ABSTRACT
1. The hypothesis was that a diet with a high starch to fat ratio (HS) impairs nutrient digestibility and growth performance, as compared to a diet with a low starch to fat ratio (LS) in Eimeria–challenged broilers. From days 10 to 29, 12 replicate pens of birds were given isocaloric and isonitrogenous steam-pelleted diets with either HS or LS, by replacing the wheat starch in one diet by a mixture of rapeseed oil and inert sand in the other. On d 17, a 10-fold dose of live vaccine strains of Eimeria spp. was administered via drinking water. Ileal samples were collected on days 16 and 29.
2. Starch content in the ileum tended to be higher on d 16 and was significantly higher on d 29 in the HS group.
3. The HS diet did not induce exceedingly high levels of starch in the ileum, suggesting there was no starch overload in the gut. Ileal starch digestibility was improved with increasing dietary starch level from 23% to 45%. This demonstrated the capacity of the broiler chicken to digest high levels of starch regardless of Eimeria spp. infection. Ileal energy digestibility was not affected by the treatments.
4. Weight gain did not differ between treatments; however, birds fed the LS diet were less efficient in feed conversion as compared to those fed the HS diet.
5. The use of isolated starch and the unintended higher extent of starch gelatinisation in the HS diet may have contributed to the higher starch digestibility in birds given the HS diet. Thus, the hypothesis that high ratios of starch to fat in pelleted diets may impair starch digestibility and production performance in Eimeria-challenged broiler chickens was not verified. Further work is required to clarify this research question, taking into consideration the physical form of starch source and the potentially confounding role of feed processing on starch availability.
Introduction
Broiler chickens are efficient at utilising starch as their main energy source (Thomas et al. Citation2008). This ability is presumably due to sufficient amylase secretion (Svihus Citation2014), high activity levels of disaccharidases shortly after hatching (Chotinsky et al. Citation2001) and a highly adaptive intestinal mechanism for glucose uptake (Suvarna et al. Citation2005). Nevertheless, starch digestibility has been observed to be low in broilers given wheat-based pelleted diets, with values ranging from 0.76 to 0.93 (Svihus Citation2001; Svihus et al. Citation2010; Abdollahi et al. Citation2011). Svihus and Hetland (Citation2001) evaluated starch digestibility in broiler chickens fed identical wheat diets that were pelleted (control), offered as mash or pelleted and diluted with 100 g/kg cellulose powder. Compared to the mash diet and the diluted diet, undiluted pelleting resulted in an overload of wheat-starch (reaching more than 200 g/kg freeze-dried ileal contents) in ileal chyme and consequently poorer starch digestibility. Accordingly, the authors proposed that reducing dietary starch level (diet dilution) or a decrease in feed intake (by changing diet structure) may be potential means to prevent excessive concentration of starch in the ileum, thereby optimising digestion.
The physiological ability to digest lipids is not fully developed in the young chick due to low lipase activity (Krogdahl Citation1985; Noy and Sklan Citation1995) and insufficient bile acid secretion (Sell Citation1996). More recently, Tancharoenrat et al. (Citation2013) confirmed this limited capacity in one-week-old chicks, and detected a significant increase in total tract digestibility (from 0.53 to 0.81) of fat at two-weeks of age independent of fat type.
Increased amounts of undigested nutrients in the digestive tract may stimulate undesirable microbial growth that could induce enteric disorders (Choct et al. Citation1999; Annett et al. Citation2002). Corroborating this, Engberg et al. (Citation2004) found a tendency for increased ileal and caecal numbers of Clostridium perfringens due to the presence of more starch and other fermentable nutrients in the small intestine of broilers fed a pelleted wheat diet. Eimeria spp. infection is another factor that may lead to microbial and intestinal dysfunctions (Yun et al. Citation2000; Hauck Citation2017), and consequently increase broiler intestinal vulnerability to other types of intestinal insults and imbalances.
Starch is the major energy-supplying source in broiler diets, but when prices are favourable, it may be preferred to replace starch with fat in the diet. Due to the rising prices of cereal grains, the use of grain-replacing, unconventional feedstuffs is increasing, and so more fat is added to increase dietary energy content. The effect of varying dietary starch to fat ratios on the performance of broilers fed isocaloric and isonitrogenous diets have been investigated and produced inconsistent results. For Veldkamp et al. (Citation2017a), Veldkamp et al. (Citation2017b) reported an improvement in feed conversion ratio (FCR) and growth performance with higher starch to fat ratio. Malheiros et al. (Citation2004) on the other hand reported slightly better FCR with lower starch to fat ratio, whereas Baéza et al. (Citation2015) found that performance parameters were not affected by the varying ratios of starch to fat.
