Harper Collins, London.
- Newton, I. & Rothery, P. 2001. Estimation and limitation of numbers of floaters in a Eurasian Sparrowhawk population. Ibis 143: 442–449.
- Nourani, E., Safi, K., Yamaguchi, N.M. & Higuchi, H. 2018. Raptor migration in an oceanic flyway: wind and geography shape the migratory route of Grey-faced Buzzards in East Asia. R. Soc. Open Sci. 5: 171555.
- R Core Team. 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Ristow, D. 2004. On the insect diet of Eleonora’s Falcon Falco eleonorae and its importance for coloniality. In Meyburg, B.-U. & Chancellor, R.D. (eds) Raptors Worldwide: Proceedings of the VI World Conference on Birds of Prey and Owls, 705–712. WWGBP/MME, Berlin.
- Singh, N.J., Allen, A.M. & Ericsson, G. 2016. Quantifying migration behavior using net squared displacement approach: clarifications and caveats. PloS One 11: e0149594.
- Smith, S.M. 1978. The ‘underworld’ in a territorial sparrow: adaptive strategy for floaters. Am. Nat. 112: 571–582.
- Strandberg, R., Klaassen, R.H.G., Hake, M., Olofsson, P. & Alerstam, T. 2008. Converging migration routes of Eurasian Hobbies Falco subbuteo crossing the African equatorial rain forest. Proc. R. Soc. B 276: 727–733.
- Tanferna, A., López-Jiménez, L., Blas, J., Hiraldo, F. & Sergio, F. 2013. Habitat selection by Black Kite breeders and floaters: implications for conservation management of raptor floaters. Biol. Conserv. 160: 1–9.
- Walter, H. 1979a. Eleonora’s Falcon: adaptions to prey and habitat in a social raptor. University of Chicago Press, Chicago.
- Walter, H. 1979b. The Sooty Falcon (Falco concolor) in Oman: results of a breeding survey, 1978. J. Oman Stud. 5: 9–60.
- Wilcove, D.S. & Wikelski, M. 2008. Going, going, gone: is animal migration disappearing. PLoS Biol. 6: e188.
- Willemoes, M., Strandberg, R., Klaassen, R.H., Tøttrup, A.P., Vardanis, Y., Howey, P.W., Thorup, K., Wikelski, M. & Alerstam, T. 2014. Narrow-front loop migration in a population of the Common Cuckoo Cuculus canorus, as revealed by satellite telemetry. PloS one 9: e83515.
- Worton, B. 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70: 164–168.
- Zefania, S. 2001. Observation of Sooty and Eleonora’s Falcons in Madagascar. In Leshem, Y., Froneman, A., Mundy, P. & Shamir, H. (eds) Wings Over Africa: Proceedings of the International Seminar on Bird Migration: Research, Conservation, Education and Flight Safety, 151–159. International Center for the Study of Bird Migration, Tel Aviv.
