ABSTRACT
1. Due to seasonal breeding, geese breeds from Southern China have low egg yield. The genetic makeup underlying performance of local breeds is largely unknown, and few studies have investigated this problem. This study integrated 21 newly generated and 50 publicly existing RNA-seq libraries, representing the hypothalamus, pituitary and testis, to identify candidate genes and importantly related pathways associated with seasonal breeding in male Lion-Head geese.
2. In total, 19, 119 and 302 differentially expressed genes (DEGs) were detected in the hypothalamus, pituitary and testis, respectively, of male Lion-Head geese between non-breeding and breeding periods. These genes were significantly involved in the neuropeptide signalling pathway, gland development, neuroactive ligand-receptor interaction, JAK-STAT signalling pathway, cAMP signalling pathway, PI3K-Akt signalling pathway and Foxo signalling pathway.
3. By integrating another 50 RNA-seq samples 4, 18 and 40 promising DEGs were confirmed in hypothalamus, pituitary and testis, respectively.
4. HOX genes were identified as having important roles in the development of testis between non-breeding and breeding periods of male Lion-Head geese.
Introduction
Lion-Head geese, originating from Raoping city of Guangdong province in China, is one of the largest species and is well-known for its meat worldwide (Zhang Citation1991; Zhao et al. Citation2019). However, because of seasonal breeding, this breed has low egg yield with an average of 25–30 eggs per year, and such low reproduction severely hinders the development of the local industry (Zhuang and Lin Citation2006). The annual cycle of reproductive quiescence and recrudescence is primarily environmentally governed by photoperiod (Bronson and Heideman Citation1994; Yoshimura Citation2013). For Lion-Head geese, reproductive activity occurs during autumn and winter with short day lengths and anoestrus occurs in spring and summer with long day lengths. To date, there are very few studies to elucidate the genetics underlying seasonal breeding of Lion-Head geese.
The hypothalamic-pituitary-gonadal (HPG) axis is the core organ responsible for the endocrine system and reproductive activities in vertebrates (Roch et al. Citation2011; Yoshimura Citation2013). For seasonal animals, the HPG axis is activated during the breeding season, and is inactivated during the non-breeding period. During the shift from reproduction to non-reproduction and vice versa, gonadal size and weight undergo dramatic change (Nicholls et al. Citation1988; Dawson et al. Citation2001; Hahn and MacDougall-Shackleton Citation2008). Correspondingly, significant changes in endocrine secretions and gene expression of the HPG axis should exist between the breeding and the non-breeding seasons. Additionally, chickens and ducks have been selected to respond less to seasonality. Thus, it is of great interest to compare transcriptomes of the HPG axis of Lion-Head geese between breeding and non-breeding periods, and further compare those with chickens, ducks and pigeons in the breeding period to detect key genes and pathways affecting seasonal breeding of Lion-Head geese.
The main objective of this study was to explore promising candidate genes and significantly-related pathways regulating seasonal breeding in male Lion-Head geese. This preliminary investigation will lay the foundation of further studying the genetic mechanism of seasonal breeding and will be helpful for the molecular selection of non-seasonal breeding.
Materials and methods
Ethical statement
The hypothalamus, pituitary and testis samples from seven male Lion-Head geese were collected while adhering to the strict codes of Animal Management Rule of the National Health and Family Planning Commission, People’s Republic of China (Documentation 55, 2001). The research protocol was reviewed and approved by the Animal Care and Use Committee of Zhongkai University of Agriculture and Engineering. Another 53 RNA-seq data sets were publicly available and downloaded from the NCBI SRA dataset.
Samples
A total of 53 RNA-seq data sets from of hypothalamic, pituitary and testicular tissues from chickens, ducks and pigeons, during the egg-laying period, were collected from the NCBI SRA database. Except for five samples of testis from pigeons, six samples for each tissue in each species were collected (Table S1).
Transcriptome data of hypothalamus, pituitary and testis was generated from male Lion-Head geese from the breeding and non-breeding periods. A total of seven healthy male geese, three from the breeding period and four from the non-breeding period, were used. Hypothalamus, pituitary and testis samples were taken after euthanasia by exsanguination at a nearby slaughterhouse. All tissue samples were immediately frozen in liquid nitrogen and stored at −80°C prior to use.