Thus, the hypothesis tested was that a diet with a high starch to fat ratio (31:1) would result in lower intestinal starch digestibility, increased concentrations of undigested starch in the posterior small intestine and impaired production performance in Eimeria–challenged broilers. The present paper focusses on nutrient digestibility and production performance, while effects on intestinal histomorphology, C. perfringens counts and toxin profile, necrotic enteritis prevalence and abundance of short-chain fatty acids are discussed in an accompanying paper (Granstad et al. Citationin press).
Materials and methods
Experimental diets and processing
Experimental diets () were processed at the Centre for Feed Technology (Fôrtek), Norwegian University of Life Sciences, Ås, Norway, and were formulated to meet or exceed Ross 308 strain recommendations for major nutrients (Aviagen Citation2014). The diets contained 5 g/kg titanium dioxide as a digestibility marker. The wheat and soybean meal (SBM) were ground to pass through a 3-mm sieve in a hammer mill (Münch-Edelstahl, Wuppertal, Germany licenced by Bliss, USA, 18.5 kW, 3000 RPM) before being mixed with other ingredients. The mash was steam-conditioned in a double pass pellet-press conditioner (Münch-Edelstahl, Wuppertal, Germany) and then pelleted using a pellet press (Münch-Edelstahl, Wuppertal, Germany, 1.2 t/h, 2 × 17 kW, RMP 350) equipped with a 60-mm-thick die with 5-mm diameter die openings. Conditioning temperature and production rates were 71°C and 700 kg/h for the diet with a high starch to fat ratio (HS), and 81°C and 800 kg/h for the diet with a low starch to fat ratio (LS). Specific energy consumption values were 45.7 and 18.5 kWh/t, and motor load was 52 and 24 A for the diet with a HS and LS, respectively. Despite the reduced conditioning temperature, post-pelleting temperatures were 95°C in the diet with a HS compared to 81.9°C for the diet with a LS, measured by collecting a sample of hot pellets from immediately below the pellet press into an insulated box fitted with a thermometer. The extent of starch gelatinisation was almost 7.3-fold higher with HS compared to LS ().
Table 1. Experimental diet composition, calculated and analysed nutrient content (g/kg as fed)
Birds and housing
The experiment was approved by the national animal research authority (Norwegian Food Safety Authority, approval ID 8824) and performed in accordance with national and international guidelines for the care and use of experimental animals.
A total of 1920 one-day-old mixed-sex Ross 308 broiler chicks obtained from a commercial hatchery (Nortura Samvirkekylling, Våler, Norway) were placed in 24-floor pens measuring 5.6 m2 with new wood shavings. Each pen housed 80 feather-sexed birds with a 50/50 male-female distribution. A room temperature of 33°C was maintained during the first week and thereafter decreased by 3–4°C weekly until the temperature reached 21°C. Water and feed were given ad libitum. The birds were exposed to 23 h light per day on the first 2 days. For the rest of the experimental period, the birds were exposed to 16 h light per day, interrupted by two, 4 h periods of darkness. All birds were fed a commercial starter diet from 0 to 9 d of age. From d 10 to day 29, the birds were randomly divided into two groups of 12 pens each and fed either an HS or an LS grower diet.
Eimeria challenge
A 10-fold dose of the vaccine Paracox-5 vet. (MSD Animal Health, Boxmeer, the Netherlands) containing live, sporulated oocysts from five attenuated strains of Eimeria spp. (one strain each of E. acervulina, E. mitis and E. tenella and two strains of E. maxima) was administered via the drinking water of all birds on d 17 post hatch.
Production performance measurements
The amount of feed per pen was weighed when allocated, and feed residues were weighed before being discarded at feed change and at the end of the experiment. Accumulated feed intake (FI) per pen from days 10–15, 15–24, 24–28 and 10–28 was calculated. Total live chicken weight per pen was recorded on d 10, 15, 24 and 28, and mean body weight gain (BWG, g/bird) and mean feed conversion ratio (FCR, g feed intake/g weight gain) per pen were calculated.
Sample collection
On days 16 and 29, two birds per pen were randomly selected and killed by a cranial blow followed by cervical dislocation. The small intestine with content was removed and placed in a zigzag pattern over an aluminium foil on a rack, and then immediately snap-frozen with liquid nitrogen and stored at −20°C for later analysis. A section from the posterior jejunum with content (5 cm anterior to Meckel’s diverticulum) was later removed and stored at −80°C until enzyme activity analysis. The jejunum was defined as the segment from the end of the duodenal loop to Meckel’s diverticulum, and the ileum as the section from Meckel’s diverticulum to the ileo-caecal junction.
