ABSTRACT
1. This review describes different classes of antimicrobial peptides (AMP) found in the gastrointestinal (GI) tract of avian species, and their antimicrobial and immunomodulatory activities. The potential benefits of synthetic AMP in poultry production are examined, in the context of the use of AMP as alternatives to antimicrobial growth promoters (AGP).
2. Since the mid-1950s, antibiotic growth promoters (AGP) have been used in feed at low prophylactic doses to modulate the homoeostasis of intestinal microbiota, decreasing the risk of intestinal dysbacteriosis and the growth of pathogens within the avian gut. Over the last three decades, AGP have faced major regulatory restrictions due to concerns of generating antimicrobial resistance (AMR). It is now well documented that the rate of infectious disease outbreaks is higher in flocks that are not fed prophylactic antibiotics, resulting in a compensatory increase in antimicrobial use for therapeutic purposes.
3. Endogenous natural AMP production is associated with the presence of microbiota and their interaction with the intestinal epithelial and lamina propria lymphoid cells. Their antimicrobial activity shapes the beneficial microbiota population and controls intestinal pathogens such Clostridium and Salmonella spp., and stimulates the development and maturation of the local immune system.
4. Similar to AGP, AMP can establish a well-balanced gut beneficial microbiota for adequate immune-competence, animal health and high growth performance parameters such as feed intake, daily weight, feed conversion and accumulated mortality.
5. Antimicrobial proteins and peptides constitute an essential part of the innate immune system of all organisms and protect the host from invading pathogenic bacteria, viruses, fungi, and parasites by interacting with the negatively charged pathogen membranes.
Introduction
Poultry producers attempt to control infectious diseases through a variety of means, including appropriate farm management practices and breeding genetically resistant lines (Sartika et al. Citation2011; Scott et al. Citation2018). Unfortunately, existing farming conditions favour increased chicken densities in poultry houses, increasing the risk of spreading entero-pathogens such as Salmonella and Clostridium spp. (FAO Citation2013). For over half a century, veterinarians and poultry nutritionists have been using low dose levels of antimicrobials in the feed to control potential enterobacterial outbreaks. Antimicrobial growth promoters (AGP) are used in subtherapeutic doses to selectively kill intestinal bacteria and modifying the intestinal microbiota of livestock. AGPs reduce competition for nutrients between bacteria and the host and, as a result, improve animal performance. In 1951, the use of AGP as poultry feed additives without veterinary prescription was authorised by the United States Food and Drug Administration (Jones and Ricke Citation2003). In 2015, van Boeckel et al. estimated that the global average consumption of antimicrobials in chickens was 148 mg/kg of meat produced. According to the Food and Agriculture Organization of the United Nations (FAO), there is an ever-increasing annual demand for poultry meat (FAO Citation2013, Citation2019). There has been a global shift towards intensive farming practices, where infections can easily spread and can significantly affect the health and productivity of a large number of animals (Graham et al. Citation2009; Van Boeckel et al. Citation2015). Addition of AGP to animal diets has been shown to control unwanted intestinal microbiota and has increased the growth rate and performance of young animals (Miles et al. Citation2006). Moreover, the composition of intestinal microbiota under intensive poultry husbandry conditions can be significantly altered by environmental factors, such as heat stress. Under such conditions, the regulation of intestinal microbiota is absolutely necessary (Hu et al. Citation2017; Shi et al. Citation2019; Zhu et al. Citation2019).
Although AGP have made a significant economic contribution to the poultry industry, there are concerns that their widespread use has resulted in the evolution and spread of antimicrobial resistant (AMR) gut pathogens (Graham et al. Citation2009; Le Devendec et al. Citation2016). Feeding AGP to poultry pathogens at subtherapeutic levels for growth promotion is a major evolutionary factor contributing to AMR in these microorganisms (Luiken et al. Citation2019). Starr and Reynolds (Citation1951) reported one of the earliest instances of AMR in poultry pathogens in 1951, when they discovered the emergence of streptomycin-resistant coliform bacteria in turkeys within three days of being fed streptomycin. Incidences of AMR have since reached alarming levels and are considered as a significant emerging threat to global public health and food security (FAO Citation2016). The earliest ban on the use of AGP in animal feeds was instituted by the government of Sweden in 1986 (Wierup Citation2001). As a consequence, several countries have restricted the use of antimicrobials in livestock feed in order to avoid any potentially harmful impacts on public health (Wegener et al. Citation1999; Kang et al. Citation2013). In January 2006, the European Union passed regulation no. 1831/2003, of which section 25 banned the use of AGP in livestock feed, except for coccidiostats and histomonostats. Several countries belonging to the Organization for Economic Co-operation and Development have carried out similar initiatives that aimed to reduce the use of antimicrobials in livestock production (European Food Safety Authority and European Centre for Disease Prevention and Control Citation2020). Such regulations have challenged researchers within the industry to find natural alternatives to replace AGP in animal feed (Van Boeckel et al. Citation2015). Although the main purpose of banning the use of antimicrobials in food-producing animals was to reduce the development of AMR in zoonotic bacteria and their potential to infect humans (More Citation2020), it is well known that the number of infectious diseases in animals without AGP has increased greatly. This has resulted in a compensatory increase in veterinary-prescribed antimicrobials use as prophylactic measures and for therapeutic purposes (Smith Citation2011; Gaucher et al. Citation2015). Therefore, there is a growing need to develop industry-friendly supplements that can be added to the feed in place of antimicrobials to reduce infections.
Antimicrobials ban impact on poultry industry
The use of antimicrobials as subtherapeutic feed additives has had significant prophylactic activity, and their withdrawal has been associated with the deterioration of flock health, in terms of increased diarrhoea, weight loss, and mortality (Casewell Citation2003). The increasing incidence of disease in animals has diverted the industry’s attention to immunological methods of disease protection. Innate mucosal immunity, as the first natural component of the immune system, can detect and respond to the conserved molecular patterns of non-infectious and infectious organisms by releasing various proinflammatory and antimicrobial agents (Peralta et al. Citation2017). Therefore, finding alternatives derived from the natural chemical secretions of host cells, such as antimicrobial peptides (AMP), may provide prophylactic control and aid in the clearing of bacterial infections. Synthetic and recombinant AMP, as feed additives, have the potential to provide several significant benefits to poultry production systems such as immunomodulation and control of potential intestinal pathogen outbreaks (Ramasamy et al. Citation2012; Wang et al. Citation2020). They have the potential to reduce incidences of AMR, minimising carcase contamination by drug-resistant pathogens and decreasing the risk of antibiotic-resistant foodborne pathogen consumption by humans.
Gut microbiota plays a crucial role in the development of the mucosal immune system and growth performance in food animals, especially in poultry, where lifespan is short. The healthy functioning of gastrointestinal (GI) microbiota in avian species is the product of homoeostasis between various chemical, physical, immunological and microbiological components (Yegani and Korver Citation2008). Under intestinal homoeostatic conditions, there is a competitive relationship between diverse bacterial communities such as Bacteroidetes, Firmicutes, Actinobacteria, and Proteobacteria spp. within the intestinal ecosystem (Mancabelli et al. Citation2016; Ferrario et al. Citation2017). These bacterial communities modify the GI micro-environment by producing different metabolites and other molecules, such as short-chain fatty acids (propionic and butyric acid), organic acids (acetic acid), vitamin K and folic acid (Shi et al. Citation2019). These metabolites can affect the whole intestinal ecology by introducing important changes in gut epithelial cell integrity, innate immune system, nutrient availability and, hence, animal performance. The AGP have the effect of reducing microbial-induced growth depression in animals, either directly or indirectly, and reduce bacterial competition for nutrients in the small intestine, decrease the production of growth-depressing metabolites like aromatic phenols, ammonia and bile degradation products. They can reduce intestinal pro-inflammatory stages, enhancing poultry production parameters and feed efficiency (Engberg et al. Citation2000; Gaskins et al. Citation2002; Apajalahti and Vienola Citation2016).
The gastrointestinal immune system and AMP
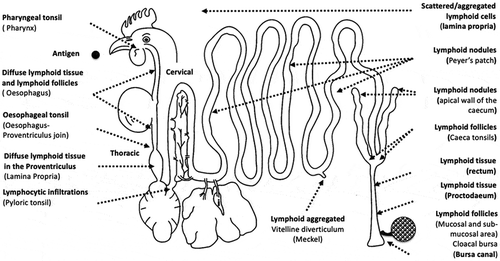
The healthy functioning of the GI tract in avian species depends on the homoeostasis between various chemical, physical, microbiological and immunological components (Yegani and Korver Citation2008). The gut associated lymphoid tissue (GALT) is composed of a diverse set of cells, including macrophages, heterophils, dendritic cells, B and T lymphocytes and specialised lymphoid tissues, such as the bursa of Fabricius, Meckel’s diverticulum, Peyer’s patches and the caecal tonsils (Peralta et al. Citation2017), as shown in . The GI tract relies on GALT to recognise antigens, such as microorganisms or metabolites, and to interact and differentiate between them to produce an immune response (Daneshmand et al. Citation2019). Furthermore, interactions between mucosal and glycocalyx components (such as AMP) result in the development of gut functional maturity, which is associated with the overall maturation of immune system. These steps generally represent the healthy development and maturation of GALT in chickens.
Figure 1. Gut associated lymphoid tissue (GALT): structures and distribution of the primary (Bursa of Fabricius), and secondary lymphoid tissue throughout the gastrointestinal tract
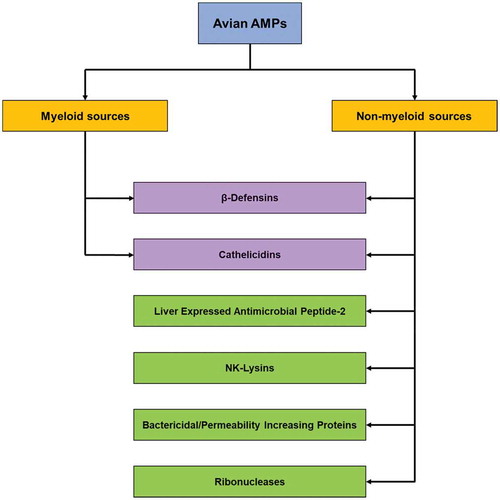
The AMP are present in chickens and other birds from embryonic stages and are continually developed in the GI tract during the bird’s lifetime. Avian AMP in the GI tract are obtained from two main sources: (a) myeloid sources and (b) non-myeloid sources. β-defensins are the first avian myeloid AMP that were isolated from turkey and chicken heterophils by Evans et al. (Citation1994), Evans et al. (Citation1995)). These have antibacterial activity against a variety of human and avian pathogens, including Staphylococcus aureus, Salmonella spp. and Escherichia coli. Heterophils are polymorphonuclear leukocytes, which constantly produce AMP, such as β-defensins, cathelicidins, lysozymes, cationic proteins and polypeptides, within the lymphoid tissues distributed throughout the GI tract (). Avian heterophils are the main source of myeloid AMP (Van Dijk et al. Citation2007; Ma et al. Citation2009; Cuperus et al. Citation2016b). Examples of non-myeloid AMP include gallinacin-3 (AvBD3), which is expressed widely in the epithelial cells of the tongue, bursa of Fabricius, trachea, skin, oesophagus, air sacs, large intestine, and kidney of chickens and turkeys (Zhao et al. Citation2001), and liver-derived antimicrobial peptide-2 (LEAP-2), which is expressed in chicken epithelial tissues, such as small intestine, liver, lung and kidney (Townes et al. Citation2004).