Total RNAs were isolated using Trizol Reagent (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s instructions. The quality and quantity of RNA samples were detected by 1.5% agarose gel electrophoresis and absorbance OD (optical density) at 260/280 nm ratio, respectively. The RNA sequencing was carried out as follows. Briefly, the libraries were prepared following TruSeq TM RNA sample preparation Kit from Illumina (San Diego, CA) by using 1 μg of total RNA. Libraries were selected for cDNA target fragments of 200–300 bp on 2% Low Range Ultra Agarose, and PCR amplification was then performed using Phusion DNA polymerase (NEB) for 15 PCR cycles. Paired-end libraries were quantified by TBS380 and then sequenced with the Illumina HiSeq PE 2 × 151bp read length.
Quantification of gene expression
A stringent and uniform bioinformatics pipeline was applied to filter RNA-seq samples and to obtain normalised gene expression values. Firstly, raw reads were filtered to remove adaptors and low-quality reads by Trimmomatic v0.39 (Bolger et al. Citation2014) with parameters: adapters/TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36. STAR (v2.7.3a) (Dobin et al. Citation2013) was employed to build the genome index, and then clean reads were mapped against the species reference genome (Table S1) with parameters: quantMode GeneCounts, chimSegmentMin 10 and outFilterMismatchNmax 3. Three RNA-seq samples were removed from duck testis due to the small number of uniquely mapped reads and the low uniquely mapping rates. All the remaining 71 samples had a uniquely mapped reads >300 K and unique mapping rates of >53% (Table S1). Finally, normalised transcripts were obtained per million (TPM) of Ensembl genes by Stringtie (v2.1.4) (Kovaka et al. Citation2019) with default parameters.
Bioinformatics analysis
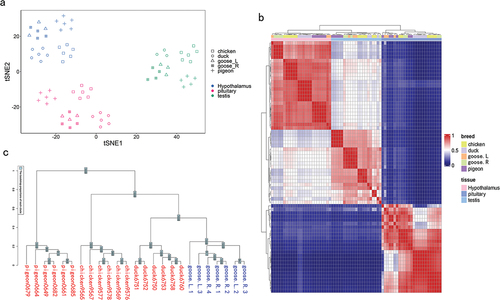
In total, 7,847 1:1 orthologous genes were extracted across the four poultry species (Figure S1(a)), and all RNA-seq samples were visualised on gene expressions log2(TPM+1) using t-Distributed Stochastic Neighbour Embedding (t-SNE) using the R package Rtsne (v0.15) (Li et al. Citation2017). The package pheatmap (v1.0.12) (Kolde Citation2012) was used to show the gene expression pattern of all RNA-seq samples. The gene expression phylogeny was analysed for each tissue across the four species by the Ape (v5.5) package (Paradis and Schliep Citation2019).
The gene differential expression analysis for geese was performed for the non-breeding period vs. the breeding period and for geese during the non-breeding period vs. other poultry (chickens, ducks or pigeons) during the breeding period by using R package DEGSeq2 (Wang et al. Citation2010). The Benjamini-Hochberg method (P < 0.05) and abs(log2(fold change)>1 were used as cut-offs for defining differentially expressed genes (DEGs) for geese during the non- breeding period vs. the breeding period, while P < 0.01 and abs(log2(fold change)>1 were used for geese during the non-breeding period vs. other poultry. Gene Ontology (GO) and KEGG enrichment analysis was conducted for DEGs using enrichGO and enrichKEGG in R package clusterProfiler v4.0.4 (Wu et al. Citation2021). The STRING v11.5 (Szklarczyk et al. Citation2019) and Cytoscape v3.8.2 (Shannon et al. Citation2003) packages were used to construct the protein-protein interaction (PPI) network for important DEGs.
Quantitative real-time PCR (qRT-PCR)
To confirm the detection of DEGs, nine were selected for qRT-PCR validation. The qRT-PCR primers were designed using Oligo 7.0 software (http://www.oligo.net/) and synthesised by Sangon Biotech (Shanghai) Co., Ltd (). The β-actin gene was used as an internal control. The reaction system (20 μl) comprised 10 μl SYBR Green PCR Master Mix, 0.5 μl forward primer, 0.5 μl reverse primer, 1 μl cDNA and 8 μl ddH2O. The reaction condition was as follows: 1 cycle at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 s and annealing at 60°C for 60 s. Three replicates were undertaken for each sample. The qRT-PCR results were analysed using the 2−△△CT method (Livak and Schmittgen Citation2001).