Chemical analyses
Representative feed samples were ground on a cutting mill (Pulverisette 19, Fritsch Industriestr. 8, 55743 Idar-Oberstein, Germany) through a 0.5 mm sieve. Dry matter and ash content of the feed and ileal samples were determined after drying overnight at 105°C and after 12 h ashing at 550°C, respectively. Gross energy was determined using an adiabatic bomb calorimeter (Parr 6400, Moline, USA) standardised with benzoic acid. Nitrogen content was determined by the Dumas method using a Vario El Cube (Elementar Analysensysteme GmbH, Hanau, Germany 2016). Dried ileal contents were pulverised using a mortar and pestle for subsequent starch, crude fat, gross energy and titanium dioxide analysis. TiO2 content of feed and ileal contents was determined as described by Short et al. (Citation1996). Crude fat was determined after extraction with 80% petroleum ether and 20% acetone in an Accelerated Solvent Extractor from Dionex (ASE200; Sunnyvale, CA, USA). Starch content of the diets was determined enzymatically based on the use of thermostable α-amylase and amylo-glucosidase (McCleary et al. Citation1994). Starch content in freeze-dried ileal samples was determined as described above after extraction with 80% ethanol (2x) to remove free sugars and oligosaccharides. Amylase activity in the jejunal chyme was assayed colorimetrically using amylase assay kit (Abcam ab102523, Cambridge, UK) according to manufacturer’s instructions. Samples for amylase activity were prepared as described by Pérez de Nanclares et al. (Citation2017) and results were expressed as unit/g of wet chyme. The degree of starch gelatinisation (DG) (as a proportion of total starch) was measured by differential scanning calorimetry (DSC 823e Module, Mettler-Toledo, Switzerland) as described by Kraugerud and Svihus (Citation2011).
Calculations
The apparent ileal digestibility coefficients of starch, fat and energy were calculated using the following formula:
Ileal digestibility coefficient = 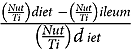 $${{\left({{{Nut } \over {Ti }}} \right) diet{\ } - \left({{{Nut } \over {Ti }}} \right) ileum} \over {\matrix{ {\left({{{Nut } \over {Ti }}} \right) d} \cr } iet}}$$
$${{\left({{{Nut } \over {Ti }}} \right) diet{\ } - \left({{{Nut } \over {Ti }}} \right) ileum} \over {\matrix{ {\left({{{Nut } \over {Ti }}} \right) d} \cr } iet}}$$
where ![]() $$\left({{{Nut } \over {Ti }}} \right)diet $$= the ratio of nutrient and TiO2 in the diet and
$$\left({{{Nut } \over {Ti }}} \right)diet $$= the ratio of nutrient and TiO2 in the diet and ![]() $$\left({{{Nut } \over {Ti }}} \right)ileum $$= the ratio of nutrient and TiO2 in the ileal digesta.
$$\left({{{Nut } \over {Ti }}} \right)ileum $$= the ratio of nutrient and TiO2 in the ileal digesta.
Statistical analysis
Statistical analyses were carried out using the statistical software R (version 2.3.2). All data sets were tested for normality using the Shapiro–Wilk test. A non-normal distribution of production performance data, nutrient content in ileal digesta, nutrient digestibility and amylase activity precluded the use of a parametric statistical test and hence these variables were compared using the two-way Wilcoxon rank-sum test (non-parametric). Differences were considered significant at P < 0.05 and results were expressed as means ± standard error. Each pen was used as the experimental unit for all data.
Results
Production performance
From d 10 to 15, no significant differences in feed intake (FI), body weight gain (BWG) and/or feed conversion ratio (FCR) were observed between dietary treatments (). From d 15 to 24, birds in both groups had similar FI, but those fed the HS diet gained more weight (P = 0.033) and as a result had a better FCR (P < 0.001). From d 24 to 28, birds fed the LS diet consumed significantly more feed than those fed the HS; however, BWG was not different (P > 0.1). Consequently, LS group had poorer FCR (P = 0.003). Over the whole experimental period (d 10 to 28), there was no difference in BWG (P > 0.05) between treatments. Still, birds in the LS group consumed more feed (P = 0.021), and thus were less efficient in feed conversion (P < 0.001) compared to the HS group.
Table 2. Effect of varying ratios of starch to fat on the overall production performance of broilers.1
Ileal digestibility coefficients and amylase activity
The freeze-dried weight of ileal digesta was significantly higher in birds fed the LS diet (containing 16.26% sand), resulting in lower ileal DM digestibility compared to those fed the HS diet (data not shown). As shown in , starch content in the ileum varied between 29 and 80 g/kg digesta, and was significantly influenced by diet composition. Starch digestibility tended to be higher on d 16 (P = 0.083), and was higher (P = 0.009) on d 29 in birds fed the HS diet. The apparent fat digestibility was significantly higher in an LS diet group at both ages, while the apparent energy digestibility was not different (P > 0.05) between the treatments. On d 29, there was a tendency (P = 0.083) for higher amylase activity (by 45%) in the jejunum of birds fed the HS diet compared to the LS diet. Whereas the digestibility of fat was improved with bird age in both diet groups, starch digestibility was increased with age in the HS group only ().
