?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This overview identified temperature and precipitation as the main seasonality triggers for changes in flotation performance. Other triggers found in the literature included total dissolved organics, amount of ultra-violent irradiation, and bacteria activity. The temperature was more frequently reported as an impactful seasonal controller, fluctuations of which cause grade, recovery, and selectivity problems in flotation: cases of laboratory and plant practices were highlighted, with some examples of ‘chemical’ solutions to low efficiency in cold flotation pulps. This overview describes seasonal cyclicity mechanisms and temperature-dependency of operations by referring to chemical and physical aspects of flotation: reactions and reagents, ore and mineral surfaces, water, bubbles, and equipment efficiency.
Cet aperçu a identifié la température et les précipitations comme principaux déclencheurs saisonniers des changements dans les rendements de flottation. D’autres déclencheurs trouvés dans la littérature incluaient les matières organiques totales dissoutes, la quantité d’irradiation à l’ultraviolet et l’activité des bactéries. La température était indiquée le plus fréquemment comme un contrôleur saisonnier influent, dont les fluctuations entraînent des problèmes de teneur, de récupération et de sélectivité dans la flottation: on a mis en évidence des cas de pratiques de laboratoire et d’usine, avec quelques exemples de solutions ‘chimiques’ à la faible efficacité des pulpes de flottation à froid. Cet aperçu décrit les mécanismes de cyclicité saisonnière et la dépendance sur la température des opérations en se référant aux aspects chimiques et physiques de la flottation: réactions et réactifs, minerai et surfaces minérales, eau, bulles et efficacité de l’équipement.
1. Introduction
Froth flotation is a complex process known for more than a century [Citation1]. It has one of the largest tonnage capacities among separation technologies [Citation2]. The process is a cornerstone of numerous industries, such as wastewater treatment (e.g. sewage treatment and water purification), the energy sector (e.g. bitumen recovery from tar sands and coal desulphurization), recycling (e.g. plastics separation [Citation3] and paper deinking), and mining and metallurgical domain (around 1 billion tons of ore is treated by froth flotation worldwide annually) [Citation4]. Despite wide application and a long history of innovation in froth flotation, some aspects of the process are still not completely understood, which makes this paper the first overview of mineral flotation comprehensively outlining challenges arising from temperature and other climate triggers [Citation1,Citation2]. Numerous flotation research and reviews highlighted temperature and climate being important parameters for wastewater flotation [Citation5,Citation6], oil sands and coal flotation [Citation7–9], plastics recycling flotation [Citation10,Citation11], and deinking flotation [Citation12,Citation13]. However, until the moment there is no review paper combining theoretical and practical aspects of temperature and climate-induced variations in relation to the flotation of different ore types.
An attempt at seasonal variation review in relation to the mineral processing industry has been made back in 1989 by Lin, who concisely summarised temperature effects on mineral processing plants, and, in the flotation subchapter, gave a couple of examples from the flotation experiments [Citation14]. The article, however, has entirely focused on temperature effects and did not elaborate on other driving mechanisms. Additionally, some new seasonal flotation mechanisms were found after publishing this review paper, such as e.g. nanobubbles on mineral surfaces and temperature-induced bacterial depression of minerals [Citation15,Citation16]. From more recent publications, a rather comprehensive review related to seasonal variations has been made by Liu and Wink, who focused on water quality effects (internally and externally sourced). Despite being well structured and containing more information than the review from Lin, it had only a short subchapter explaining external factors in an exclusive water quality context [Citation17]. Another review on the topic by Bhattacharya and Pascoe has also set significantly narrower borders by limiting the paper to one mineral commodity (coal) and one external effect (temperature) [Citation9]. Thus, the presented review on ‘Temperature and climate-induced fluctuations: an overview of different ore types’ aims at providing a comprehensive overview of the causes, mechanisms, consequences, and solutions to the negative impact of seasonal external factors on flotation.