The AMP have been demonstrated to improve growth performance, activate immune cells, improve intestinal morphology and increase the population of commensal bacteria, such as Lactobacillus spp. (Hu et al. Citation2017; Daneshmand et al. Citation2019). Due to their antimicrobial properties, AMP can serve as natural alternatives as additive in animal feed to replace therapeutic antibiotics and AGP in poultry production, with the potential to control of AMR strains within flocks. Since most AMP target the cell membranes of pathogens, it is more difficult for pathogens to develop resistance, as evolutionary changes in the cell membranes occur very slowly (Lee et al. Citation2016). Therefore, understanding the role of naturally occurring avian AMP in innate gut mucosal immunity is essential to develop synthetic and recombinant AMP as feed supplements to control enteric pathogen populations in livestock.
Avian antimicrobial peptides, polypeptides and proteins
β-defensins
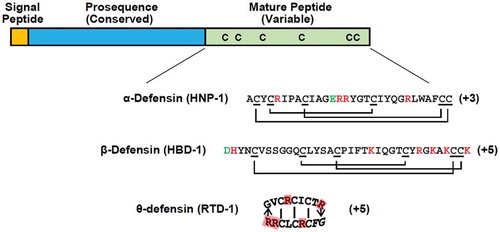
Defensins are a large family of cysteine-rich, cationic AMP that are found in vertebrates. Defensins consist of a β-sheet core that is stabilised by three disulphide bonds formed from six conserved cysteine residues within the peptide structure, as shown in . Based on the differences in the spacing between the residues of AMP, defensins have been further classified into three subfamilies, namely α-, β-, and θ-defensins. α-defensins are found in mammals, and form disulphide bridges between Cys1-Cys6, Cys2-Cys4 and Cys3-Cys5. The θ-defensins have been isolated from the leukocytes of rhesus monkeys and baboons, and have a distinctive cyclic structure, with disulphide bonds between Cys1-Cys6, Cys2-Cys5 and Cys3-Cys4. The β-defensins are present in all vertebrates and form disulphide bridges between Cys1-Cys5, Cys2-Cys4 and Cys3-Cys6 (Zhao et al. Citation2001; Liu et al. Citation2018a).
Figure 3. General structure of defensins consists of the signal peptide (yellow), the conserved prosequence (blue), and the cysteine-rich mature peptide (green)
Among avian species, more than 40 different avian β-defensins (AvBDs) have been discovered so far. Among these, 14 AvBDs have been discovered in chickens (AvBD1-14) and are collectively known as gallinacins. Among these, AvBD8, 10, 11 and 13 are present specifically in the intestinal mucosa, while AvBD1, 2, 4 and 6 are found in the caecal tonsils (Akbari et al. Citation2008), and AvBD9 is highly expressed in the bursa (Van Dijk et al. Citation2007). The expression of AvBD genes varies among different commercial broiler lines. For example, Ross-line chickens express higher levels of AvBD1, 6, 8 and 10 than Cobb-line chickens, which may explain the differences in disease resistance among different commercial breeds (Hong et al. Citation2012).
In most AMP, membrane disruption is the major mechanism of microbicidal activity in AvBDs. Therefore, the net cationic charge of these peptides is directly related to their potency against microbes (Hu et al. Citation2017). In chickens, the net charge of AvBDs ranges from +0.37 (AvBD8) to +8.0 (AvBD1),; however,the average net charge of all chicken defensins is +4.69 (Cheng et al. Citation2015). The net charges of all 14 chicken AvBDs are listed in . Net cationic charge is shown to have a significant effect on the activity of AvBDs against Gram-negative, but not Gram-positive, bacteria. For example, AvBD2 (net charge of +4.18) has a lower minimum inhibitory concentration (MIC) against Gram-negative bacteria than AvBD1 and AvBD7 (net charges of +8.0 and +6.0, respectively). However, the MIC of AvBD2 in Gram-positive bacteria is virtually identical to that of AvBD1 and 7 (Derache et al. Citation2009). It has been shown that synthetic AvBD10 (net charges of +2.09) has a comparable antimicrobial effect to AvBD4 (net charge of +6.08) on various strains of pathogenic bacteria and fungi, despite having a much lower cationic charge (Yacoub et al. Citation2015). This suggested that the antibacterial activities of AvBDs dependon other factors, such as optimal ratios of hydrophobic to cationic residues and the presence and location of disulphide bridges within AMP sequences.
Table 1. Net charges of chicken AvBDs at pH = 7 (Cheng et al. Citation2015)
The presence of conserved disulphide bridges within the AMP sequence plays an important role in the mechanism of action of AvBDs; however, antimicrobial activity is dependent on the types of AvBDs. For example, reduction of AvBD2 by S-alkylation of thiol groups of cysteine has been shown to reduce antibacterial activity of peptide towards both Gram-positive and Gram-negative bacteria (Derache et al. Citation2009). However, the reduction of disulphide bridges by thioredoxin reductase in AvBD6 and 12 only altered the mechanism of action of peptides; hence, their magnitude of antibacterial activities was not significantly reduced. The reduction of disulphide bridges in AvBDs has been shown to reduce their capacity to neutralise lipopolysaccharides (LPS), which maintains the structural integrity of cell membranes in Gram-negative bacteria and is responsible for the inflammatory responses in the host cells (Yang et al. Citation2010).
The AvBDs display chemotactic effects on multiple avian leukocytes and have been shown to modulate the activity of monocytes in many non-avian species. Yang et al. (Citation2010) demonstrated that AvBD13 expressed in chicken intestinal epithelial cells could bind with toll-like receptor (TLR) 4 in the peripheral blood mononuclear cells of mice. This binding upregulated the expression of costimulatory molecules and promoted maturation and proliferation of immune cells in mammals and birds. The TLR4 plays an important role in pathogen recognition, as it binds with pathogen-associated molecular patterns (PAMP) and produces intracellular signals for cytokine production in leukocytes. The AvBDs play a positive role in anti-inflammatory processes, such as blocking LPS-induced inflammation in the avian GI tract (Rengaraj et al. Citation2018). Data has revealed that injection of LPS results in relatively high expression of AvBD9, and AvBD4 in the intestines of chicken (Lu et al. Citation2014).
Cathelicidins
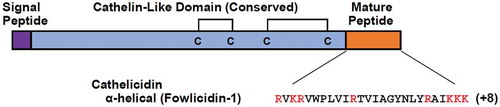
Cathelicidins are a family of cationic AMP that are characterised by the presence of an overall α-helical structure consisting of a highly conserved signal peptide, a cysteine-rich cathelin-like domain at the N-terminus and a highly diverse mature sequence at the C-terminus of the peptide, as shown in . Upon activation, cathelicidin precursors are proteolytically cleaved to remove the cathelin domain from the mature peptide, releasing the C-terminal domain, leading to antimicrobial activity. However, cathelin domain of peptide is thought to play a role in immunomodulation (Xiao et al. Citation2006a, Citation2006b; Cheng et al. Citation2015).
Figure 4. General structure of avian cathelicidins with the signal peptide (purple), the cysteine-rich cathelin-like domain (blue), and the mature peptide sequence (orange) (Zhang and Sunkara Citation2014)
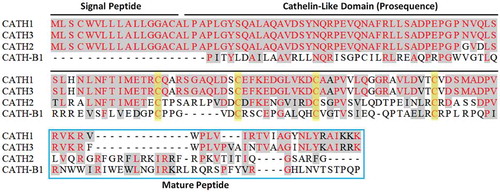
Four different cathelicidins have been isolated from chickens, namely, CATH1, CATH2, CATH3, and CATH-B1, and CATH1, −2 and −3 are collectively known as fowlicidins and are highly expressed in the lungs and in bone marrow heterophils (Cheng et al. Citation2015; Cuperus et al. Citation2016b). The expression of CATH-B1 was discovered in the epithelium of bursa of Fabricius. CATH-1 and 3 are the most closely related AMP among chicken cathelicidins and share 98% structural homology. CATH-B1 is a distant member of the cathelicidin family, as it shares only ~40% homology with other cathelicidins and contains an uncharacteristic cathelin domain (Goitsuka et al. Citation2007), which is not present in other cathelicidin members. shows the amino acid sequences of the four chicken cathelicidins, with conserved sequences highlighted in red and cysteine residues highlighted in yellow.
Figure 5. Amino acid sequences of chicken cathelicidins, with conserved sequences highlighted in red and cysteine residues highlighted in yellow (Zhang and Sunkara Citation2014)
All mature cathelicidins are highly cationic, with CATH-2 possessing the highest net charge of +10, which implies that membrane disruption is the major mechanism of their antimicrobial activity. Although the mature forms of all cathelicidins are cationic, the cathelin-like propieces (pieces of the peptide precursor) are anionic in nature and play a crucial role in preventing auto-cytotoxicity by neutralising the cationic character of the mature peptides. For example, the inactive form of CATH-2 has the lowest net charge among avian cathelicidins; however, the mature form of CATH-2 exhibits the highest net positive charge among all isoforms (Cheng et al. Citation2015). Similarly, mature forms of CATH-1 and −3 have been shown to be cytotoxic towards Madin-Darby canine kidney (MDCK) epithelial cells and display haemolytic activity against human erythrocytes in serum media. This could potentially limit the application of cathelicidins as feed antimicrobials. However, the addition of 10% foetal bovine serum (FBS) was found to reduce cytotoxicity of both CATH-1 and CATH-3 towards MDCK and human erythrocytes, while still retaining >80% activity against Gram-negative E. coli and Gram-positive S. aureus (Bommineni et al. Citation2007).
Avian cathelicidins, similar to human cathelicidin LL-37, play a variety of roles in immunomodulation and are known to be involved in chemotaxis, differentiation of leukocytes, and modulation of cytokine responses (Bommineni et al. Citation2007). For example, CATH-2 has been shown to inhibit LPS-induced interleukin-1β (IL-1β) and nitric oxide production in chicken macrophages. Avian CATH-3 displays dose-dependent inhibition of IL-1β, CC chemokine ligand 2 (CCL2)/monocyte chemotactic protein-1 (MCP-1), and CCL3/monocyte inflammatory protein-1α (MIP-1α) in mouse macrophages (Kraaij et al. Citation2017). In ovo administration of a D-analogue of CATH-2 (D-CATH-2) prevented E. coli induced lymphopenia by increasing the total number of leukocytes and heterophils during pathogenic E. coli infection in chicken embryos (Cuperus et al. Citation2016a). Moreover, L-CATH-2 and D-CATH-2 increase the number of mononuclear phagocytes, expression of mannose receptor (MRC1) and antigen presentation markers (MHCII, CD40 and CD86) on mononuclear phagocytes, increasing their antigen presentation capacity (Kraaij et al. Citation2017).
Liver expressed antimicrobial peptide-2
Liver-expressed antimicrobial peptide-2 (LEAP-2) was first isolated from human blood ultrafiltrate and its orthologues were subsequently found in other mammals, including primates, mice, cattle, swine and in avian species, such as chickens. The chicken LEAP-2 (cLEAP-2) gene is located on chromosome 13 of the chicken genome and encodes a peptide precursor of 76 residues, in which residues 37–76 are enzymatically cleaved to give a mature LEAP-2 peptide of 40 residues. Mature cLEAP-2 contains two disulphide bonds formed by four cysteine residues, where the position of cysteine is conserved in LEAP-2 homologues (Townes et al. Citation2004). shows the amino acid sequences of LEAP-2 found in humans, mice, and chickens, with the mature peptides highlighted in red.