Table 1. Primers used for quantitative real-time PCR.
Results
Gene expression pattern across four poultry species
As shown in , samples were clearly clustered by tissue type in terms of gene expression patterns. Within each tissue, samples were further clustered by breed. The heatmap of gene expression across all samples showed a similar pattern to that from PCA (). The gene expression tree showed breed-specific divergences for all tissues, which was consistent with the known avian phylogeny (, S1(b,c)).
Differential expression analysis
By comparing gene expression in male Lion-Head geese between non-breeding and breeding periods, 19, 119 and 302 DEGs were detected in hypothalamus, pituitary and testis, respectively (, Table S2). By using gene expression from geese in the non-breeding period as the benchmark, the number of up-regulated DEGs were 11, 67 and 22 in the hypothalamus, pituitary and testis, respectively, while down-regulated DEGs were 8, 52 and 279.
Figure 2. The overall analysis of differentially expressed genes (DEGs). (a) The number of DEGs including up-regulated and down-regulated genes for male Lion-Head geese within breeding and non-breeding periods. (b) The number of overlapping DEGs between geese within the non-breeding period (goose.R) and chickens, ducks or pigeons within the breeding period in the hypothalamus. (c) Top 10 pathways in the KEGG enrichment of 119 DEGs for the pituitary. (d) Top 10 pathways in the KEGG enrichment of 302 DEGs for the testis.
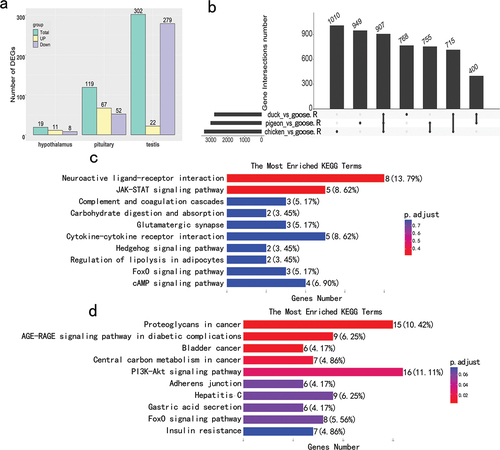
Due to the small number of (n = 19) DEGs detected in hypothalamus, GO and KEGG enrichment analysis were only performed for those detected in the pituitary and testis. Among the top 10 KEGG enriched pathways, Neuroactive ligand-receptor interaction, JAK-STAT signalling pathway and cytokine-cytokine receptor interaction were mostly enriched in pituitary (), while proteoglycans in cancer, PI3K-Akt signalling pathway and AGE-RAGE signalling in diabetic complications were enriched in testis (). Among the top 10 GO biological processes, negative regulation of proteolysis, negative regulation of peptidase activity and adenylate cyclase-modulating G protein-coupled receptor signalling pathway were mostly enriched in pituitary, while pattern specification process, skeletal system development and gland development for testis (Figure S2(a,b)).
Through differential expression analysis of genes from Lion-Head geese in the non-breeding period and other poultry (i.e. chicken, duck and pigeon) in the breeding period, several DEGs in hypothalamus (907), pituitary (1087) and testis (711) were found that shared all three pairwise comparisons by the gene intersection analysis (, S3(a,b)).
Candidate genes for seasonal breeding
In total, 4, 18 and 40 promising DEGs in hypothalamus, pituitary and testis were obtained from Lion-Head geese between non-breeding and breeding periods which overlapped with DEGs between geese in the non-breeding period and other poultry species in the breeding period (, Table S3). In the hypothalamus, compared to geese in the non-breeding period, the expression of tyrosine hydroxylase (TH) gene in geese and the other three poultry species during breeding was down-regulated. In contrast, Solute Carrier Family 24 Member 4 (SLC24A4), WAS/WASL Interacting Protein Family Member 3 (WIPF3) and Leucine Rich Repeat Transmembrane Neuronal 2 (LRRTM2) were up-regulated. Similarly, among 18 DEGs in pituitary and 40 DEGs in testis, there were six and 36 down-regulated genes, and three and one up-regulated genes, respectively. The gene expression distributions of 40 and 18 DEGs in testis and pituitary across poultry species are shown in and S4(a), respectively.