The necessity of such a review is also dictated by an expected increase in the impact of climate change on the mining and metallurgical domain (through more frequent and intense manifestations of climate extremes). A strategy developed for combating climate change involves the clean energy transition, which heavily relies on the sustainable production of required minerals and metals [Citation18] making this paper particularly useful for consulting the industrial flotation practices and laboratory-scale investigations of different mineral commodities. Clean energy transition and the associated expected increase in the demand for raw materials [Citation19] are in line with predicted growth in flotation equipment and flotation reagent production markets [Citation20,Citation21]. The nomenclature of flotation reagents used in mineral separation is extensive as a response to climate-induced challenges and the increased complexity of the ores. A summary of the chemical reagents highlighted in this review article is given in .
Table 1. Summary of reagents mentioned in literature.
Increased complexity of the ores derives from the decrease in the major metal reserves, which consequently leads to the mining and processing of low-grade ores with fine dissemination of the valuable component [Citation59–61]. From the point of view of climate-induced challenges, several mineral processing plants across Canada have reported seasonally attributed metal losses during flotation [Citation28,Citation62–65], with examples of seasonal variations in flotation for two Canadian processing plants given in .
Figure 1. Examples of seasonal drop: (a) in gold recovery on Hudson Bay Mining and Smelting, 2000–2003; (b) in Cu + Ni grade on Clarabelle mill, adapted from Refs. [Citation28,Citation63].
![Figure 1. Examples of seasonal drop: (a) in gold recovery on Hudson Bay Mining and Smelting, 2000–2003; (b) in Cu + Ni grade on Clarabelle mill, adapted from Refs. [Citation28,Citation63].](/cms/asset/f01ec768-04e3-4ceb-8adf-815ae1bd2912/ycmq_a_2127788_f0001_oc.jpg)
Historically, similar fluctuations resulted in revenue loss accounting for millions of dollars with JCI plants in South Africa taken as an example [Citation14,Citation66,Citation67]. Therefore, it is vital to investigate seasonal effects to develop forecasting models and flexible flotation solutions for securing mining businesses, maintaining mining competitiveness, and expanding resource-efficient practices in the Arctic, Subarctic, and mountainous regions [Citation68]. This is particularly important for Canada’s development of Arctic and Sub-arctic regions, supported by Quebec’s ‘Plan Nord’ strategy, Chinese mining expansions in mountainous Tibet, or new mining projects in Fenno-Scandinavia [Citation69–71]. Investigations into more stable and environmentally friendly methods could be a pivotal point in the development of territories with drastic temperature fluctuations. For example, in Canada, mines could experience temperature variations from below −40°C during arctic winters to above +30°C during hot summers (for example, Raglan mine experiences a temperature swing of more than 30°C over the calendar year) [Citation72]. To fully account for seasonal effects in flotation, an investigation of individual flotation factors and their contribution to seasonal metallurgical patterns is vital. This would help to develop technical solutions improving the performances of plants subjected to rigid climatic conditions and assisting in understanding fluctuations’ driving mechanisms.
Flotation systems and individual components can be (and have been) investigated by measuring such parameters as water hardness, dissolved oxygen level, pH, suspended fines, viscosity, surface tension, etc. All these parameters have been shown to be influenced by temperature variations [Citation73], leading to cyclicity in flotation performance [Citation14]. Flotation performance, as a rule, is characterised through grade, recovery, and selectivity terms. In the example of the Canatuan Cu-Zn plant in the Philippines, a decrease in viscosity at elevated temperatures was believed to improve flotation selectivity [Citation74]. Good flotation performance is achieved when both the chemical and physical aspects of the flotation process are optimised.
Froth flotation combines chemical reactions (reagent adsorption, or conditioning) and physical separation (particle transport to the concentrate with bubbles) steps [Citation75]. From the chemical perspective, Van’t Hoff EquationEquation (1(1)
(1) ) [Citation76], relates flotation reaction equilibrium constants with medium temperatures:
(1)
(1) where
is enthalpy (temperature effect of a chemical reaction) in J mol−1, R is the universal gas constant (8.3145 J mol−1·K−1), T is the temperature (in Kelvin), and Kd is an equilibrium dissociation constant. Collector adsorption through physisorption is normally achieved at negative enthalpy values, while chemisorption increases with temperature [Citation77]. The importance of the adsorption mechanism may be demonstrated by fluorite flotation/adsorption tests, which revealed that at room temperature, fluorite recovery with oleic acid was found lower than that at higher temperatures [Citation77]. Investigations of collector adsorption on the mineral surface at room temperature revealed that it was washed off more easily from the surface compared to higher temperatures. These observations led to the conclusion that at a higher temperature, a change in the adsorption mechanism from physisorption to chemisorption of some part of the collector allowed the production of a more stable hydrophobic layer, which improved flotation [Citation77,Citation78].