Figure 6. Amino acid sequences of LEAP-2 in humans, mice, and chickens; with the mature peptide sequences highlighted in red (Townes et al. Citation2004)

The cLEAP-2 is expressed in a number of chicken epithelial tissues, such as in small intestine, liver, lung and kidney. At physiological pH, mature cLEAP-2 is cationic with a net charge of +3.8. The peptide functions to prevent interactions with and invasion of potentially pathogenic microbes in epithelial surfaces by inducing membrane permeabilisation (Townes et al. Citation2009; Michailidis Citation2010). Expression of cLEAP-2 gene is pathogen dependent, and infection of Goldline chickens with S. enterica resulted in upregulation (Townes et al. Citation2004). In contrast, infection with intestinal protozoan Eimeria maxima resulted in downregulation of cLEAP-2 in the intestinal epithelia. Eimeria spp. is responsible for coccidiosis in poultry, and downregulation of cLEAP-2 in intestinal epithelia is responsible for the severity of this infection (Casterlow et al. Citation2011); hence, prophylactic administration of cLEAP-2 through feed may help in controlling this disease.
NK-lysins
The NK-lysins are cationic AMP that were first isolated from porcine intestinal tissue and are found in the granules of T-lymphocytes and natural killer (NK) cells (Lee et al. Citation2016). They are a member of the saposin-like protein fly with α-helical structures, which possess microbicidal tumour cytolytic and immunomodulatory activities in vitro (Lee et al. Citation2014). The amino acid sequence of chicken NK-lysin (cNKL) is shown in , with the mature peptide highlighted in red.
Figure 7. Amino acid sequence of cNKL, with the mature peptide highlighted in red and cysteine residues highlighted in yellow (Lee et al. Citation2014)

The cNKL and its synthetic derivatives, cNK-2 and cNK-3, show considerable bactericidal and parasiticidal activities against E. coli and against various species of sporozoites (Lee et al. Citation2012, Citation2014). In comparison with cNKL, cNK-2 displays higher parasiticidal activity and immunomodulatory functions in chicken cells (Hong et al. Citation2008; Lee et al. Citation2013). The cNK-2 significantly upregulates the expression of cytokines, chemokines (such as CCL4, CCL5) and specifically upregulates the expression of IL-1β in HD-11 chicken cells and CCL4 and CCL5 in primary monocytes. However, upregulation of pro-inflammatory cytokines has been seen to be suppressed in the presence of LPS, indicating that synthesis of cNKL analogues of optimised amino acid sequences may improve immunomodulatory profiles of AMP in infected tissues (Kim et al. Citation2017; Kim and Lillehoj Citation2019). shows the amino acid sequences of mature cNKL and some of its synthetic analogues.
Table 2. Amino acid sequences and properties of cNKL and its synthetic analogues
Bactericidal/permeability-increasing proteins
Bactericidal/permeability-increasing protein (BPI) is an antimicrobial and anti-inflammatory protein with high LPS binding and neutralisation efficacies, which actively inhibits the invasion of Gram-negative bacteria in host tissues. Chicken BPI (cBPI) is widely expressed within the GI tract and the salivary glands (Chiang et al. Citation2011) and exhibits extremely complex regulation of immune response within the intestinal epithelium. This is because of the continuous exposure of intestinal epithelial cells to various gut microbiota and pathogenic bacteria derived PAMPs, which makes it difficult for the epithelial cells to recognise pathogenic bacteria versus commensal microbiota (Balakrishnan and Chakravortty Citation2017). Introducing synthetic analogues of cBPI into the GI tract of poultry may help to improve the immunomodulatory activities of intestinal epithelial cells and may improve overall intestinal health.
Ribonucleases
The RNase A ribonucleases have been implicated in a wide variety of physiological functions, including angiogenesis, cellular apoptosis and as anti-tumour and anti-pathogen host defense agents. However, all members of the RNase A ribonuclease gene family maintain invariant disulphide bonds and catalytic components that are necessary for RNA degradation (Nitto et al. Citation2006). Angiogenin is a member of the RNase A superfamily which has an endothelial-binding motif which, when combined with its endonuclease enzyme activity, produces a potent stimulus for blood vessel formation. Physiologically, angiogenin is induced during inflammation and exhibits wound healing properties, microbicidal activity and confers host immunity (Tello-Montoliu et al. Citation2006). The expression of angiogenin 4 (Ang4) in a non-mammalian species was first discovered in intestinal epithelial and lymphoid cells of chickens. The chicken Ang4 (chAng4) is a new class of microbicidal protein that is involved in innate immunity and regulation of gut microflora, and possess 97–99% homology with RNase A genes present in humans and rodents (Rodriguez-Lecompte et al. Citation2008; Losada-Medina et al. Citation2020). Birds fed probiotics and organic acids exhibited strong expression of Ang4 across the entire GI tract and showed significantly higher expression in the bursa of Fabricius (Rodríguez-Lecompte et al. Citation2012). In recent studies, Ang4-derived AMP have shown in vitro antimicrobial activity against E. coli and S. enterica (Nazeer CitationForthcoming). Therefore, detailed characterisation of chAng4 in the GI tract may help in the development of bactericidal and immunomodulatory peptides against intestinal pathogenic microbes.
Gut pathogens
The GI tract health is defined as the ability of the gut to normally perform physiological functions which assist in enduring infectious and non-infectious stressors (Kogut et al. Citation2017). Gut health has been recognised as a major factor influencing the growth, well-being and other productive parameters in poultry flocks. The optimum intestinal microbiota population and ecosystem balance supports gut health and integrity. Nevertheless, as mentioned before, current poultry production conditions have led to a constant exposure of GI tract and to stressors and entero-pathogens that can trigger dysbiosis, and negatively affect gastrointestinal integrity and health. Many pathogens have been recognised as key stressors in the development of systemic diseases in poultry GI tract, by increasing feed conversion ratio and extending the period to slaughter. Some of these key pathogens include enteric viruses such as avian adenovirus, infectious bronchitis virus, reovirus, and rotavirus (Mettifogo et al. Citation2014), bacteria such avian pathogenic E. coli, S. enterica Gallinarum and Pullorum, Clostridium perfringens and parasites such as Eimeria. Under such conditions, the regulation of intestinal microbiota is necessary to control the spread of pathogens within the barns (Hu et al. Citation2017; Shi et al. Citation2019; Zhu et al. Citation2019). However, due to the increasing incidences of AMR in poultry entero-pathogens (Nhung et al. Citation2017), natural, synthetic and recombinant AMP have been studied in recent years as an alternative to relieve AGP use (Rodríguez-Lecompte et al. Citation2012; Yitbarek et al. Citation2015). The potential effect of AMP in livestock to combat avian pathogenic E. coli, Salmonella, Clostridium and Eimeria spp. is discussed below.
Escherichia coli
E. coli is a ubiquitous commensal bacterium in humans and animals; however, the avian pathogenic species (APEC) is commonly seen in intestinal, respiratory and systemic infections in poultry (Dominick and Jensen Citation1984; Antão et al. Citation2008; Alber et al. Citation2020). Respiratory viruses, such as infectious bronchitis and immune suppressive viruses, such as infectious bursal disease, predispose birds to APEC infection (Antão et al. Citation2008). However, this manuscript does not aim to review intestinal viruses and their effect on poultry. Defining an APEC pathotype has been challenging due to the different E. coli serotypes that cause disease (Dziva et al. Citation2013; Kemmett et al. Citation2013). Enteric disease resulting from APEC colonisation triggers gut inflammation, gut integrity loss, bacterial translocation into the circulation and, further, perihepatitis, pericarditis and airsaculitis (Kemmett et al. Citation2013). At the slaughterhouse, APEC has increased in the past decades causing cellulitis and septicaemic lesions that affect carcase quality (Coura et al. Citation2017; Oliveira et al. Citation2020). Although the poultry industry has attempted to control APEC strains spread by feeding AGP, the current limitations have resulted in increases of colibacillosis outbreaks worldwide. Feed supplementation with novel alternatives, such as AMP, has shown promising results. Antimicrobial peptides, polypeptides and protein supplementation has been shown to significantly reduce pathogenic bacteria loads within the avian gut, while increasing the population of beneficial bacteria (Daneshmand et al. Citation2019); The antimicrobial effect of AMP is accomplished through their cationic or amphipathic charge, which allows interaction with the negatively charged APEC membrane (Wimley Citation2010). The production of a recombinant peptide derived from camel milk has shown a strong antimicrobial effect against two different E. coli strains in birds (Tanhaiean et al. Citation2018), and in pigs by reducing intestinal colonisation (Tang et al. Citation2008). Others have reported a similar effect of AMP derived from different sources on reducing bacterial infections in chicken flocks (Sochacki et al. Citation2011; Choi et al. Citation2013b)
Moreover, AMP can modulate the intestinal expression of proinflammatory cytokines such as IL-2 and IL-6 and anti-proinflammatory molecules such as IL-10, as demonstrated in cell culture models of bacterial toxins challenged macrophages treated with CATH-B1 (Daneshmand et al. Citation2019; Peng et al. Citation2020). Besides, as a result of AMP supplementation during in vivo experiments, the intestinal morphometric parameters such as villus height and villus surface area of chickens were significantly greater when compared to the controls (Choi et al. Citation2013b; Daneshmand et al. Citation2019), and productive parameters, such as feed conversion ratio, were comparable to the AGP supplemented group (Wang et al. Citation2009; Daneshmand et al. Citation2019). Notably, supplementing exogenous AMP mimics the physiological release of endogenous AMP, such as cathelicidin-B1, after immune cells respond to APEC presence (Peng et al. Citation2020). Therefore, AMP has become a major alternative to relieve the use of AGP to maintain the intestinal eubiosis in avian species.
Salmonella enterica serovars Gallinarum and Pullorum
The host specialist serovars of S. enterica Gallinarum and Pullorum, are non-mobile avian pathogens (Kwon et al. Citation2000) that cause fowl typhoid and pullorum disease, respectively (Shivaprasad Citation2000). Both serovars can be horizontally and vertically transmitted (Berchieri et al. Citation2001). Chickens under three weeks post-hatch are more often infected by the Pullorum, developing a systemic disease, accompanied with white diarrhoea and high mortality rates (Tessari et al. Citation2012). Moreover, Gallinarum causes a systemic septicaemic disease, leading in liver and spleen gross lesions. Salmonella spp. are known to translocate from the intestinal and reproductive tract into intestinal tissue and, through systemic circulation, affect different organs, thus, provoking clinical lesions that vary depending on the health and immune status of the flock (Freitas Neto et al. Citation2007). Infection can cause villus atrophy in the small intestine, affecting the absorptive surface of the intestine (Fasina et al. Citation2010), decreasing flock performance and causing significant economic losses (Hajam et al. Citation2018). The antibiotic treatment of infected birds results in prolonged and higher shedding levels in the faeces and is not a viable solution for controlling the spread of the infection in chickens (Smith and Tucker Citation1975, Citation1980; Barrow et al. Citation2012). The α-helical AMP, such as cathelicidins exhibit potent activity against Salmonella spp. strains. For example, synthetic human cathelicidin LL-37, released by neutrophils, has demonstrated vigorous antimicrobial activity against different strains (Turner et al. Citation1998). One of the most potent cathelicidins discovered thus far is cathelicidin-BF from the venom of the snake Bungarus fasciatus. Its administration in mice was found to reduce infection by streptomycin-resistant Salmonella spp. (Xia et al. Citation2015). Similarly, chicken-derived CATH-2 were found to exhibit potent antibacterial activity against both S. enterica and S. enterica Typhimurium (Veldhuizen et al. Citation2013), and synthetic analogues of Ang4 are currently being evaluated for their potential to impart antibacterial activity against Salmonella spp. (Hooper et al. Citation2003; Walker et al. Citation2013). Moreover, Townes et al. (Citation2009) demonstrated that the LEAP-2 peptide is able to disrupt the outer membrane of several S. enterica serovars including Typhimurium. Other avian AMP have also shown anti-Salmonellosis effects; for example, infection has been reported to upregulate the expression of AvBD-1, 4 and 10. Moreover, evidence suggests that the mechanism of AMP release in the chicken intestine can be modulated by feeding prebiotic and probiotic substances to chicken flocks (Shao et al. Citation2016). However, the potential of several avian AMP to reduce Salmonellosis infections in the poultry industry is yet to be explored.