Table 3. Effect of varying ratios of starch: fat on amylase activity (Unit/g jejunal chyme), nutrient concentration in ileal digesta1 and ileal digestibility of nutrients1 and energy
Table 4. Relationships between age and the apparent ileal digestibility coefficients 1 of starch and fat in broilers
Discussion
The current experiment demonstrated the large flexibility of broilers in terms of capacity to thrive on diets containing large variations in the ratios of starch to fat and high level of sand as an inert filler. Compared to the LS diet, feeding the HS diet was expected to cause a reduction in starch digestibility, which, in turn, might impair production performance and intestinal health. However, the HS diet was associated with improved, rather than impaired, starch digestibility and production performance.
Although ileal starch levels were higher in HS birds than LS birds, none of the examined bird groups were recorded with average concentrations higher than 80 g/kg ileal DM. Previous studies (Svihus and Hetland Citation2001; Svihus et al. Citation2010) which examined the association between dietary manipulations (pellets vs. mash and ground wheat vs. whole wheat) and ileal concentration of starch indicated that treatments associated with low (0.79–0.82) starch digestibility coefficients had mean ileal starch concentrations ranging from 222 to 250 g/kg ileal dry matter, whereas treatments associated with high (0.95) starch digestibility had starch concentrations ranging from 88 to 101 g/kg. These experiments were conducted with dietary starch levels ranging from 42% to 52%, as compared to 45% starch in our HS diet. These data indicate that ileal starch contents in the present experiment were similar to or lower than those found in bird groups with satisfactory starch digestibility in previous studies. Based on these data it was concluded that the intake of starch did not imply an overload in the gut in any experimental group in the current study. Poor starch digestibility in wheat diets has been attributed to several different factors, including the soluble fibre-fraction in wheat (Annison Citation1993), wheat hardness (Carré et al. Citation2002), resistant cell wall material (Meng et al. Citation2005), and a lower starch gelatinisation degree (Zimonja and Svihus Citation2009). The wheat in the current experiment was finely ground, and the diets were supplied with fibre-degrading enzymes to eliminate any potential effect of the cell wall or insoluble fibre fraction on nutrient encapsulation and digesta viscosity.
The surprisingly higher starch digestibility obtained with feeding the HS diet and the unanticipated lower starch digestibility associated with feeding the LS diet may be explained by unintended confounding factors, not least the observed higher extent of gelatinisation (by 7.3-fold) in the HS diet compared with the LS diet. A high degree of gelatinisation increases the susceptibility of starch to enzymatic hydrolysis (Mollah et al. Citation1983; Holm et al. Citation1988; Ankrah et al. Citation1999; Zimonja and Svihus Citation2009). The 14% difference in hot pellet temperature between the diets clearly indicated that, like soy oil (Cutlip et al. Citation2008), rapeseed oil in the LS diet had a lubricating effect, and as a result, decreased friction in the pellet die, which was the only source of heat at that point. This was supported by the pellet mill throughput and energy consumption data. In contrast, the very low oil content in the HS diet led to increased friction in the die, i.e., higher pellet temperature, and consequently higher degree of starch gelatinisation (Thomas et al. Citation1998). It is important to note that, although the LS diet resulted in lower starch digestibility, the average concentration of undigested starch in ileal contents was not higher than 58 g/kg, and tended to be lower (on d 16) or was significantly lower (on d 29) than ileal starch levels in the HS group.
It has been shown that starch gelatinisation can be modified, delayed or inhibited by the presence of lipids (Larsson Citation1980; Eliasson et al. Citation1981; Lund and Lorenz Citation1984). Lipids are known to form inclusion compounds with amylose (Putseys et al. Citation2010; López et al. Citation2012) during processing or in the intestine (Holm et al. Citation1983) which potentially, hinders starch digestion. Due to its hydrophobic nature, fat may interfere with the hydration of feed components, for example, by coating starch granules and limiting steam penetration (Zimonja et al. Citation2007), thus repressing swelling and solubilisation (Eliasson et al. Citation1981; Svihus et al. Citation2005) and reducing the rate of starch hydrolysis (Tufvesson et al. Citation2001). Therefore, fat digestibility, or, in other words, the amount of undigested fat remaining in the intestine may have an impact on starch digestion. In fact, fat digestibility improved with age and was significantly higher with a low ratio of starch to fat. Although not evaluated, this may be due to an increase in fatty acid-binding protein activity, lipase activity and bile salt secretion (Krogdahl Citation1985; Krogdahl and Sell Citation1989). Compared to d 16, birds killed on d 29 in both dietary-groups had higher fat digestibility, i.e., less fat was present to complex with starch (Crowe et al. Citation2000). This would make the starch more available for amylase digestion especially since amylase activity was similar at both ages. Despite this, starch digestibility did not improve significantly with age in birds fed the LS diet. This suggested that the low ratio of starch to fat (high dietary level of fat) was not optimal for efficient starch utilisation under the experimental conditions applied. Several researchers (Nitsan et al. Citation1997; Veldkamp et al. Citation2017b) reported a decrease in starch digestibility with low compared to the high ratio of starch to fat in the diet.