The adsorption process could be characterised by its speed (adsorption kinetics) and completeness (adsorption equilibrium capacity). The adsorption kinetics of flotation reagents depends on temperature, and may be described through the Arrhenius Equationequation (2(2)
(2) ) [Citation79]:
(2)
(2) where Ea is the activation energy (J mol−1) required to trigger the reaction (i.e. to overcome electron repulsive forces, as well as weakening bonds of reactants), kmax is the reaction rate constant at Ea = 0, k is the reaction rate constant, and T is the temperature (Kelvin).
Low temperatures and high activation energies could result in no pronounced interaction of reactants even when thermodynamic criteria are satisfied [Citation80]. Favourable conditions for collector adsorption are found when the total energy of mineral-collector aggregate is lower than the sum of collector and mineral surface energies [Citation81].
After nano-scale processes of conditioning and reagent adsorption on a mineral surface, hydrophobized particles should be collected by bubbles and rise to the froth zone. The microscale description of the flotation process could be also summarised by temperature-dependent equations, where bubble (B) and particle (p) ‘react’ to produce flotula (particle-bubble aggregate – pB), as illustrated in Equations (3) and (4) [Citation82]:
(3)
(3)
(4)
(4) where
denotes the activity of the reaction product and reactants,
is Gibbs free energy in J mol−1 (characterises direction and limits of flotation reaction, it is negative for adsorption reactions [Citation83]).
Overall, as it derives from previous paragraphs, the current understanding of seasonal variations in flotation is heavily based on the established thermodynamic perceptions, some of which are adapted to describe flotation specifics. Combating the adverse effects of seasonal triggers on flotation performance follows two main methods: through controlled temperature manipulations, and/or by expanding (optimising) the nomenclature of chemicals used (e.g. collectors, modifiers). Combining numerous factors, flotation scenarios and industrial cases in this article should reveal knowledge gaps, and spin-off new research targeting more efficient flotation recipes leading to higher grades and recoveries of valuable minerals.
Apart from the analysis of grade and recovery drops under varying temperatures, seasonal impacts are known to arise the following challenges:
Discrepancies in flotation selectivity: e.g. a less efficient sphalerite-chalcopyrite separation was observed in summer at the Prieska Copper Mines concentrator in South Africa.
Changes in slurry viscosity: higher slurry viscosity was recorded during winter at South African mines including Daggafontein, Vaal Reefs, and Welkom [Citation84]. These changes have implications on entrainment, froth drainage issues, and bubble size [Citation7,Citation85], ultimately affecting concentrate quality.
Fluctuations in flotation kinetics: for example, a kinetics study into sulphide flotation showed that the flotation rate constant increases with temperature [Citation86].
Deterioration of reagent efficiencies: e.g. an increase in the required dosages of depressants on plants in Finland, Sweden, and Norway [Citation29].
A review of existing literature allowed the grouping of the data regarding seasonality in flotation into four main categories, comprising principal flotation components:
Seasonal fluctuations in water, gas, and froth phases
Interactions between equipment, water, and ore in different seasons
Flotation reagents and their competition under seasonal constraints
Ore interaction with water under varying temperature conditions.
1.1. Water properties
Significant inter-seasonal temperature changes (of more than 10°C) could have a pronounced effect on dissolved oxygen (DO) content and slurry viscosity [Citation14]. Oxygen is an important component for the oxidation of mineral surfaces and flotation chemicals. DO has temperature-dependent solubility ((a)), affecting flotation pulp pH and Eh. Gas dissolution in water can be described by Henri’s law and Van’t Hoff-type equations [Citation87,Citation88]:
(5)
(5)
(6)
(6) where
is a gas molar concentration (mol L−1),
is the partial pressure (atm) of the gas above the solution,
is Henry’s constant (l·atm mol−1),
is absorbed heat (J mol−1) during evaporation,
is the individual gas constant in l·atm mol−1. From these equations, it can be observed that an increase in temperature increases Henry’s constant, and subsequently the molar gas concentrations, which could lead to a DO deficit in flotation pulps.