Clostridium perfringens
Necrotic enteritis is one of the most common intestinal disease in poultry and is caused by C. perfringens, a spore-forming, anaerobic, gram-positive bacteria known to produce enterotoxins (Van Immerseel et al. Citation2004; Lanckriet et al. Citation2010). One of the main predisposing factors for necrotic enteritis is the epithelial damage caused by coccidiosis (Lanckriet et al. Citation2010), however, diets that increase the viscosity of intestinal lumen content are another major predisposing factor for the disease (Van Immerseel et al. Citation2004; Moore Citation2016). After intestinal colonisation and abnormal proliferation, alpha and spore-forming toxins are released within the gut lumen, causing extended necrotic lesions throughout the intestinal epithelium surface of the bird, as observed in clinical cases (Van Immerseel et al. Citation2009; Lee et al. Citation2011). On the other hand, a mild intestinal infection has been described, and focal necrotic areas in the intestinal epithelium and a reduction in the flock productive parameters are characteristic of the subclinical disease (Parish Citation1961; Skinner et al. Citation2010). The associated enterotoxins disrupt the intestinal cell membrane through phospholipid hydrolysis, and this activates innate immune proinflammatory pathways in the GALT, such as the arachidonic acid pathway (Van Immerseel et al. Citation2004). This triggers the production of proinflammatory mediators such as cytokines (IL-2, IL-8, TNF-alpha), leukotrienes and thromboxane that are involved in the pathogenesis of the disease (Sakurai et al. Citation2004; Park et al. Citation2008; Sakurai and Oda Citation2011). Intestinal necrosis and loss of gut integrity reduce the absorptive surface and allow the appearance of clinical signs, which involve depression, diarrhoea, and increased feed conversion ratio (Latorre et al. Citation2018). Mortality rates can reach values over 50% in the affected flock.
The recent reports of emerging C. perfringens AMR strains are directly linked to the increasing incidences of clinical and subclinical cases infection-associated necrotic enteritis in the poultry industry (Lanckriet et al. Citation2010) causing major economic losses (Skinner et al. Citation2010; Mwangi et al. Citation2019). New supplements, such as AMP, have been investigated to reduce the impact of AMR. Sublancin, an antimicrobial peptide extracted from Bacillus subtilis, for example, have been shown to reduce the caecal C. perfringens content by attacking its negatively charged membrane, decreasing proinflammatory molecules (IL-1β, IL-6, and TNF-α) production and enhancing histological integrity of the intestinal epithelium surface during in vivo experiments (Wang et al. Citation2015). Similar results for the intestinal histological parameters of AMP fed birds were reported by other authors (Bao et al. Citation2009; Choi et al. Citation2013a). Moreover, the same authors demonstrated the ability of antimicrobial peptide-A3 to reduce the excretion of C. perfringens at different ages during in vivo experiments in broilers, and AMP-A3 improved weight gain of the birds (Choi et al. Citation2013a). In addition, C. perfringens has been demonstrated to be susceptible to other peptides, such as enniatins produced by some species of the Fusarium genus, and has been demonstrated to reduce C. perfringens growth at different concentrations (Meca et al. Citation2011).
Eimeria
Eimeria parasites belong to the Apicomplexan phylum. These parasites infect the poultry intestinal tract causing coccidiosis, a disease seen across the world with significant economic relevance due to reducing the feed intake, feed efficiency and the bodyweight gain of infected birds (Allen and Fetterer Citation2002; Casterlow et al. Citation2011; Broom and Kogut Citation2019). Several Eimeria spp. have been described; however, seven are known to be pathogenic in poultry; three of them (E. acervulina, E. maxima, and E. tenella) are widely distributed and are the most common cause of clinical coccidiosis (Liu et al. Citation2018b). The susceptible host consumes sporulated oocysts and sporozoites are released within the GI tract. Sporozoites, the first of several stages (sexual or asexual) in the Eimeria life cycle, occur within the intestinal epithelium cells. These result in clinical signs, such as mucoid or bloody diarrhoea, dehydration, ruffled feathers, thickening of intestine, necrotic enteritis and suboptimal production parameters (Sharma et al. Citation2015; Song et al. Citation2015). Nevertheless, Eimeria spp. infections do not always cause clinical disease. Clinical signs are more likely to be present in young animals, although, sporadically, they can be seen in adult flocks. Such infections can trigger necrotic enteritis due to intestinal mucosa disruption (Sumners et al. Citation2011; Wu et al. Citation2014; Hardy et al. Citation2020).
Like AGP, prophylactic feed supplementation with anticoccidials, such as ionophores (salinomycin, medermycin and narasin) and synthetic compounds (sulphonamides, amprolium, and nicarbazin), have been used since the late 11940s to control spread (Chapman Citation2009). Anticoccidial use in Europe is controlled through the regulation (EC) No 1831/2003 and are generally classified as safe products. Moreover, Europe and the US have still classified anticoccidials as feed additives, and a veterinary prescription for their use has not been necessary yet (Kadykalo et al. Citation2018). Nevertheless, the absence of AGP in the feed can increase the incidence of avian coccidiosis and necrotic enteritis (Gaucher et al. Citation2015). The primary concern about ionophore antibiotics and their use as prophylactic anticoccidial feed supplements is the development of AMR in bacteria (Nisbet et al. Citation2008; Nilsson et al. Citation2012; Song et al. Citation2015). For example, it has been shown that vancomycin-resistant genes in a plasmid together with nnarasin-resistant genes can be transferred between Enterococci faecium (Nilsson et al. Citation2012), leading to the development of AMR for both vancomycin and narasin (Peek and Landman Citation2003, Citation2011). Similarly, Peek and Landman (Citation2003) demonstrated Eimeria spp. resistance against some of the most common anticoccidial drugs currently used in the poultry industry, such as monensin, nicarbazin, salinomycin and narazin.
The AMP have been tested as anticoccidials agents and have shown remarkable efficacies (Hong et al. Citation2008; Casterlow et al. Citation2011). The NK-lysin polypeptide, for example, is expressed in the jejunum of chickens after E. maxima infection (Hong et al. Citation2006), and this peptide has shown significant reduction in E. maxima and E. acervulina infections, in vitro (Hong et al. Citation2008). However, Eimeria spp. infection in chickens was shown to downregulate the natural intestinal expression of LEAP-2 (Casterlow et al. Citation2011), and CATHL3 (Sumners et al. Citation2011). Therefore, as the natural innate immune production of AMPs may be downregulated by coccidiosis, it is necessary to evaluate synthetic AMP mimics as a feed supplement alternative to control spread without the use of prophylactic anticoccidials.
Synthetic antimicrobial peptides as new antimicrobial alternatives for poultry
The AMP derived from intestinal mucosal cells are a potentially emerging source to treat various foodborne pathogens. The peptides and proteins present in intestinal mucosa of chickens provide excellent templates for designing and developing synthetic peptides as alternatives to AGP. Research has revealed that synthetic modification of natural AMP often results in significantly improved antimicrobial and immunomodulatory efficacies compared to natural analogues. Synthetic AMP as feed additives have the potential of improving animal health, while safeguarding human health by preventing the spread of antibiotic resistant foodborne pathogens. Synthetic AMP in feed can provide several major benefits to poultry production systems, such as immunomodulation and controlling the spread of foodborne pathogens at the farm level (Hasenstein et al. Citation2006). In addition, they have the potential to reduce incidences of antibiotic resistance, minimising carcase contamination by drug-resistant pathogens. Moreover, synthetic analogues of natural AMP improve the antimicrobial and immunomodulatory response against specific pathogenic microbes (Zelezetsky and Tossi Citation2006). For example, incorporation of a synthetic AMP to the GI tract of chicken was found to downregulate LEAP-2 produced by E. maxima in the poultry intestine (Casterlow et al. Citation2011), which suggested their potential as immunomodulatory materials. The recent advances in solid-phase peptide synthesis (SPPS) techniques and development of fully automated synthesisers have allowed facile and large-scale production of synthetic peptides (Ahmed Citation2017; Mijalis et al. Citation2017) with defined amino acid sequences. These can be prepared at reduced costs and exhibit exceptional antimicrobial activities against emerging drug resistant bacterial strains. In short, the availability of a wide variety of naturally occurring AMP in the GI tracts of poultry as a template, and rapid advances in peptide synthesis technology for the large-scale production makes the future prospect of industrial application of synthetic AMP in poultry promising.
Conclusions
The ban on AGP in some parts of the world has revealed that this has been associated with the deterioration of animal health and performance. There is a growing need to develop other alternatives as AGP replacements in poultry diets to help the industry transition to a cleaner and eco-friendly production while successfully meeting the growing market demand for organic poultry products. The innate gut mucosal immunity in the chicken is an excellent source of new products to replace traditional antibiotics in poultry diets. The cells of the avian GI tract produce a wide variety of naturally occurring AMP and antimicrobial proteins that have antimicrobial activity against a broad range of pathogens after millions of years of evolutionary selection. These provide excellent templates for designing and developing synthetic peptides as alternatives to AGP. Research has revealed that synthetic modification of natural AMP often results in significantly improved antimicrobial and immunomodulatory efficacies compared to natural analogues. Synthetic AMP as feed additives, therefore, have the potential to modulate host cells immunity and provide safe alternatives to prevent the spread of antibiotic resistant, foodborne pathogens in the human population. In addition, rapid advances in peptide synthesis techniques and availability of large-scale production of polypeptides by simple methods can provide a promising avenue to apply these products in the poultry industry.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed, M. 2017. “Peptides, Polypeptides and Peptide–Polymer Hybrids as Nucleic Acid Carriers.” Biomaterials Science 5 (11): 2188–2211. doi:https://doi.org/10.1039/C7BM00584A.
- Akbari, M. R., H. R. Haghighi, J. R. Chambers, J. Brisbin, L. R. Read, and S. Sharif. 2008. “Expression of Antimicrobial Peptides in Cecal Tonsils of Chickens Treated with Probiotics and Infected with Salmonella Enterica Serovar Typhimurium.” Clinical and Vaccine Immunology 15 (11): 1689–1693. doi:https://doi.org/10.1128/CVI.00242-08.
- Alber, A., K. M. Morris, K. J. Bryson, K. M. Sutton, M. S. Monson, C. Chintoan-Uta, D. Borowska, et al. 2020. “Avian Pathogenic Escherichia Coli (APEC) Strain-Dependent Immunomodulation of Respiratory Granulocytes and Mononuclear Phagocytes in CSF1R-Reporter Transgenic Chickens.” Frontiers in Immunology 10. doi:https://doi.org/10.3389/fimmu.2019.03055.
- Allen, P. C., and R. H. Fetterer. 2002. “Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry.” Clinical Microbiology Reviews 15 (1): 58–65. doi:https://doi.org/10.1128/CMR.15.1.58-65.2002.
- Antão, E., S. Glodde, G. Li, R. Sharifi, T. Homeier, C. Laturnus, I. Diehl, et al. 2008. “The Chicken as a Natural Model for Extraintestinal Infections Caused by Avian Pathogenic Escherichia Coli (APEC).” Microbial Pathogenesis 45 (5–6): 361–369. doi:https://doi.org/10.1016/j.micpath.2008.08.005.
- Apajalahti, J., and K. Vienola. 2016. “Interaction between Chicken Intestinal Microbiota and Protein Digestion.” Animal Feed Science and Technology 221: 323–330. doi:https://doi.org/10.1016/J.ANIFEEDSCI.2016.05.004.