Figure 3. The survey of key differentially expressed genes (DEGs). (a) Venn diagrams of two kinds of DEGs for the hypothalamus, the pituitary and the testis. A: The number of DEGs shared in geese within the non-breeding period (goose.R) and chickens, ducks and pigeons, B: The number of DEGs for male Lion-Head geese within breeding and non-breeding periods. (b) The distribution of expression of 40 DEGs across poultry in the testis. (c) The protein-protein interaction network for 4 DEGs of the hypothalamus. (d) qPCR verification of 4 DEGs in the hypothalamus of male Lion-Head geese. (d) qPCR verification of 5 HOX DEGs in the testis of male Lion-Head geese. L: male Lion-Head geese within the breeding period, R: male Lion-Head geese within the non-breeding period.
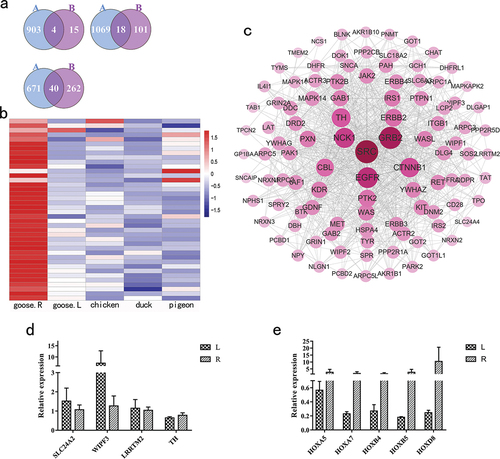
The DEGs detected in pituitary and testis were significantly involved in some important GO and KEGG pathways (Table S4). For example, Prostaglandin E Receptor 3 (PTGER3), Somatostatin Receptor 5 (SSTR5) and Hyperpolarisation Activated Cyclic Nucleotide Gated Potassium and Sodium Channel 2 (HCN2) are involved in the cAMP signalling pathway in pituitary, while Phosphoenolpyruvate Carboxykinase 1(PCK1), Homeobox A3 (HOXA3), Forkhead Box A1(FOXA1) and Homeobox A5 (HOXA5) are related to gland development in testis. The PPI network showed that TH was the core gene among the 4 DEGs in hypothalamus, while Heat Shock Protein 90 Alpha Family Class B Member 1 (HSP90AB1) was central for the 18 DEGs in pituitary and Apolipoprotein B (APOB) for the 40 DEGs in testis ( and S4(b,c)). These findings indicated that these DEGs might play important roles in controlling seasonal reproductive activities.
Next, qPCR was used to confirm the expression profiles obtained from RNA-seq for SLC24A4, WIPF3, LRRTM2 and TH in hypothalamus and five HOX genes in testis. For male Lion-Head geese within non-breeding and breeding periods, the expression patterns of nine genes obtained by qPCR were consistent with those obtained by RNA-seq (). The similar expression patterns of these nine genes, except for HOXB4, were further validated in male Magang geese during non-breeding and breeding periods (Figure S4(a,b)).
Discussion
Seasonal breeding is a reproductive strategy in most of local Chinese goose breeds (Huang et al. Citation2008; Shi et al. Citation2008). To date, there are few studies investigating the genetics underlying seasonal breeding of Chinese goose breeds. This study is the first in Lion-Head geese which has identified important genes and related pathways associated with seasonal breeding by integrating RNA-seq data of HPG axis across geese, chickens, ducks and pigeons. The four genes from the hypothalamus and five HOX genes in the testis were validated in Lion-Head geese and Magang geese by qPCR.
Among these, four key DEGs in the hypothalamus, SLC24A4, WIPF3 and LRRTM2 were all up-regulated in geese and the other three poultry species within the breeding period compared with thin the non-breeding period, while TH was down-regulated. TH is the rate limiting enzyme in dopamine biosynthesis in the hypothalamus, a core tissue controlling seasonal reproduction of birds (Kang et al. Citation2007; El Halawani et al. Citation2009; Kang et al. Citation2010). There is little information regarding the genes SLC24A4, WIPF3 and LRRTM2 concerning their role in reproduction, but the current findings suggested their potentially regulating seasonal activities of Lion-Head geese.