Figure 2. (a) DO solubility in distilled water at different temperatures, adapted from Ref. [Citation90]; (b) effect of temperature on water viscosity and bubble size, adapted from Ref. [Citation91].
![Figure 2. (a) DO solubility in distilled water at different temperatures, adapted from Ref. [Citation90]; (b) effect of temperature on water viscosity and bubble size, adapted from Ref. [Citation91].](/cms/asset/4cce6af5-24ff-459d-ba1a-8eb4d1d005fe/ycmq_a_2127788_f0002_oc.jpg)
Another important water-related flotation parameter is viscosity, which impacts the entrainment rate of gangue minerals leading to poor flotation selectivity [Citation63]. Viscosity is believed to change turbulence, energy dissipation, and bubble-particle interaction patterns in a flotation cell [Citation89].
The change of viscosity in a liquid system with respect to temperature ((b)) may be described by Barrier’s equation:
(7)
(7) where a and b are constants,
is dynamic viscosity in centipoise (cP, 1 cP = 1 mPa⋅s) and T is the temperature in Kelvin [Citation92]. Additionally, in the case of viscous pulps, any temperature-driven viscosity changes will impact collector emulsification and, as a result, attachment to mineral particles. For example, molybdenite recovery on the Climax plant in the USA was increased by flotation pulp heating and the application of emulsification additives [Citation93].
Viscosity has been also shown to have a strong impact on bubble size (Equation (8)) [Citation91]. Bubble diameter (, in mm) is one of the key controllers of the flotation rate constant through collision efficiency (
) [Citation94] and bubble surface area flux (
, in s−1). The flotation rate constant is directly proportional to recovery (
), as shown in Equation (11) [Citation95–97]:
(8)
(8)
(9)
(9)
(10)
(10)
(11)
(11) where
is the Sauter mean bubble diameter (mm);
is pulp viscosity (μPa·s),
is the pulp viscosity at a reference temperature (20°C); K, a, and b are coefficients;
is the superficial gas velocity (cm s−1);
is particle diameter (mm);
is collision efficiency;
is attachment efficiency;
is detachment efficiency;
is collection efficiency,
is residence time (s), and T is a water temperature (°C). A seasonality study of zinc grades at the Matagami concentrator (Canada) attempted to link seasonal metallurgy with bubble size variations. It was found that in summer the zinc grade was on average 2.5% higher and the bubble size was approximately two times larger compared to winter () [Citation64].
Figure 3. Zinc concentrate grade fluctuations at Matagami concentrator, adapted from Ref. [Citation64].
![Figure 3. Zinc concentrate grade fluctuations at Matagami concentrator, adapted from Ref. [Citation64].](/cms/asset/ebaecc68-fc62-4441-a860-bb4c4f5d9ef0/ycmq_a_2127788_f0003_oc.jpg)
Nesset et al. [Citation64] proposed the following explanation: an increase in the water recovery (and hence entrained gangue minerals) by smaller bubbles in winter, and higher froth stability caused by smaller bubbles and increased water viscosity (and hence poorer water drainage). Another explanation could be the changes in frother concentrations due to high evaporation rates in summer [Citation1,Citation98].
Frother adsorption on the water-gas interface is also temperature dependent and is closely linked to the surface tension (, in N m−1) by Gibbs’ adsorption equation [Citation99]:
(12)
(12) where
is surfactant adsorption (mol m−2),
is the surfactant concentration (mol m−3),
is the temperature (Kelvin). Drzymala [Citation100] reported (at laboratory scale) the use of frother concentration as an incentive parameter in the Arrhenius-type equation to describe the kinetics of collectorless chalcopyrite flotation from copper shales:
(13)
(13) where kn is a rate constant, c0 and c are reference and actual frother concentrations expressed in the same units (e.g. mol L−1). The response of frothers to temperature changes is revealed by increased froth stability and height in cold environments [Citation7]. Liu et al. studied the temperature effect on pyrite flotation with NaBX as the collector and ‘# 2 oil’ (alcohol group frother with general formula ROH), showing that at 23°C, the dosage of frother required to reach maximum recovery was 5 mg L−1, compared to 10 mg L−1 at 0°C [Citation32].