- Balakrishnan, A., and D. Chakravortty. 2017. “Epithelial Cell Damage Activates Bactericidal/permeability Increasing-protein (BPI) Expression in Intestinal Epithelium.” Frontiers in Microbiology 8: 1567. doi:https://doi.org/10.3389/fmicb.2017.01567.
- Bao, H., R. She, T. Liu, Y. Zhang, K. S. Peng, D. Luo, Z. Yue, et al. 2009. “Effects of Pig Antibacterial Peptides on Growth Performance and Intestine Mucosal Immune of Broiler Chickens.” Poultry Science 88 (2): 291–297. doi:https://doi.org/10.3382/ps.2008-00330.
- Barrow, P. A., M. A. Jones, A. L. Smith, and P. Wigley. 2012. “The Long View: Salmonella – The Last Forty Years.” Avian Pathology 41 (5): 413–420. doi:https://doi.org/10.1080/03079457.2012.718071.
- Berchieri, A., C. K. Murphy, K. Marston, and P. A. Barrow. 2001. “Observations on the Persistence and Vertical Transmission of Salmonella Enterica Serovars Pullorum and Gallinarum in Chickens: Effect of Bacterial and Host Genetic Background.” Avian Pathology: Journal of the W.V.P.A 30 (3): 221–231. doi:https://doi.org/10.1080/03079450120054631.
- Bommineni, Y. R., H. Dai, Y. Gong, J. L. Soulages, S. C. Fernando, U. DeSilva, O. Prakash, et al. 2007. “Fowlicidin-3 Is an α-helical Cationic Host Defense Peptide with Potent Antibacterial and Lipopolysaccharide-neutralizing Activities.” FEBS Journal 274 (2): 418–428. doi:https://doi.org/10.1111/j.1742-4658.2006.05589.x.
- Broom, L. J., and M. H. Kogut. 2019. “Deciphering Desirable Immune Responses from Disease Models with Resistant and Susceptible Chickens.” Poultry Science 98 (4): 1634–1642. doi:https://doi.org/10.3382/ps/pey535.
- Casewell, M. 2003. “The European Ban on Growth-promoting Antibiotics and Emerging Consequences for Human and Animal Health.” Journal of Antimicrobial Chemotherapy 52 (2): 159–161. doi:https://doi.org/10.1093/jac/dkg313.
- Casterlow, S., H. Li, E. R. Gilbert, R. A. Dalloul, A. P. McElroy, D. A. Emmerson, and E. A. Wong. 2011. “An Antimicrobial Peptide Is Downregulated in the Small Intestine of Eimeria Maxima-infected Chickens.” Poultry Science 90 (6): 1212–1219. doi:https://doi.org/10.3382/ps.2010-01110.
- Chapman, H. D. 2009. “A Landmark Contribution to Poultry science—Prophylactic Control of Coccidiosis in Poultry.” Poultry Science 88 (4): 813–815. doi:https://doi.org/10.3382/ps.2008-00316.
- Cheng, Y., M. D. Prickett, W. Gutowska, R. Kuo, K. Belov, and D. W. Burt. 2015. “Evolution of the Avian β-defensin and Cathelicidin Genes.” BMC Evolutionary Biology 15 (1): 188. doi:https://doi.org/10.1186/s12862-015-0465-3.
- Chiang, S., E. J. A. Veldhuizen, F. A. Barnes, C. J. Craven, H. P. Haagsman, and C. D. Bingle. 2011. “Identification and Characterisation of the BPI/LBP/PLUNC-like Gene Repertoire in Chickens Reveals the Absence of a LBP Gene.” Developmental and Comparative Immunology 35: 285–295. doi:https://doi.org/10.1016/j.dci.2010.09.013.
- Choi, S. C., B. J. Chae, I. K. Kwon, J. S. Kim, S. L. Ingale, and Y. K. Park. 2013b. “Effects of Dietary Supplementation with an Antimicrobial Peptide-P5 on Growth Performance, Nutrient Retention, Excreta and Intestinal Microflora and Intestinal Morphology of Broilers.” Animal Feed Science and Technology 185: 78–84. doi:https://doi.org/10.1016/j.anifeedsci.2013.07.005.
- Choi, S. C., S. L. Ingale, J. S. Kim, Y. K. Park, I. K. Kwon, and B. J. Chae. 2013a. “An Antimicrobial peptide-A3: Effects on Growth Performance, Nutrient Retention, Intestinal and Faecal Microflora and Intestinal Morphology of Broilers.” British Poultry Science 54 (6): 738–746. doi:https://doi.org/10.1080/00071668.2013.838746.
- Coura, F. M., A. S. Diniz, M. X. Silva, T. L. M. Arcebismo, S. Minharro, A. C. F. Feitosa, A. P. Lage, T. Knöbl, J. M. S. Mussi, and M. B. Heinemann. 2017. “Phylogenetic Group of Escherichia Coli Isolates from Broilers in Brazilian Poultry Slaughterhouse.” The Scientific World Journal 2017. doi:https://doi.org/10.1155/2017/5898701.
- Cuperus, T., A. Van Dijk, R. M. Dwars, and H. P. Haagsman. 2016b. “Localization and Developmental Expression of Two Chicken Host Defense Peptides: Cathelicidin-2 and Avian β-defensin 9.” Developmental and Comparative Immunology 61: 48–59. doi:https://doi.org/10.1016/j.dci.2016.03.008.
- Cuperus, T., A. Van Dijk, M. G. R. Matthijs, E. J. A. Veldhuizen, and H. P. Haagsman. 2016a. “Protective Effect of in Ovo Treatment with the Chicken Cathelicidin Analog D-CATH-2 against Avian Pathogenic E. Coli.” Scientific Reports 6: 26622. doi:https://doi.org/10.1038/srep26622.
- Daneshmand, A., H. Kermanshahi, M. H. Sekhavati, A. Javadmanesh, and M. Ahmadian. 2019. “Antimicrobial Peptide, cLF36, Affects Performance and Intestinal Morphology, Microflora, Junctional Proteins, and Immune Cells in Broilers Challenged with E. Coli.” Scientific Reports 9: 14176. doi:https://doi.org/10.1038/s41598-019-50511-7.
- Derache, C., V. Labas, V. Aucagne, H. Meudal, C. Landon, A. F. Delmas, T. Magallon, et al. 2009. “Primary Structure and Antibacterial Activity of Chicken Bone Marrow-derived β-defensins.” Antimicrobial Agents and Chemotherapy 53: 4647–4655. doi:https://doi.org/10.1128/AAC.00301-09.
- Dominick, M. A., and A. E. Jensen. 1984. “Colonization and Persistence of Escherichia Coli in Axenic and Monoxenic Turkeys.” American Journal of Veterinary Research 45: 2331–2335.
- Dziva, F., H. Hauser, T. R. Connor, P. M. Van Diemen, G. Prescott, G. C. Langridge, S. Eckert, et al. 2013. “Sequencing and Functional Annotation of Avian Pathogenic Escherichia Coli Serogroup O78 Strains Reveal the Evolution of E. Coli Lineages Pathogenic for Poultry via Distinct Mechanisms.” Infection and Immunity 81: 838–849. doi:https://doi.org/10.1128/IAI.00585-12.
- Engberg, R. M., M. S. Hedemann, T. D. Leser, and B. B. Jensen. 2000. “Effect of Zinc Bacitracin and Salinomycin on Intestinal Microflora and Performance of Broilers.” Poultry Science 79 (9): 1311–1319. doi:https://doi.org/10.1093/ps/79.9.1311.
- European Food Safety Authority and European Centre for Disease Prevention and Control. 2020. “The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017/2018.” EFSA Journal 18(3). doi: https://doi.org/10.2903/j.efsa.2020.6007.
- Evans, E. W., F. G. Beach, K. M. Moore, M. W. Jackwood, J. R. Glisson, and B. G. Harmon. 1995. “Antimicrobial Activity of Chicken and Turkey Heterophil Peptides CHP1, CHP2, THP1, and THP3.” Veterinary Microbiology 47 (3–4): 295–303. doi:https://doi.org/10.1016/0378-1135(95)00126-3.
- Evans, E. W., G. G. Beach, J. Wunderlich, and B. G. Harmon. 1994. “Isolation of Antimicrobial Peptides from Avian Heterophils.” Journal of Leukocyte Biology 56 (5): 661–665. doi:https://doi.org/10.1002/jlb.56.5.661.
- Fasina, Y. O., F. J. Hoerr, S. R. McKee, and D. E. Conner. 2010. “Influence of Salmonella Enterica Serovar Typhimurium Infection on Intestinal Goblet Cells and Villous Morphology in Broiler Chicks.” Avian Diseases 54 (2): 841–847. doi:https://doi.org/10.1637/9055-090809-Reg.1.
- Ferrario, C., R. Statello, L. Carnevali, L. O. Mancabelli, C. Milani, M. Mangifesta, S. Duranti, et al. 2017. “How to Feed the Mammalian Gut Microbiota: Bacterial and Metabolic Modulation by Dietary Fibers.” Frontiers in Microbiology 8. doi:https://doi.org/10.3389/fmicb.2017.01749.
- FAO (Food and Agriculture Organization). 2013. Poultry Development Review. Rome: FAO.
- FAO (Food and Agriculture Organization). 2016. Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production. Rome: FAO.
- FAO (Food and Agriculture Organization). 2019. Meat Market Review. Rome: FAO.
- Freitas Neto, O. C., W. Arroyave, A. C. Alessi, J. J. Fagliari, and A. Berchieri. 2007. “Infection of Commercial Laying Hens with Salmonella Gallinarum: Clinical, Anatomopathological and Haematological Studies.” Brazilian Journal of Poultry Science 9 (2): 133–141. doi:https://doi.org/10.1590/S1516-635X2007000200010.
- Gaskins, H. R., C. T. Collier, and D. B. Anderson. 2002. “Antibiotics as Growth Promotants: Mode of Action.” Animal Biotechnology 13: 29–42. doi:https://doi.org/10.1081/ABIO-120005768.
- Gaucher, M.-L., S. Quessy, A. Letellier, J. Arsenault, and M. Boulianne. 2015. “Impact of a Drug-Free Program on Broiler Chicken Growth Performances, Gut Health, Clostridium Perfringens and Campylobacter Jejuni Occurrences at the Farm Level.” Poultry Science 94: 1791–1801. doi:https://doi.org/10.3382/ps/pev142.
- Goitsuka, R., C. H. Chen, L. Benyon, Y. Asano, D. Kitamura, and M. D. Cooper. 2007. “Chicken cathelicidin-B1, an Antimicrobial Guardian at the Mucosal M Cell Gateway.” Proceedings of the National Academy of Sciences of the United States of America 104: 15063–15068. doi:https://doi.org/10.1073/pnas.0707037104.
- Graham, J. P., S. L. Evans, L. B. Price, and E. K. Silbergeld. 2009. “Fate of Antimicrobial-resistant Enterococci and Staphylococci and Resistance Determinants in Stored Poultry Litter.” Environmental Research 109: 682–689. doi:https://doi.org/10.1016/j.envres.2009.05.005.
- Hajam, I. A., K. Jehyoung, and J. H. Lee. 2018. “Oral Immunization with a Novel Attenuated Salmonella Gallinarum Encoding Infectious Bronchitis Virus Spike Protein Induces Protective Immune Responses against Fowl Typhoid and Infectious Bronchitis in Chickens.” Veterinary Research 49. doi:https://doi.org/10.1186/s13567-018-0588-9.