In the pituitary, six out of 18 DEGs (i.e. dehydrogenase/reductase11 (DHRS11), DNAJ heat shock protein family (Hsp40) member C12 (DNAJC12), Prostaglandin E Receptor 3 (PTGER3), Somatostatin Receptor 5 (SSTR5), GTP binding protein overexpressed in skeletal muscle (GEM) and Solute carrier family 35 member F3 (SLC35F3)) were down-regulated in poultry within the breeding period. Three DEGs (i.e. Netrin 1 (NTN1), Prostaglandin E receptor 4 (PTGER4) and 24-dehydrocholesterol reductase (DHCR24)) were up-regulated. Another nine DEGs, i.e. Solute Carrier Family 7 Member 5 (SLC7A5), Heat Shock Protein 90 Alpha Family Class B Member 1 (HSP90AB1), Glutaminyl-Peptide Cyclotransferase (QPCT), RAS p21 Protein Activator 3 (RASA3), SPARC Related Modular Calcium Binding 2 (SMOC2), Hyperpolarization Activated Cyclic Nucleotide Gated Potassium and Sodium Channel 2 (HCN2), Leucine Zipper Tumour Suppressor 1 (LZTS1), Limb Development Membrane Protein 1 Like (LMBR1 L) and LanC Like 2 (LANCL2) were not consistent between geese and the other three species. The species studied may have different core gene expression patterns of regulating reproductive activities. The pituitary may be an important organ to control the reproductive differences across poultry. The PTGER3, PTGER4 and SSTR5 genes were involved in neuroactive ligand-receptor interaction and the cAMP signalling pathway, which was consistent with previous studies which showed that PTGER3 and PTGER4 play important roles in the influence of polyunsaturated fatty acids on male and female reproduction. Receptor subtype of SSTR5 may be an important determinant of GH secretion in the pituitary (Greenman and Melmed Citation1994; Wathes et al. Citation2007).
The phenomenon that the gonadal size changed more than a hundred-fold between breeding and non-breeding periods allowed detection of more DEGs in testes compared with the hypothalamus and pituitary. Among 40 DEGs, there were 36 genes, such as PCK1, HOXA3, FOXA1 and HOXA5, down-regulated in geese and the other three species within the breeding period compared with geese within the non-breeding period. Another four DEGs (i.e. Sortilin Related Receptor 1 (SORL1), Thromboxane A2 Receptor (TBXA2 R), Zinc Finger Protein 521 (ZNF521) and Fibronectin type III Domain Containing 5 (FNDC5)) were not consistent between geese and other birds in the breeding period (Table S3).
Interestingly, many HOX genes, such as HOXB4, HOXA3 and HOXD8 were detected, and were related to gland development, skeletal system morphogenesis and pattern specification process. Previous studies have reported that HOX genes are highly conserved between species and play important roles in function of the female reproductive tract and human implantation (Taylor Citation2000a, Citation2000b; Du and Taylor Citation2015). The current study demonstrated the important role of HOX genes in the development of testis across poultry species, but analysis of their specific function needs deduction.
For GO pathways, the adenylate cyclase-modulating G protein-coupled receptor signalling pathway and neuropeptide signalling pathway in the pituitary were reported to be important for hormone secretion functions of the pituitary (Tatsuno et al. Citation1991). The testis had several important GO terms of pattern specification process, skeletal system development and gland development, which confirmed previous studies. These pathways were reported to be involved in proliferation and apoptosis of testicular cells and the development of the testis (Cool and Capel Citation2009). In the KEGG pathways, there was a Neuroactive ligand-receptor interaction, JAK-STAT signalling pathway, cAMP signalling pathway and Foxo signalling pathway in pituitary, and PI3K-Akt signalling pathway, AGE-RAGE signalling pathway in diabetic complications and Foxo signalling pathway in testis. The neuroactive ligand-receptor interaction pathway was reported to be important for reproduction in chickens (Zhang et al. Citation2019), ducks (Tao et al. Citation2017) and geese (Ouyang et al. Citation2020). The JAK-STAT signalling pathway is indispensable and pivotal in many biological processes including immunity and inflammatory response (Yang and Zhang Citation2017). It has been reported as a mediator in human spermatozoa function (Lachance and Leclerc Citation2011). The cAMP signalling and PI3K-Akt signalling pathways were reportedly involved in oocyte maturation and ovulation in mammals (Conti et al. Citation2012). The Foxo signalling pathway was reported to regulate reproductive activities (Brosens et al. Citation2009; Edmonds et al. Citation2010; Christian et al. Citation2011).