Another plausible cause for the high starch digestibility associated with the HS diet was the use of the isolated wheat starch. This source was added to increase starch content in the diet, which was hypothesised to cause high concentrations of starch in the lower intestinal tract. Evidently, isolated wheat starch was not challenging enough for the birds, suggesting a fast rate of degradation in the upper intestinal tract. Compared to wheat, isolated wheat-starch was found to be hydrolysed more readily in vitro (Wiseman et al. Citation2000) and was completely digestible in vivo (Rogel et al. Citation1987), independent of the wheat characteristics (high or low AME).
Amylase results showed a trend characterised by an increase or decrease in activity depending on the amount of substrate in the digesta, as demonstrated previously (Karasov and Hume Citation1997). This physiological adaptation (Murugesan et al. Citation2014) may, at least partly, explain the high capacity of the birds to digest high levels of starch in the diet.
The lower apparent fat digestibility of the HS diet may have been attributed to the low content of dietary fat (14.2 g/kg) and a relatively higher contribution of endogenous losses, such as bile acid esters, cholesterol or structural lipids from desquamated cells (Jørgensen et al. Citation1993). It may be that broilers have a large capacity to utilise fat; however, due to the very low-fat content in the HS diet, fat digestibility from this group may have been unreliable.
The two diets differed significantly with regard to overall feed conversion ratio, but not with regard to body weight gain and ileal energy digestibility. A possible explanation could be that the amount of metabolisable energy was slightly different between the diets, although this was not intended. Both diets were formulated to be isoenergetic and isonitrogenous, assuming an AMEn value of 37.7 MJ/kg or 8843 kcal/kg for the rapeseed oil (Sauvant et al. Citation2004). However, the energetic value of rapeseed oil has been reported to vary considerably (8000–8500 kcal/kg rapeseed oil) (Scheele et al. Citation1997), and thus, the value used in the current trial calculations may have overestimated the true amount of metabolisable energy. Another factor which may have accounted in part for the better feed conversion in birds fed the HS diet was the decreased ingredient segregation (higher gelatinisation) and the resulting reduction of energy expenditure from feed intake. The potential role of an Eimeria spp. infection as an additional factor that may have influenced the production performance results is discussed in the accompanying paper (Granstad et al. Citationin press).
The use of isolated wheat starch and the unintentionally higher extent of starch gelatinisation may have contributed to the high starch digestibility in birds given the HS diet. Thus, the hypothesis that high ratio of starch to fat in a pelleted diet may impair starch digestibility and production performance in Eimeria-challenged broiler chickens was not verified. Further work is required to clarify this research question, taking into consideration the physical form of starch source and the potentially confounding role of feed processing on starch availability.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Abdollahi, M., V. Ravindran, T. Wester, G. Ravindran, and D. Thomas. 2011. “Influence of Feed Form and Conditioning Temperature on Performance, Apparent Metabolisable Energy and Ileal Digestibility of Starch and Nitrogen in Broiler Starters Fed Wheat-based Diet.” Animal Feed Science and Technology 168 (1): 88–99. doi:10.1016/j.anifeedsci.2011.03.014.
- Ankrah, N., G. Campbell, R. Tyler, B. Rossnagel, and S. Sokhansanj. 1999. “Hydrothermal and β-glucanase Effects on the Nutritional and Physical Properties of Starch in Normal and Waxy Hull-less Barley.” Animal Feed Science and Technology 81 (3–4): 205–219. doi:10.1016/S0377-8401(99)00084-X.
- Annett, C., J. Viste, M. Chirino-Trejo, H. Classen, D. Middleton, and E. Simko. 2002. “Necrotic Enteritis: Effect of Barley, Wheat and Corn Diets on Proliferation of Clostridium Perfringens Type A.” Avian Pathology 31 (6): 598–601. doi:10.1080/0307945021000024544.
- Annison, G. 1993. “The Role of Wheat Non-starch Polysaccharides in Broiler Nutrition.” Crop and Pasture Science 44 (3): 405–422. doi:10.1071/AR9930405.
- Aviagen, R. 2014. 308 Nutrition Specifications. Scotland, UK: Aviagen.
- Baéza, E., F. Gondret, P. Chartrin, E. Le Bihan-Duval, C. Berri, I. Gabriel, A. Narcy, M. Lessire, S. Métayer-Coustard, and A. Collin. 2015. “The Ability of Genetically Lean or Fat Slow-growing Chickens to Synthesize and Store Lipids Is Not Altered by the Dietary Energy Source.” Animal 9 (10): 1643–1652. doi:10.1017/S1751731115000683.