From the perspective of even smaller bubbles, recent investigations with a Pb–Zn ore from Gorevskoye mill (Russia) showed that nanobubbles formed on ore mineral surfaces after sharp temperature fluctuations have the potential to improve recovery [Citation15]. Mikhlin et al. noted that this factor should be considered when analysing plant performance in winter when heated milling products are mixed with cold process waters [Citation15]. The formation of nanobubbles on a mineral surface will hydrophobize it, and facilitate more efficient collection by larger bubbles.
Collection efficiency from Equation (10) could be also seen as a parameter controlled by temperature-dependent induction time (, in μs) [Citation1]: particle-bubble attachment time reduces with increasing temperature as a result of increased water fluidity, facilitating the extrusion of a water layer between a mineral and a bubble [Citation101].
(14)
(14) where D and J are constants. Increased collection of quartz through improved bubble-particle collision has been also found when increasing temperature from 20°C up to 40°C [Citation102]. The bubble rise velocity (
) increased by approximately 40%, increasing bubble kinetic energy and reducing the size of the largest particle feasible to float (
, in cm) (Equation (15) [Citation103]):
(15)
(15) where
is a constant (dyn),
is surface tension (dyn cm−1),
is the contact angle (degrees),
is a media density (g cm−3), and
is the bubble rise velocity in cm s−1. Bubble rise velocity could explain certain variations in flotation performance. For example, He et al. suggested it as one possible explanation for reduced galena recovery in cold pulps. They blamed low pulp temperatures and the associated increase in slurry viscosity for reduced bubble rise velocity and lower collision efficiency between bubbles and particles [Citation104]. More slow bubble rise velocity hypothesis has been also used by O'Connor et al. to explain a reduction of pyrite mass transfer from the pulp to the froth, leading to a decreased pyrite flotation performance in winter months on a pyrite flotation plant in South Africa () [Citation66].
Figure 4. Monthly pyrite production on a flotation plant in South Africa in relation to the temperature, adapted from [Citation66].
![Figure 4. Monthly pyrite production on a flotation plant in South Africa in relation to the temperature, adapted from [Citation66].](/cms/asset/53093ad4-ffcf-42a7-a3ae-d20ee826da35/ycmq_a_2127788_f0004_oc.jpg)
Another effect of altered pulp viscosity is a change in froth rheology. A relationship between bubble size and pulp viscosity with froth properties may be described through Equation (16) [Citation105,Citation106]:
(16)
(16) where
is the apparent froth viscosity (mPa·s),
is the volume fraction of air,
is the Sauter mean bubble diameter (mm),
is the shear rate (s−1),
is a constant that depends on the continuous phase viscosity and surface tension,
is a superficial fraction of lamella that is covered with solids. Increased froth viscosity has been reported to increase copper grade, which was explained by increased residence and froth drainage times [Citation106–108] ().
Figure 5. (a) Concentrate quality depends on froth rheological properties; (b) changes in water and pyrite pulp viscosities, and water surface tension as a function of temperature. Adapted from Refs. [Citation66,Citation92].
![Figure 5. (a) Concentrate quality depends on froth rheological properties; (b) changes in water and pyrite pulp viscosities, and water surface tension as a function of temperature. Adapted from Refs. [Citation66,Citation92].](/cms/asset/40fa99e0-a2c7-42a4-b169-bd3c2e287bb9/ycmq_a_2127788_f0005_oc.jpg)
Surface tension is another parameter, closely related to viscosity (Equation (17)) [Citation109], which shows a similar trend as a function of temperature, while the best fit described in the literature was achieved with the Ramsay-Shields EquationEquation (18(18)
(18) ) [Citation110]:
(17)
(17)
(18)
(18) where
is surface tension in N m−1, a and b are liquid-specific constants,
is the critical temperature in Kelvin,
is actual temperature in Kelvin,
is liquid density in kg m−3,
is the molar mass of the liquid in kg mol−1,
is association degree of liquid, K is Eotvos-Ramsay Coefficient in J (K·mol)−2/3. Some laboratory investigations into the collectorless flotation of sulphides have discovered that different minerals have a certain optimum surface tension range where the highest recoveries are achieved () [Citation111]. Moreover, above a critical surface tension, stable wetting layers form, which increases the induction time for particle-bubble attachment [Citation112,Citation113].