The study generated RNA-seq data from hypothalamus, pituitary and testis of male Lion-Head geese in non-breeding and breeding periods to detect DEGs and related pathways affecting seasonal breeding. Furthermore, more promising candidate genes from these DEGs were identified by comparing public RNA-seq data related to the HPG axis from chickens, ducks and pigeons in the breeding period with Lion-Head geese in the non-breeding period. Interestingly, most of these candidate genes had the same direction of expression across all four poultry species. These may play important roles in the seasonal breeding of Lion-Head geese. Investigating the genetic network underlying seasonal breeding will become easier as more RNA-seq data of the HPG axis from quail, sheep or other seasonal breeders, both in non-breeding and breeding periods, become available.
Table S1-4
Download MS Excel (74.8 KB)Supplementary Figures S1-S5
Download MS Word (2.6 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The RNA-seq datasets of hypothalamus, pituitary and testis from geese are available in the Sequence Read Archive of the National Center for Biotechnology Information (accession number: PRJNA885911).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00071668.2022.2152651
Additional information
Funding
References
- Bolger, A. M., M. Lohse, and B. Usadel. 2014. “Trimmomatic: A Flexible Trimmer for Illumina Sequence Data.” Bioinformatics 30 (15): 2114–2120. doi:10.1093/bioinformatics/btu170.
- Bronson, F. H., and P. D. Heideman. 1994. “Seasonal Regulation of Reproduction in mammals.” The Physiology of Reproduction 2: 541–583.
- Brosens, J. J., M. S. Wilson, and E. W. Lam. 2009. “FOXO Transcription Factors: From Cell Fate Decisions to Regulation of Human Female Reproduction.” Advances in Experimental Medicine and Biology 665: 227–241. doi:10.1007/978-1-4419-1599-3_17.
- Christian, M., E. W. Lam, M. S. Wilson, and J. J. Brosens. 2011. “FOXO Transcription Factors and Their Role in Disorders of the Female Reproductive Tract.” Current Drug Targets 12 (9): 1291–1302. doi:10.2174/138945011796150253.
- Conti, M., M. Hsieh, A. M. Zamah, and J. S. Oh. 2012. “Novel Signalling Mechanisms in the Ovary During Oocyte Maturation and Ovulation.” Molecular and Cellular Endocrinology 356 (1–2): 65–73. doi:10.1016/j.mce.2011.11.002.
- Cool, J., and B. Capel. 2009. “Mixed Signals: Development of the Testis.” Seminars in Reproductive Medicine 27 (1): 5–13. doi:10.1055/s-0028-1108005.
- Dawson, A., V. M. King, G. E. Bentley, and G. F. Ball. 2001. “Photoperiodic Control of Seasonality in Birds.” Journal of Biological Rhythms 16 (4): 365–380. doi:10.1177/074873001129002079.
- Dobin, A., C. A. Davis, F. Schlesinger, J. Drenkow, C. Zaleski, S. Jha, P. Batut, M. Chaisson, and T. R. Gingeras. 2013. “STAR: ultrafast universal RNA-seq aligner.” Bioinformatics 29 (1): 15–21. doi:10.1093/bioinformatics/bts635.
- du, H., and H. S. Taylor. 2015. “The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility.” Cold Spring Harbor Perspectives in Medicine 6 (1): a023002. doi:10.1101/cshperspect.a023002.
- Edmonds, J. W., J. K. Prasain, D. Dorand, Y. Yang, H. D. Hoang, J. Vibbert, H. M. Kubagawa, and M. A. Miller. 2010. “Insulin/FOXO Signalling Regulates Ovarian Prostaglandins Critical for Reproduction.” Developmental Cell 19 (6): 858–871. doi:10.1016/j.devcel.2010.11.005.