- Hardy, S. P., S. L. Benestad, I. S. Hamnes, T. Moldal, B. David, J. R. Barta, J. Reperant, and M. Kaldhusdal. 2020. “Developing an Experimental Necrotic Enteritis Model in Turkeys - the Impact of Clostridium Perfringens, Eimeria Meleagrimitis and Host Age on Frequency of Severe Intestinal Lesions.” BMC Veterinary Research 16: 63. doi:https://doi.org/10.1186/s12917-020-2270-5.
- Hasenstein, J. R., G. Zhang, and S. J. Lamont. 2006. “Analyses of Five Gallinacin Genes and the Salmonella Enterica Serovar Enteritidis Response in Poultry.” Infection and Immunity 74: 3375–3380. doi:https://doi.org/10.1128/IAI.00027-06.
- Hong, Y. H., H. S. Lillehoj, S. H. Lee, R. A. Dalloul, and E. P. Lillehoj. 2006. “Analysis of Chicken Cytokine and Chemokine Gene Expression following Eimeria Acervulina and Eimeria Tenella Infections.” Veterinary Immunology and Immunopathology 114: 209–223. doi:https://doi.org/10.1016/j.vetimm.2006.07.007.
- Hong, Y. H., H. S. Lillehoj, G. R. Siragusa, D. D. Bannerman, and E. P. Lillehoj. 2008. “Antimicrobial Activity of Chicken NK-lysin against Eimeria Sporozoites.” Avian Diseases 52: 302–305. doi:https://doi.org/10.1637/8083-072307-ResNote.1.
- Hong, Y. H., W. Song, S. H. Lee, and H. S. Lillehoj. 2012. “Differential Gene Expression Profiles of β-defensins in the Crop, Intestine, and Spleen Using a Necrotic Enteritis Model in 2 Commercial Broiler Chicken Lines.” Poultry Science 91: 1081–1088. doi:https://doi.org/10.3382/ps.2011-01948.
- Hooper, L., T. Stappenbeck, C. Hong, and J. Gordon. 2003. “Angiogenins: A New Class of Microbicidal Proteins Involved in Innate Immunity.” Nature Immunology 4: 269–273. doi:https://doi.org/10.1038/ni888.
- Hu, F., X. Gao, R. She, J. Chen, J. Mao, P. Xiao, and R. Shi. 2017. “Effects of Antimicrobial Peptides on Growth Performance and Small Intestinal Function in Broilers under Chronic Heat Stress.” Poultry Science 96: 798–806. doi:https://doi.org/10.3382/ps/pew379.
- Jones, F. T., and S. C. Ricke. 2003. “Observations on the History of the Development of Antimicrobials and Their Use in Poultry Feeds.” Poultry Science 82: 613–617. doi:https://doi.org/10.1093/ps/82.4.613.
- Kadykalo, S., T. Roberts, M. Thompson, J. Wilson, M. Lang, and O. Espeisse. 2018. “The Value of Anticoccidials for Sustainable Global Poultry Production.” International Journal of Antimicrobial Agents 51 (3): 304–310. doi:https://doi.org/10.1016/j.ijantimicag.2017.09.004.
- Kang, H. K., H. M. Salim, N. Akter, D. W. Kim, J. H. Kim, H. T. Bang, M. J. Kim, et al. 2013. “Effect of Various Forms of Dietary Chlorella Supplementation on Growth Performance, Immune Characteristics, and Intestinal Microflora Population of Broiler Chickens.” Journal of Applied Poultry Research 22 (1): 100–108. doi:https://doi.org/10.3382/japr.2012-00622.
- Kemmett, K., T. Humphrey, S. Rushton, A. Close, P. Wigley, N. J. Williams, and D. Bassham. 2013. “A Longitudinal Study Simultaneously Exploring the Carriage of APEC Virulence Associated Genes and the Molecular Epidemiology of Faecal and Systemic E. Coli in Commercial Broiler Chickens.” PLOS ONE 8 (6): e67749. doi:https://doi.org/10.1371/journal.pone.0067749.
- Kim, W. H., and H. S. Lillehoj. 2019. “Immunity, Immunomodulation, and Antibiotic Alternatives to Maximize the Genetic Potential of Poultry for Growth and Disease Response.” Animal Feed Science and Technology 250: 41–50. doi:https://doi.org/10.1016/j.anifeedsci.2018.09.016.
- Kim, W. H., H. S. Lillehoj, and W. Min. 2017. “Evaluation of the Immunomodulatory Activity of the Chicken NK-lysin-derived Peptide cNK-2.” Scientific Reports 7 (1): 45099. doi:https://doi.org/10.1038/srep45099.
- Kogut, M. H., Y. X. Nan., Y. J. Min, and L. Broom. 2017. “Gut Health in Poultry.” CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 12. doi:https://doi.org/10.1079/PAVSNNR201712031.
- Kraaij, M. D., A. Van Dijk, and H. P. Haagsman. 2017. “CATH-2 and LL-37 Increase Mannose Receptor Expression, Antigen Presentation and the Endocytic Capacity of Chicken Mononuclear Phagocytes.” Molecular Immunology 90: 118–125. doi:https://doi.org/10.1016/j.molimm.2017.07.005.
- Kwon, H. J., K. Y. Park, H. S. Yoo, J. Y. Park, Y. H. Park, and S. J. Kim. 2000. “Differentiation of Salmonella Enterica Serotype Gallinarum Biotype Pullorum from Biotype Gallinarum by Analysis of Phase 1 Flagellin C Gene (Flic).” Journal of Microbiological Methods 40: 33–38. doi:https://doi.org/10.1016/s0167-7012(99)00129-3.
- Lanckriet, A., L. Timbermont, M. De Gussem, M. Marien, D. Vancraeynest, F. Haesebrouck, R. Ducatelle, and F. Van Immerseel. 2010. “The Effect of Commonly Used Anticoccidials and Antibiotics in a Subclinical Necrotic Enteritis Model.” Avian Pathology 39: 63–68. doi:https://doi.org/10.1080/03079450903505771.
- Latorre, J. D., B. Adhikari, S. H. Park, K. D. Teague, L. E. Graham, B. D. Mahaffey, M. F. A. Baxter, et al. 2018. “Evaluation of the Epithelial Barrier Function and Ileal Microbiome in an Established Necrotic Enteritis Challenge Model in Broiler Chickens.” Frontiers in Veterinary Science 5. doi:https://doi.org/10.3389/fvets.2018.00199.
- Le Devendec, L., G. Mourand, S. Bougeard, J. Léaustic, E. Jouy, A. Keita, W. Couet, N. Rousset, and I. Kempf. 2016. “Impact of Colistin Sulfate Treatment of Broilers on the Presence of Resistant Bacteria and Resistance Genes in Stored or Composted Manure.” Veterinary Microbiology 194: 98–106. doi:https://doi.org/10.1016/j.vetmic.2015.11.012.
- Lee, K. W., H. S. Lillehoj, W. Jeong, H. Y. Jeoung, and D. J. An. 2011. “Avian Necrotic Enteritis: Experimental Models, Host Immunity, Pathogenesis, Risk Factors, and Vaccine Development.” Poultry Science 90: 1381–1390. doi:https://doi.org/10.3382/ps.2010-01319.
- Lee, M. O., H. Jang, J. Y. Han, and J. E. Womack. 2014. “Chicken NK-lysin Is an Alpha-helical Cationic Peptide that Exerts Its Antibacterial Activity through Damage of Bacterial Cell Membranes.” Poultry Science 93: 864–870. doi:https://doi.org/10.3382/ps.2013-03670.
- Lee, M. O., H. Jang, D. Rengaraj, S. Yang, J. Y. Han, S. J. Lamont, and J. E. Womack. 2016. “Tissue Expression and Antibacterial Activity of Host Defense Peptides in Chicken.” BMC Veterinary Research 12: 231. doi:https://doi.org/10.1186/s12917-016-0866-6.
- Lee, M. O., E. Kim, H. Jang, M. N. Park, H. Woo, J. H. Han, and J. H. Womack. 2012. “Effects of a Single Nucleotide Polymorphism in the Chicken NK-lysin Gene on Antimicrobial Activity and Cytotoxicity of Cancer Cells.” Proceedings of the National Academy of Sciences of the United States of America 109: 12087–12092. doi:https://doi.org/10.1073/pnas.1209161109.
- Lee, S. H., H. S. Lillehoj, W. Tuo, C. A. Murphy, Y. H. Hong, and E. P. Lillehoj. 2013. “Parasiticidal Activity of a Novel Synthetic Peptide from the Core α-helical Region of NK-lysin.” Veterinary Parasitology 197: 113–121. doi:https://doi.org/10.1016/j.vetpar.2013.04.020.
- Liu, C., L. Jiang, L. Liu, L. Sun, W. Zhao, Y. Chen, T. Qi, et al. 2018a. “Induction of Avian β-defensin 2 Is Possibly Mediated by the P38 MAPK Signal Pathway in Chicken Embryo Fibroblasts after Newcastle Disease Virus Infection.” Frontiers in Microbiology 9: 751. doi:https://doi.org/10.3389/fmicb.2018.00751.
- Liu, J., L. Liu, L. Li, D. Tian, W. Li, L. Xu, R. Yan, X. Li, and X. Song. 2018b. “Protective Immunity Induced by Eimeria Common Antigen 14–3-3 against Eimeria Tenella, Eimeria Acervulina and Eimeria Maxima.” BMC Veterinary Research 14: 337. doi:https://doi.org/10.1186/s12917-018-1665-z.
- Losada-Medina, D., A. Yitbarek, N. Nazeer, S. Uribe-Diaz, M. Ahmed, and J. C. Rodriguez-Lecompte. 2020. “Identification, Tissue Characterization and Innate Immune Role of Angiogenin-4 Expression in Young Broiler Chickens.” Poultry Science 99: 2992–3000. doi:https://doi.org/10.1016/j.psj.2020.03.022.
- Lu, Y., H. Zhao, J. Sun, Y. Liu, X. Zhou, R. C. Beier, G. Wu, et al. 2014. “Characterization of Multidrug-Resistant Salmonella Enterica Serovars Indiana and Enteritidis from Chickens in Eastern China.” PLoS One 9: e96050. doi:https://doi.org/10.1371/journal.pone.0096050.
- Luiken, R. E. C., L. Van Gompel, P. Munk, S. Sarrazin, P. Joosten, R. Al. Dorado-García, B. Hansen, et al. 2019. “Associations between Antimicrobial Use and the Faecal Resistome on Broiler Farms from Nine European Countries.” The Journal of Antimicrobial Chemotherapy 74: 2596–2604. doi:https://doi.org/10.1093/jac/dkz235.
- Ma, D., W. Liao, R. Wang, Z. Han, and S. Liu. 2009. “Two Novel Duck Antibacterial Peptides, Avian β-defensins 9 and 10, with Antimicrobial Activity.” Journal of Microbiology and Biotechnology 19: 1447–1455. doi:https://doi.org/10.4014/jmb.0904.4028.
- Mancabelli, L., C. Ferrario, C. Milani, M. Mangifesta, F. Turroni, S. Duranti, G. Lugli, et al. 2016. “Insights into the Biodiversity of the Gut Microbiota of Broiler Chickens.” Environmental Microbiology.” Environmental Microbiology 18 (12): 4727–4738. doi:https://doi.org/10.1111/1462-2920.13363.
- Meca, G., I. Sospedra, M. A. Valero, J. Mañes, G. Font, and M. J. Ruiz. 2011. “Antibacterial Activity of the Enniatin B, Produced by Fusarium Tricinctum in Liquid Culture, and Cytotoxic Effects on Caco-2 Cells.” Toxicology Mechanisms and Methods 21 (7): 503–512. doi:https://doi.org/10.3109/15376516.2011.556202.