- Carré, B., A. Idi, S. Maisonnier, J.-P. Melcion, F.-X. Oury, J. Gomez, and P. Pluchard. 2002. “Relationships Between Digestibilities Of Food Components And Characteristics Of Wheats (Triticum aestivum) introduced as the only cereal source in a broiler chicken diet.” British Poultry Science 43 (3): 404–415. doi:10.1080/00071660120103684.
- Choct, M., R. Hughes, and M. Bedford. 1999. “Effects of a Xylanase on Individual Bird Variation, Starch Digestion Throughout the Intestine, and Ileal and Caecal Volatile Fatty Acid Production in Chickens Fed Wheat.” British Poultry Science 40 (3): 419–422. doi:10.1080/00071669987548.
- Chotinsky, D., E. Toncheva, and Y. Profirov. 2001. “Development of Disaccharidase Activity in the Small Intestine of Broiler Chickens.” British Poultry Science 42 (3): 389–393. doi:10.1080/00071660120055386.
- Crowe, T. C., S. A. Seligman, and L. Copeland. 2000. “Inhibition of Enzymic Digestion of Amylose by Free Fatty Acids in Vitro Contributes to Resistant Starch Formation.” The Journal of Nutrition 130 (8): 2006–2008. doi:10.1093/jn/130.8.2006.
- Cutlip, S., J. Hott, N. Buchanan, A. Rack, J. Latshaw, and J. Moritz. 2008. “The Effect of Steam-conditioning Practices on Pellet Quality and Growing Broiler Nutritional Value.” Journal of Applied Poultry Research 17 (2): 249–261. doi:10.3382/japr.2007-00081.
- de Nanclares, P., M. M. Trudeau, J. Hansen, L. Mydland, P. Urriola, G. Shurson, C. Åkesson, N. Kjos, M. Arntzen, and M. Øverland. 2017. “High-fiber Rapeseed Co-product Diet for Norwegian Landrace Pigs: Effect on Digestibility.” Livestock Science 203: 1–9. doi:10.1016/j.livsci.2017.06.008.
- Eliasson, A. C., K. Larsson, and Y. Miezis. 1981. “On the Possibility of Modifying the Gelatinization Properties of Starch by Lipid Surface Coating.” Starch‐Stärke 33 (7): 231–235. doi:10.1002/star.19810330704.
- Engberg, R. M., M. S. Hedemann, S. Steenfeldt, and B. B. Jensen. 2004. “Influence of Whole Wheat and Xylanase on Broiler Performance and Microbial Composition and Activity in the Digestive Tract.” Poultry Science 83 (6): 925–938. doi:10.1093/ps/83.6.925.
- Granstad, S., K. Itani, Ø. Øines, B. Svihus, and M. Kaldhusdal. in press. Varying Starch to Fat Ratios in Pelleted Diets: II. Effects on Intestinal Histomorphometry, Clostridium Perfringens and Short-chain Fatty Acids in Eimeria-challenged Broiler Chickens. Submitted to British Poultry Science.
- Hauck, R. 2017. “Interactions between Parasites and the Bacterial Microbiota of Chickens.” Avian Diseases 61 (4): 428–436. doi:10.1637/11675-051917-Review.1.
- Holm, J., I. Björck, and A.-C. Eliasson. 1988. “Effects of Thermal Processing of Wheat on Starch: I. Physico-chemical and Functional Properties.” Journal of Cereal Science 8 (3): 249–260. doi:10.1016/S0733-5210(88)80036-5.
- Holm, J., I. Björck, S. Ostrowska, A. C. Eliasson, N. G. Asp, K. Larsson, and I. Lundquist. 1983. “Digestibility of Amylose‐lipid Complexes In‐vitro and In‐vivo.” Starch‐Stärke 35 (9): 294–297. doi:10.1002/star.19830350902.
- Jørgensen, H., K. Jakobsen, and B. Eggum. 1993. “Determination of Endogenous Fat and Fatty Acids at the Terminal Ileum and on Faeces in Growing Pigs.” Acta Agriculturae Scandinavica Section A-Animal Sciences 43 (2): 101–106. doi:10.1080/09064709309410151.
- Karasov, W. H., and I. D. Hume. 1997. “Vertebrate Gastrointestinal System.” Comprehensive Physiology 1: 409–480. doi:10.1002/cphy.cp130107.
- Kraugerud, O., and B. Svihus. 2011. “Tools to Determine the Degree of Starch Gelatinization in Commercial Extruded Salmon Feeds.” Journal of the World Aquaculture Society 42 (6): 914–920. doi:10.1111/j.1749-7345.2011.00522.x.
- Krogdahl, Å. 1985. “Digestion and Absorption of Lipids in Poultry.” The Journal of Nutrition 115 (5): 675–685. doi:10.1093/jn/115.5.675.