- el Halawani, M. E., S. W. Kang, B. Leclerc, S. Kosonsiriluk, and Y. Chaiseha. 2009. “Dopamine-Melatonin Neurons in the Avian Hypothalamus and Their Role as Photoperiodic Clocks.” General & Comparative Endocrinology 163 (1–2): 123–127. doi:10.1016/j.ygcen.2008.11.030.
- Greenman, Y., and S. Melmed. 1994. “Expression of Three Somatostatin Receptor Subtypes in Pituitary Adenomas: Evidence for Preferential SSTR5 Expression in the Mammosomatotroph Lineage.” Journal of Clinical Endocrinology & Metabolisam 79 (3): 724–729. doi:10.1210/jcem.79.3.7521350.
- Hahn, T. P., and S. A. MacDougall-shackleton. 2008. “Adaptive Specialization, Conditional Plasticity and Phylogenetic History in the Reproductive Cue Response Systems of Birds.” Philosophical Transactions-Royal Society Biological Sciences 363 (1490): 267–286. doi:10.1098/rstb.2007.2139.
- Huang, Y. M., Z. D. Shi, Z. Liu, Y. Liu, and X. W. Li. 2008. “Endocrine Regulations of Reproductive Seasonality, Follicular Development and Incubation in Magang Geese.” Animal Reproduction Science 104 (2–4): 344–358. doi:10.1016/j.anireprosci.2007.02.005.
- Kang, S. W., B. Leclerc, S. Kosonsiriluk, L. J. Mauro, A. Iwasawa, and M. E. el Halawani. 2010. “Melanopsin Expression in Dopamine-Melatonin Neurons of the Premammillary Nucleus of the Hypothalamus and Seasonal Reproduction in Birds.” Neuroscience 170 (1): 200–213. doi:10.1016/j.neuroscience.2010.06.082.
- Kang, S. W., A. Thayananuphat, T. Bakken, and M. E. el Halawani. 2007. “Dopamine-Melatonin Neurons in the Avian Hypothalamus Controlling Seasonal Reproduction.” Neuroscience 150 (1): 223–233. doi:10.1016/j.neuroscience.2007.08.031.
- Kolde, R. 2012. “Pheatmap: Pretty Heatmaps.” R Package 1 (2): 726.
- Kovaka, S., A. V. Zimin, G. M. Pertea, R. Razaghi, S. L. Salzberg, and M. Pertea. 2019. “Transcriptome Assembly from Long-Read RNA-Seq Alignments with StringTie2.” Genome Biology 20 (1): 278. doi:10.1186/s13059-019-1910-1.
- Lachance, C., and P. Leclerc. 2011. “Mediators of the Jak/STAT Signalling Pathway in Human Spermatozoa.” Biology of Reproduction 85 (6): 1222–1231. doi:10.1095/biolreprod.111.092379.
- Li, W., J. E. Cerise, Y. Yang, and H. Han. 2017. “Application of T-SNE to Human Genetic Data.” Journal of Bioinformatics and Computational Biology 15 (4): 1750017. doi:10.1142/s0219720017500172.
- Livak, K. J., and T. D. Schmittgen. 2001. “Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2− ΔΔCT Method.” Methods 25 (4): 402–408. doi:10.1006/meth.2001.1262.
- Nicholls, T. J., A. R. Goldsmith, and A. Dawson. 1988. “Photorefractoriness in Birds and Comparison with Mammals.” Physiological Reviews 68 (1): 133–176. doi:10.1152/physrev.1988.68.1.133.
- Ouyang, Q., S. Hu, G. Wang, J. Hu, J. Zhang, L. Li, B. Hu, et al. 2020. “Comparative Transcriptome Analysis Suggests Key Roles for 5-Hydroxytryptamlne Receptors in Control of Goose Egg Production.” Genes (Basel) 11 (4): 455. doi:10.3390/genes11040455.
- Paradis, E., and K. Schliep. 2019. “Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R.” Bioinformatics 35 (3): 526–528. doi:10.1093/bioinformatics/bty633.
- Roch, G. J., E. R. Busby, and N. M. Sherwood. 2011. “Evolution of GnRh: Diving Deeper.” General & Comparative Endocrinology 171 (1): 1–16. doi:10.1016/j.ygcen.2010.12.014.