- Mettifogo, E., L. F. N. Nuñez, J. L. Chacón, S. H. Santander Parra, C. S. Astolfi-Ferreira, J. A. Jerez, R. C. Jones, and A. J. Piantino Ferreira. 2014. “Emergence of Enteric Viruses in Production Chickens Is a Concern for Avian Health.” The Scientific World Journal 2014: 1–8. doi:https://doi.org/10.1155/2014/450423.
- Michailidis, G. 2010. “Expression of Chicken LEAP-2 in the Reproductive Organs and Embryos and in Response to Salmonella Enterica Infection.” Veterinary Research Communications 34 (5): 459–471. doi:https://doi.org/10.1007/s11259-010-9420-3.
- Mijalis, A. J., D. A. Thomas, M. D. Simon, A. Adamo, R. Beaumont, K. F. Jensen, and B. L. Pentelute. 2017. “A Fully Automated Flow-Based Approach for Accelerated Peptide Synthesis.” Nature Chemical Biology 13: 464–466. doi:https://doi.org/10.1038/nchembio.2318.
- Miles, R. D., G. D. Butcher, P. R. Henry, and R. C. Littell. 2006. “Effect of Antibiotic Growth Promoters on Broiler Performance, Intestinal Growth Parameters, and Quantitative Morphology.” Poultry Science 85: 476–485. doi:https://doi.org/10.1093/ps/85.3.476.
- Moore, R. J. 2016. “Necrotic Enteritis Predisposing Factors in Broiler Chickens.” Avian Pathology 45: 275–281. doi:https://doi.org/10.1080/03079457.2016.1150587.
- More, S. J. 2020. “European Perspectives on Efforts to Reduce Antimicrobial Usage in Food Animal Production.” Irish Veterinary Journal 73 (1): 2. doi:https://doi.org/10.1186/s13620-019-0154-4.
- Mwangi, S., J. Timmons, S. Fitz-Coy, and S. Parveen. 2019. “Characterization of Clostridium Perfringens Recovered from Broiler Chicken Affected by Necrotic Enteritis.” Poultry Science 98: 128–135. doi:https://doi.org/10.3382/ps/pey332.
- Nazeer, N. Forthcoming. “Antimicrobial and Immunomodulatory Activities of Ang4 Derived Peptides.” PhD diss., University of Prince Edward Island.
- Nhung, N. T., N. Chansiripornchai, and J. J. Carrique-Mas. 2017. “Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review.” Frontiers in Veterinary Science 4: 126. doi:https://doi.org/10.3389/fvets.2017.00126.
- Nilsson, O., C. Greko, B. Bengtsson, and S. Englund. 2012. “Genetic Diversity among VRE Isolates from Swedish Broilers with the Coincidental Finding of Transferrable Decreased Susceptibility to Narasin.” Journal of Applied Microbiology 112: 716–722. doi:https://doi.org/10.1111/j.1365-2672.2012.05254.x.
- Nisbet, D. J., T. R. Callaway, T. S. Edrington, R. C. Anderson, and T. L. Poole. 2008. “Effects of Ionophores on Enterococcus Faecalis and E. Faecium Growth in Pure and Mixed Ruminal Culture.” Foodborne Pathogens and Disease 5: 193–198. doi:https://doi.org/10.1089/fpd.2007.0058.
- Nitto, T., K. D. Dyer, M. Czapiga, and H. F. Rosenberg. 2006. “Evolution and Function of Leukocyte RNase A Ribonucleases of the Avian Species, Gallus Gallus.” Journal of Biological Chemistry 281: 25622–25634. doi:https://doi.org/10.1074/jbc.M604313200.
- Oliveira, A. L., D. M. Newman, Y. Sato, A. Noel, B. Rauk, L. K. Nolan, N. L. Barbieri, and C. M. Logue. 2020. “Characterization of Avian Pathogenic Escherichia Coli (APEC) Associated with Turkey Cellulitis in Iowa.” Frontiers in Veterinary Science 7. doi:https://doi.org/10.3389/fvets.2020.00380.
- Parish, W. E. 1961. “Necrotic Enteritis in the Fowl (Gallus Gallus Domesticus). I. Histopathology of the Disease and Isolation of a Strain of Clostridium Welchii.” Journal of Comparative Pathology 71: 377–393. doi:https://doi.org/10.1016/S0368-1742(61)80043-X.
- Park, S. S., H. S. Lillehoj, P. C. Allen, D. W. Park, S. FitzCoy, D. A. Bautista, and E. P. Lillehoje. 2008. “Immunopathology and Cytokine Responses in Broiler Chickens Coinfected with Eimeria Maxima and Clostridium Perfringens with the Use of an Animal Model of Necrotic Enteritis.” Avian Diseases 52: 14–22. doi:https://doi.org/10.1637/7997-041707-Reg.
- Peek, H. W., and W. J. M. Landman. 2003. “Resistance to Anticoccidial Drugs of Dutch Avian Eimeria Spp. Field Isolates Originating from 1996, 1999 and 2001.” Avian Pathology 32: 391–401. doi:https://doi.org/10.1080/0307945031000121149.
- Peek, H. W., and W. J. M. Landman. 2011. “Coccidiosis in Poultry: Anticoccidial Products, Vaccines and Other Prevention Strategies.” Veterinary Quarterly 31: 143–161. doi:https://doi.org/10.1080/01652176.2011.605247.
- Peng, L., M. R. Scheenstra, R. M. Van Harten, H. P. Haagsman, and E. J. A. Veldhuizen. 2020. “The Immunomodulatory Effect of Cathelicidin-B1 on Chicken Macrophages.” Veterinary Research 51: 122. doi:https://doi.org/10.1186/s13567-020-00849-y.
- Peralta, M. F., A. Magnoli, F. Alustiza, A. Nilson, R. Miazzo, and A. Vivas. 2017. “Gut-associated Lymphoid Tissue: A Key Tissue inside the Mucosal Immune System of Hens Immunized with Escherichia Coli F4.” Frontiers in Immunology 8: 568. doi:https://doi.org/10.3389/fimmu.2017.00568.
- Ramasamy, T. K., P. Verma, and M. R. Reddy. 2012. “Differential Gene Expression of Antimicrobial Peptides β Defensins in the Gastrointestinal Tract of Salmonella Serovar Pullorum Infected Broiler Chickens.” Veterinary Research Communications 36: 57–62. doi:https://doi.org/10.1007/s11259-011-9512-8.
- Rengaraj, D., A. D. Truong, H. S. Lillehoj, J. Y. Han, and Y. H. Hong. 2018. “Expression and Regulation of Avian Beta-defensin 8 Protein in Immune Tissues and Cell Lines of Chickens.” Asian-Australasian Journal of Animal Sciences 31: 1516–1524. doi:https://doi.org/10.5713/ajas.17.0836.
- Rodriguez-Lecompte, J. C., J. D. House, M. Jing, A. Bandegan, and W. Guenter. 2008. “Molecular Cloning and Characterization of a New Class of Microbicidal Protein Involved in Innate Immunity in Chickens.” 10th Avian Immunology Research Group. (Oral presentation).
- Rodríguez-Lecompte, J. C., A. Yitbarek, J. Brady, S. Sharif, M. D. Cavanagh, G. Crow, W. Guenter, et al. 2012. “The Effect of Microbial-nutrient Interaction on the Immune System of Young Chicks after Early Probiotic and Organic Acids Administration.” Journal of Animal Science 90 (7): 2246–2254. doi:https://doi.org/10.2527/jas.2011-4184.
- Sakurai, J., M. Nagahama, and M. Oda. 2004. “Clostridium Perfringens Alpha-Toxin: Characterization and Mode of Action.” Journal of Biochemistry 136: 569–574. doi:https://doi.org/10.1093/jb/mvh161.
- Sakurai, J., and M. Oda. 2011. “Effect of Macrolide Antibiotics on Biological Activities Induced by Clostridium Perfringens Alpha-Toxin.” Gangrene - Current Concepts and Management Options. doi:https://doi.org/10.5772/22404.
- Sartika, T., S. Sulandari, and M. S. A. Zein. 2011. “Selection of Mx Gene Genotype as Genetic Marker for Avian Influenza Resistance in Indonesian Native Chicken.” BMC Proceedings 5: S37. doi:https://doi.org/10.1186/1753-6561-5-S4-S37.
- Scott, A. B., M. Singh, P. Groves, M. Hernandez-Jover, B. Barnes, K. Glass, B. Moloney, et al. 2018. “Biosecurity Practices on Australian Commercial Layer and Meat Chicken Farms: Performance and Perceptions of Farmers.” PLoS One 13 (4): e0195582. doi:https://doi.org/10.1371/journal.pone.0195582.
- Shao, Y., Z. Wang, X. Tian, Guo, H. Zhang, and Y. Guo. 2016. “Yeast β-d-Glucans Induced Antimicrobial Peptide Expressions against Salmonella Infection in Broiler Chickens.” International Journal of Biological Macromolecules 85: 573–584. doi:https://doi.org/10.1016/j.ijbiomac.2016.01.031.
- Sharma, S., S. Azmi, A. Iqbal, N. Nasirudullah, and I. Mushtaq. 2015. “Pathomorphological Alterations Associated with Chicken Coccidiosis in Jammu Division of India.” Journal of Parasitic Diseases: Official Organ of the Indian Society for Parasitology 39: 147–151. doi:https://doi.org/10.1007/s12639-013-0302-9.
- Shi, D., L. Bai, Q. Qu, S. Zhou, M. Yang, S. Guo, Q. Li, and C. Liu. 2019. “Impact of Gut Microbiota Structure in Heat-Stressed Broilers.” Poultry Science 98: 2405–2413. doi:https://doi.org/10.3382/ps/pez026.
- Shivaprasad, H. L. 2000. “Fowl Typhoid and Pullorum Disease.” Revue Scientifique Et Technique 19: 405–424. doi:https://doi.org/10.20506/rst.19.2.1222.
- Skinner, J. T., S. Bauer, V. Young, G. Pauling, and J. Wilson. 2010. “An Economic Analysis of the Impact of Subclinical (Mild) Necrotic Enteritis in Broiler Chickens.” Avian Diseases 54: 1237–1240. doi:https://doi.org/10.1637/9399-052110-Reg.1.
- Smith, H. W., and J. F. Tucker. 1975. “The Effect of Feeding Diets Containing Permitted Antibiotics on the Faecal Excretion of Salmonella Typhimurium by Experimentally Infected Chickens.” Journal of Hygiene 75: 293–301. doi:https://doi.org/10.1017/s0022172400047318.
- Smith, H. W., and J. F. Tucker. 1980. “Further Observations on the Effect of Feeding Diets Containing Avoparcin, Bacitracin and Sodium Arsenilate on the Colonization of the Alimentary Tract of Poultry by Salmonella Organisms.” The Journal of Hygiene 84: 137–150. doi:https://doi.org/10.1017/S0022172400026620.
- Smith, J. A. 2011. “Experiences with Drug-free Broiler Production.” Poultry Science 90 (11): 2670–2678. doi:https://doi.org/10.3382/ps.2010-01032.
- Sochacki, K. A., K. J. Barns, R. Bucki, and J. C. Weisshaar. 2011. “Real-time Attack on Single Escherichia Coli Cells by the Human Antimicrobial Peptide LL-37.” Proceedings of the National Academy of Sciences of the United States of America 108: E77–81. doi:https://doi.org/10.1073/pnas.1101130108.
- Song, X., Z. Ren, R. Yan, L. Xu, and X. Li. 2015. “Induction of Protective Immunity against Eimeria Tenella, Eimeria Necatrix, Eimeria Maxima and Eimeria Acervulina Infections Using Multivalent Epitope DNA Vaccines.” Vaccine 33: 2764–2770. doi:https://doi.org/10.1016/j.vaccine.2015.04.052.