- Krogdahl, Å., and J. L. Sell. 1989. “Influence of Age on Lipase, Amylase, and Protease Activities in Pancreatic Tissue and Intestinal Contents of Young Turkeys.” Poultry Science 68 (11): 1561–1568. doi:10.3382/ps.0681561.
- Larsson, K. 1980. “Inhibition of Starch Gelatinization by Amylose‐lipid Complex Formation. Behinderung Der Stärkeverkleisterung Durch Bildung Eines Amylose‐lipidkomplexes.” Starch‐Stärke 32 (4): 125–126. doi:10.1002/star.19800320407.
- López, C. A., A. H. de Vries, and S. J. Marrink. 2012. “Amylose Folding under the Influence of Lipids.” Carbohydrate Research 364: 1–7. doi:10.1016/j.carres.2012.10.007.
- Lund, D., and K. J. Lorenz. 1984. “Influence of Time, Temperature, Moisture, Ingredients, and Processing Conditions on Starch Gelatinization.” Critical Reviews in Food Science & Nutrition 20 (4): 249–273. doi:10.1080/10408398409527391.
- Malheiros, R., V. Moraes, A. Collin, G. Janssens, E. Decuypere, and J. Buyse. 2004. “Dietary Macronutrients and Performance and Plasma Hormone and Metabolite Levels of Broiler Chickens Fat by Carbohydrate Substitution.” Archiv fur Geflugelkunde 68 (2): 87–93. https://www.european-poultry-science.com/artikel.dll/s-087-093_OTk5OA.PDF?UID=E2F39C5F3B198F8DFAA3A34A9F6E542FBC6EBE642644E9B1.
- McCleary, B., V. Solah, and T. Gibson. 1994. “Quantitative Measurement of Total Starch in Cereal Flours and Products.” Journal of Cereal Science 20 (1): 51–58. doi:10.1006/jcrs.1994.1044.
- Meng, X., B. Slominski, C. Nyachoti, L. Campbell, and W. Guenter. 2005. “Degradation of Cell Wall Polysaccharides by Combinations of Carbohydrase Enzymes and Their Effect on Nutrient Utilization and Broiler Chicken Performance.” Poultry Science 84 (1): 37–47. doi:0.1093/ps/84.1.37.
- Mollah, Y., W. Bryden, I. Wallis, D. Balnave, and E. Annison. 1983. “Studies on Low Metabolisable Energy Wheats for Poultry Using Conventional and Rapid Assay Procedures and the Effects of Processing.” British Poultry Science 24 (1): 81–89. doi:10.1080/00071668308416716.
- Murugesan, G. R., L. F. Romero, and M. E. Persia. 2014. “Effects of Protease, Phytase and a Bacillus Sp. Direct-fed Microbial on Nutrient and Energy Digestibility, Ileal Brush Border Digestive Enzyme Activity and Cecal Short-chain Fatty Acid Concentration in Broiler Chickens.” PloS One 9 (7): e101888. doi:10.1371/journal.pone.0101888.
- Nitsan, Z., A. Dvorin, Z. Zoref, and S. Mokady. 1997. “Effect of Added Soyabean Oil and Dietary Energy on Metabolisable and Net Energy of Broiler Diets.” British Poultry Science 38 (1): 101–106. doi:10.1080/00071669708417948.
- Noy, Y., and D. Sklan. 1995. “Digestion and Absorption in the Young Chick.” Poultry Science 74 (2): 366–373. doi:10.3382/ps.0740366.
- Putseys, J., L. Lamberts, and J. Delcour. 2010. “Amylose-inclusion Complexes: Formation, Identity and Physico-chemical Properties.” Journal of Cereal Science 51 (3): 238–247. doi:10.1016/j.jcs.2010.01.011.
- Rogel, A., E. Annison, W. Bryden, and D. Balnave. 1987. “The Digestion of Wheat Starch in Broiler Chickens.” Australian Journal of Agricultural Research 38 (3): 639–649. doi:10.1071/AR9870639.
- Sauvant, D., J. M. Perez, and G. Tran. 2004. Tables of Composition and Nutritional Value of Feed Materials: Pigs, Poultry, Cattle, Sheep, Goats, Rabbits, Horses and Fish. The Netherlands, Wageningen: Wageningen Academic Publishers.
- Scheele, C., C. Kwakernaak, J. Van Der Klis, and G. Bakker. 1997. “Effects of Different Factors Including Enzymes on the Nutritional Value of Fats for Poultry.” In Recent Advances in Animal Nutrition, 59–75.
- Sell, J. L. 1996. “Physiological Limitations and Potential for Improvement in Gastrointestinal Tract Function of Poultry.” Journal of Applied Poultry Research 5 (1): 96–101. doi:10.1093/japr/5.1.96.
- Short, F., P. Gorton, J. Wiseman, and K. Boorman. 1996. “Determination of Titanium Dioxide Added as an Inert Marker in Chicken Digestibility Studies.” Animal Feed Science and Technology 59 (4): 215–221. doi:10.1016/0377-8401(95)00916-7.