- Shannon, P., A. Markiel, O. Ozier, N. S. Baliga, J. T. Wang, D. Ramage, N. Amin, B. Schwikowski, and T. Ideker. 2003. “Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks.” Genome Research 13 (11): 2498–2504. doi:10.1101/gr.1239303.
- Shi, Z. D., Y. B. Tian, W. Wu, and Z. Y. Wang. 2008. “Controlling Reproductive Seasonality in the Geese: A Review.” World’s Poultry Science Journal 64 (3): 343–355. doi:10.1017/S0043933908000081.
- Szklarczyk, D., A. L. Gable, D. Lyon, A. Junge, S. Wyder, J. Huerta-cepas, M. Simonovic, et al. 2019. “STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets.” Nucleic Acids Research 47 (D1): D607–613. doi:10.1093/nar/gky1131.
- Tao, Z., W. Song, C. Zhu, W. Xu, H. Liu, S. Zhang, and L. Huifang. 2017. “Comparative Transcriptomic Analysis of High and Low Egg-Producing Duck Ovaries.” Poultry Science 96 (12): 4378–4388. doi:10.3382/ps/pex229.
- Tatsuno, I., A. Somogyvari-vigh, K. Mizuno, P. E. Gottschall, H. Hidaka, and A. Arimura. 1991. “Neuropeptide Regulation of Interleukin-6 Production from the Pituitary: Stimulation by Pituitary Adenylate Cyclase Activating Polypeptide and Calcitonin Gene-Related Peptide.” Endocrinology 129 (4): 1797–1804. doi:10.1210/endo-129-4-1797.
- Taylor, H. S. 2000a. “The Role of HOX Genes in Human Implantation.” Human Reproduction Update 6 (1): 75–79. doi:10.1093/humupd/6.1.75.
- Taylor, H. S. 2000b. “The Role of HOX Genes in the Development and Function of the Female Reproductive Tract.” Seminars in Reproductive Medicine 18 (1): 81–89. doi:10.1055/s-2000-13478.
- Wang, L., Z. Feng, X. Wang, X. Wang, and X. Zhang. 2010. “DEGseq: An R Package for Identifying Differentially Expressed Genes from RNA-Seq Data.” Bioinformatics 26 (1): 136–138. doi:10.1093/bioinformatics/btp612.
- Wathes, D. C., D. R. Abayasekara, and R. J. Aitken. 2007. “Polyunsaturated Fatty Acids in Male and Female Reproduction.” Biology of Reproduction 77 (2): 190–201. doi:10.1095/biolreprod.107.060558.
- Wu, T., E. Hu, S. Xu, M. Chen, P. Guo, Z. Dai, T. Feng, et al. 2021. “clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data.” Innovation 2 (3): 100141. doi:10.1016/j.xinn.2021.100141.
- Yang, L., and Y. J. Zhang. 2017. “Antagonizing Cytokine-Mediated JAK-STAT Signalling by Porcine Reproductive and Respiratory Syndrome Virus.” Veterinary Microbiology 209: 57–65. doi:10.1016/j.vetmic.2016.12.036.
- Yoshimura, T. 2013. “Thyroid Hormone and Seasonal Regulation of Reproduction.” Frontiers in Neuroendocrinology 34 (3): 157–166. doi:10.1016/j.yfrne.2013.04.002.
- Zhang, C. 1991. “Development of Lion Head Goose Breeding Resources (in Chinese).” Shantou Technology 1: 26–33.
- Zhang, T., L. Chen, K. Han, X. Zhang, G. Zhang, G. Dai, J. Wang, and K. Xie. 2019. “Transcriptome Analysis of Ovary in Relatively Greater and Lesser Egg Producing Jinghai Yellow Chicken.” Animal Reproduction Science 208: 106114. doi:10.1016/j.anireprosci.2019.106114.
- Zhao, Q., J. Chen, X. Zhang, Z. Xu, Z. Lin, H. Li, W. Lin, and Q. Xie. 2019. “Genome-Wide Association Analysis Reveals Key Genes Responsible for Egg Production of Lion Head Goose.” Frontiers in Genetics 10: 1391. doi:10.3389/fgene.2019.01391.
- Zhuang, Y., and Z. Lin. 2006. “Industrialization Prospects of Lion Head Goose (in Chinese).” Poultry Husbandry Disease & Control 2: 36–37.