- Starr, M. P., and D. M. Reynolds. 1951. “Streptomycin Resistance of Coliform Bacteria from Turkeys Fed Streptomycin.” American Journal of Public Health and the Nations Health 41: 1375–1380. doi:https://doi.org/10.2105/AJPH.41.11_Pt_1.1375.
- Sumners, L. H., K. B. Miska, M. C. Jenkins, R. H. Fetterer, C. M. Cox, S. Kim, and R. A. Dalloul. 2011. “Expression of Toll-like Receptors and Antimicrobial Peptides during Eimeria Praecox Infection in Chickens.” Experimental Parasitology 127: 714–718. doi:https://doi.org/10.1016/j.exppara.2010.12.002.
- Tang, Z., Y. Yin, Y. Zhang, R. Huang, Z. Sun, T. Li, W. Chu, et al. 2008. “Effects of Dietary Supplementation with an Expressed Fusion Peptide Bovine Lactoferricin–Lactoferrampin on Performance, Immune Function and Intestinal Mucosal Morphology in Piglets Weaned at Age 21 D.” British Journal of Nutrition 101: 998–1005. doi:https://doi.org/10.1017/S0007114508055633.
- Tanhaiean, A., M. Azghandi, R. J. Razmyar, E. Mohammadi, and M. H. Sekhavati. 2018. “Recombinant Production of a Chimeric Antimicrobial Peptide in E. Coli and Assessment of Its Activity against Some Avian Clinically Isolated Pathogens.” Microbial Pathogenesis 122: 73–78. doi:https://doi.org/10.1016/j.micpath.2018.06.012.
- Tello-Montoliu, A., J. V. Patel, and G. Y. Lip. 2006. “Angiogenin: A Review of the Pathophysiology and Potential Clinical Applications.” Journal of Thrombosis and Haemostasis 4 (9): 1864–1874. doi:https://doi.org/10.1111/j.1538-7836.2006.01995.x.
- Tessari, E. N., A. M. Castiglioni, I. Kanashiro, G. F. Z. Stoppa, R. L. Luciano, A. Guilherme, M. De Castro, and A. L. S. P. Cardoso. 2012. “Important Aspects of Salmonella in the Poultry Industry and in Public Health.” Salmonella - A Dangerous Foodborne Pathogen. doi:https://doi.org/10.5772/30812.
- Townes, C. L., G. Michailidis, and J. Hall. 2009. “The Interaction of the Antimicrobial Peptide cLEAP-2 and the Bacterial Membrane.” Biochemical and Biophysical Research Communications 387 (3): 500–503. doi:https://doi.org/10.1016/j.bbrc.2009.07.046.
- Townes, C. L., G. Michailidis, C. J. Nile, and J. Hall. 2004. “Induction of Cationic Chicken Liver-expressed Antimicrobial Peptide 2 in Response to Salmonella Enterica Infection.” Infection and Immunity 72 (12): 6987–6993. doi:https://doi.org/10.1128/IAI.72.12.6987-6993.2004.
- Turner, J., Y. Cho, Dinh, N. N. Waring, and R. I. Lehrer. 1998. “Activities of LL-37, a Cathelin-Associated Antimicrobial Peptide of Human Neutrophils.” Antimicrobial Agents and Chemotherapy 42: 2206–2214. doi:https://doi.org/10.1128/AAC.42.9.2206.
- Van Boeckel, T. P., C. Brower, M. Gilbert, B. T. Grenfell, S. A. Levin, T. P. Robinson, A. Teillant, et al. 2015. “Global Trends in Antimicrobial Use in Food Animals.” Proceedings of the National Academy of Sciences of the United States of America 112 (18): 5649–5654. doi:https://doi.org/10.1073/pnas.1503141112.
- Van Dijk, A., E. J. A. Veldhuizen, S. I. C. Kalkhove, J. L. M. Tjeerdsma-van Bokhoven, R. A. Romijn, and H. P. Haagsman. 2007. “The β-defensin Gallinacin-6 Is Expressed in the Chicken Digestive Tract and Has Antimicrobial Activity against Food-borne Pathogens.” Antimicrobial Agents and Chemotherapy 51: 912–922. doi:https://doi.org/10.1128/AAC.00568-06.
- Van Immerseel, F., V. De Buck, J. Pasmans, F. Huyghebaert, G. Haesebrouck, and F. R. Ducatelle. 2004. “Clostridium Perfringens in Poultry: An Emerging Threat for Animal and Public Health.” Avian Pathology 33: 537–549. doi:https://doi.org/10.1080/03079450400013162.
- Van Immerseel, F., J. I. Rood, R. J. Moore, and R. W. Titball. 2009. “Rethinking Our Understanding of the Pathogenesis of Necrotic Enteritis in Chickens.” Trends in Microbiology 17 (1): 32–36. doi:https://doi.org/10.1016/j.tim.2008.09.005.
- Veldhuizen, E. J. A., E. C. Brouwer, V. A. F. Schneider, and A. C. Fluit. 2013. “Chicken Cathelicidins Display Antimicrobial Activity against Multiresistant Bacteria without Inducing Strong Resistance.” PLoS One 8: e61964. doi:https://doi.org/10.1371/journal.pone.0061964.
- Walker, C. R., I. Hautefort, J. E. Dalton, K. Overweg, C. E. Egan, R. J. Bongaerts, D. J. Newton, et al. 2013. “Intestinal Intraepithelial Lymphocyte-enterocyte Crosstalk Regulates Production of Bactericidal Angiogenin 4 by Paneth Cells upon Microbial Challenge.” PLoS One 8: e84553. doi:https://doi.org/10.1371/journal.pone.0084553.
- Wang, D., W. Ma, R. She, Q. Sun, Y. Liu, Y. Hu, L. Liu, Y. Yang, and K. Peng. 2009. “Effects of Swine Gut Antimicrobial Peptides on the Intestinal Mucosal Immunity in Specific-Pathogen-Free Chickens.” Poultry Science 88: 967–974. doi:https://doi.org/10.3382/ps.2008-00533.
- Wang, S., X. F. Zeng, Q. W. Wang, J. L. Zhu, Q. Peng, C. L. Hou, P. Thacker, and S. Y. Qiao. 2015. “The Antimicrobial Peptide Sublancin Ameliorates Necrotic Enteritis Induced by Clostridium Perfringens in Broilers.” Journal of Animal Science 93: 4750–4760. doi:https://doi.org/10.2527/jas.2015-9284.
- Wang, Y., M. Wang, A. Shan, and X. Feng. 2020. “Avian Host Defense Cathelicidins: Structure, Expression, Biological Functions, and Potential Therapeutic Applications.” Poultry Science 99: 6434–6445. doi:https://doi.org/10.1016/j.psj.2020.09.030.
- Wegener, H. C., F. M. Aarestrup, L. B. Jensen, A. M. Hammerum, and F. Bager. 1999. “Use of Antimicrobial Growth Promoters in Food Animals and Enterococcus Faecium Resistance to Therapeutic Antimicrobial Drugs in Europe.” Emerging Infectious Diseases 5: 329–335. doi:https://doi.org/10.3201/eid0503.990303.
- Wierup, M. 2001. “The Swedish Experience of the 1986 Year Ban of Antimicrobial Growth Promoters, with Special Reference to Animal Health, Disease Prevention, Productivity, and Usage of Antimicrobials.” Microbial Drug Resistance 7 (2): 183–190. doi:https://doi.org/10.1089/10766290152045066.
- Wimley, W. C. 2010. “Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model.” ACS Chemical Biology 5 (10): 905–917. doi:https://doi.org/10.1021/cb1001558.
- Wu, S., D. Stanley, N. Rodgers, R. A. Swick, and R. J. Moore. 2014. “Two Necrotic Enteritis Predisposing Factors, Dietary Fishmeal and Eimeria Infection, Induce Large Changes in the Caecal Microbiota of Broiler Chickens.” Veterinary Microbiology 169 (3–4): 188–197. doi:https://doi.org/10.1016/j.vetmic.2014.01.007.
- Xia, X., L. Zhang, and Y. Wang. 2015. “The Antimicrobial Peptide cathelicidin-BF Could Be a Potential Therapeutic for Salmonella Typhimurium Infection.” Microbiological Research 171: 45–51. doi:https://doi.org/10.1016/j.micres.2014.12.009.
- Xiao, Y., Y. Cai, Y. R. Bommineni, S. C. Fernando, S. C. Prakash, S. E. Gilliland, Zhang, and G. Zhang. 2006b. “Identification and Functional Characterization of Three Chicken Cathelicidins with Potent Antimicrobial Activity.” Journal of Biological Chemistry 281 (5): 2858–2867. doi:https://doi.org/10.1074/jbc.M507180200.
- Xiao, Y., H. Dai, Y. R. Bommineni, J. L. Soulages, Y. Gong, O. Prakash, and G. Zhang. 2006a. “Structure–activity Relationships of Fowlicidin-1, a Cathelicidin Antimicrobial Peptide in Chicken.” FEBS Journal 273: 2581–2593. doi:https://doi.org/10.1111/j.1742-4658.2006.05261.x.
- Yacoub, H. A., A. M. Elazzazy, O. A. H. Abuzinadah, A. M. Al-Hejin, M. M. Mahmoud, and S. M. Harakeh. 2015. “Antimicrobial Activities of Chicken β-defensin (4 and 10) Peptides against Pathogenic Bacteria and Fungi.” Frontiers in Cellular and Infection Microbiology 17. doi:https://doi.org/10.3389/fcimb.2015.00036.
- Yang, Y., Y. Jiang, Q. Yin, H. Liang, and R. She. 2010. “Chicken Intestine Defensins Activated Murine Peripheral Blood Mononuclear Cells through the TLR4-NF-κB Pathway.” Veterinary Immunology and Immunopathology 133 (1): 59–65. doi:https://doi.org/10.1016/j.vetimm.2009.07.008.
- Yegani, M., and D. R. Korver. 2008. “Factors Affecting Intestinal Health in Poultry.” Poultry Science 87: 2052–2063. doi:https://doi.org/10.3382/ps.2008-00091.
- Yitbarek, A., H. Echeverry, P. Munyaka, and J. C. Rodriguez-Lecompte. 2015. “Innate Immune Response of Pullets Fed Diets Supplemented with Prebiotics and Synbiotics.” Poultry Science 94: 1802–1811. doi:https://doi.org/10.3382/ps/pev147.
- Zelezetsky, I., and A. Tossi. 2006. “Alpha-Helical Antimicrobial Peptides–Using a Sequence Template to Guide Structure-Activity Relationship Studies.” Biochimica Et Biophysica Acta 1758: 1436–1449. doi:https://doi.org/10.1016/j.bbamem.2006.03.021.
- Zhang, G., and L. T. Sunkara. 2014. “Avian Antimicrobial Host Defense Peptides: From Biology to Therapeutic Applications.” Pharmaceuticals 7: 220–247. doi:https://doi.org/10.3390/ph7030220.
- Zhao, C. Q., T. Nguyen, L. Liu, R. E. Sacco, K. A. Brogden, R. I. Lehrer, and R. N. Moore. 2001. “Gallinacin-3, an Inducible Epithelial β-defensin in the Chicken.” Infection and Immunity 69 (4): 2684–2691. doi:https://doi.org/10.1128/IAI.69.4.2684-2691.2001.
- Zhu, N., J. Wang, L. Yu, Q. Zhang, K. Chen, and B. Liu. 2019. “Modulation of Growth Performance and Intestinal Microbiota in Chickens Fed Plant Extracts or Virginiamycin.” Frontiers in Microbiology 10. doi:https://doi.org/10.3389/fmicb.2019.01333.