- Suvarna, S., V. Christensen, D. Ort, and W. Croom. 2005. “High Levels of Dietary Carbohydrate Increase Glucose Transport in Poult Intestine.” Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 141 (3): 257–263. doi:10.1016/j.cbpb.2005.03.008.
- Svihus, B. 2001. “Research Note: A Consistent Low Starch Digestibility Observed in Pelleted Broiler Chicken Diets Containing High Levels of Different Wheat Varieties.” Animal Feed Science and Technology 92 (1): 45–49. doi:10.1016/S0377-8401(01)00251-6.
- Svihus, B. 2014. “Starch Digestion Capacity of Poultry.” Poultry Science 93 (9): 2394–2399. doi:10.3382/ps.2014-03905.
- Svihus, B., and H. Hetland. 2001. “Ileal Starch Digestibility in Growing Broiler Chickens Fed on a Wheat-based Diet Is Improved by Mash Feeding, Dilution with Cellulose or Whole Wheat Inclusion.” British Poultry Science 42 (5): 633–637. doi:10.1080/00071660120088461.
- Svihus, B., A. Sacranie, V. Denstadli, and M. Choct. 2010. “Nutrient Utilization and Functionality of the Anterior Digestive Tract Caused by Intermittent Feeding and Inclusion of Whole Wheat in Diets for Broiler Chickens.” Poultry Science 89 (12): 2617–2625. doi:10.3382/ps.2010-00743.
- Svihus, B., A. K. Uhlen, and O. M. Harstad. 2005. “Effect of Starch Granule Structure, Associated Components and Processing on Nutritive Value of Cereal Starch: A Review.” Animal Feed Science and Technology 122 (3): 303–320. doi:10.1016/j.anifeedsci.2005.02.025.
- Tancharoenrat, P., V. Ravindran, F. Zaefarian, and G. Ravindran. 2013. “Influence of Age on the Apparent Metabolisable Energy and Total Tract Apparent Fat Digestibility of Different Fat Sources for Broiler Chickens.” Animal Feed Science and Technology 186 (3): 186–192. doi:10.1016/j.anifeedsci.2013.10.013.
- Thomas, D., V. Ravindran, and G. Ravindran. 2008. “Nutrient Digestibility and Energy Utilisation of Diets Based on Wheat, Sorghum or Maize by the Newly Hatched Broiler Chick.” British Poultry Science 49 (4): 429–435. doi:10.1080/00071660802213467.
- Thomas, M., T. Van Vliet, and A. Van Der Poel. 1998. “Physical Quality of Pelleted Animal Feed 3. Contribution of Feedstuff Components.” Animal Feed Science and Technology 70 (1–2): 59–78. doi:10.1016/S0377-8401(97)00072-2.
- Tufvesson, F., V. Skrabanja, I. Björck, H. L. Elmståhl, and A.-C. Eliasson. 2001. “Digestibility of Starch Systems Containing Amylose–glycerol Monopalmitin Complexes.” LWT-Food Science and Technology 34 (3): 131–139. doi:10.1006/fstl.2000.0727.
- Veldkamp, T., R. Dekker, A. Smit-Heinsbroek, A. Van Der Lee, and A. Jansman. 2017a. Effect of Iso-energetic Exchange of Dietary Fat and Starch on Growth Performance and Body Composition of Broilers; Experiment 2. Wageningen UR Livestock Research.
- Veldkamp, T., T. Schamp, J. Van Harn, R. Dekker, M. Sosef, and A. Jansman. 2017b. Effect of Iso-energetic Exchange of Dietary Fat and Starch on Growth Performance and Body Composition of Broilers; Experiment 1. Wageningen UR Livestock Research.
- Wiseman, J., N. Nicol, and G. Norton. 2000. “Relationship between Apparent Metabolisable (Ame) Values and in Vivo/in Vitro Starch Digestibility of Wheat for Broilers.” World’s Poultry Science Journal 56 (4): 305–318. doi:10.1079/WPS20000022.
- Yun, C., H. Lillehoj, and E. Lillehoj. 2000. “Intestinal Immune Responses to Coccidiosis.” Developmental & Comparative Immunology 24 (2–3): 303–324. doi:10.1016/S0145-305X(99)00080-4.
- Zimonja, O., A. Stevnebø, and B. Svihus. 2007. “Nutritional Value of Diets for Broiler Chickens as Affected by Fat Source, Amylose Level and Diet Processing.” Canadian Journal of Animal Science 87 (4): 553–562. doi:10.4141/CJAS07044.
- Zimonja, O., and B. Svihus. 2009. “Effects of Processing of Wheat or Oats Starch on Physical Pellet Quality and Nutritional Value for Broilers.” Animal Feed Science and Technology 149 (3): 287–297. doi:10.1016/j.anifeedsci.2008.06.010.