Figure 6. Flotation recovery of selected sulphide minerals in relation to liquid-vapour surface tension, adapted from Ref. [Citation111].
![Figure 6. Flotation recovery of selected sulphide minerals in relation to liquid-vapour surface tension, adapted from Ref. [Citation111].](/cms/asset/16e7beb2-23c0-4126-ab3f-635bdf70ffed/ycmq_a_2127788_f0006_oc.jpg)
As the contact angle depends on surface tension [Citation9,Citation114], it could be concluded that the angle is also a temperature-dependent parameter, and could be used to determine the free energy of the system (Equation (19)). Young’s Equationequation (20(20)
(20) ) describes the contact angle-surface tension relationship:
(19)
(19)
(20)
(20) where
,
,
, are the surface tensions at vapor–liquid, solid–vapor, and solid–liquid interfaces respectively. Similar to mineral recovery at different surface tensions, a contact angle measured for copper-activated sphalerite and galena conditioned with potassium amyl xanthate (PAX) have indicated an optimal temperature [Citation115]. The largest contact angle (floatability) was found in the range of 30–35°C for both tested minerals. An and Zhang [Citation116] showed that for chalcopyrite conditioned with PAX, the contact angle steadily increased over the temperature range of 25–60°C.
1.2. Temperature-induced equipment issues
Temperature changes also influence the output of other mineral processing units serving flotation circuits, namely: grinding, classifiers (such as hydrocyclones), and thickeners (water recycling). Increased water viscosity due to a seasonal temperature drop in milling leads to less efficient grinding. Theoretically, this produces fewer fines in a mill discharge and reduces milling capacity [Citation14]. Regarding classification, slurry viscosity changes the hydrocyclone cut point during the summer. A comparison of a hydrocyclone cut point for iron ore at 3.3°C and 20°C revealed coarser output in cold water: 25 µm as opposed to 20 µm [Citation85]. The result of sending coarser material to the flotation circuit is that flotation performance will be negatively impacted due to poorer mineral liberation and a coarser feed [Citation14].
Another possible seasonal issue is grinding media, which contaminates the pulp with iron, and plays a critical role in sulphide flotation depression, especially for fine fractions through iron hydroxide coverage on mineral particles [Citation117]. Iron dissolution takes place more readily at elevated oxygen levels in the pulp, through a cathodic reaction [Citation118]. Thus, for marmatite, it has been reported that iron (III) hydroxide from grinding media adsorbed on the copper-activated surface hinders collector adsorption [Citation119].
Regarding thickener efficiency, a drop from 29°C to 13°C has the potential to increase residence time by 33% as a consequence of decreased settling rate in more viscous pulps [Citation14]. Such seasonal changes should be taken into consideration in the plant design stage as recycling the finest material back to the flotation could render the whole process inefficient.
2. Sulphide ores
Seasonal variations are an important factor in most sulphide flotation circuits, with temperature changes impacting numerous flotation plants [Citation120], or being manipulated to improve process selectivity [Citation121,Citation122]. Dunne et al. outlined that temperature regulation has limited potential for sulphide plants with heating operations being impractical, and noted limited industrial application – mostly for molybdenite separation from copper minerals or in sphalerite flotation [Citation120]. In the separation of molybdenite from copper minerals, pulp heating is used to decelerate Na2S degradation, which improves the depression of copper minerals [Citation123].
Nevertheless, it does not negate the fact that cyclicity in sulphide flotation within annual operation cycles (between summer and winter seasons), and even on a daily production scale (between day and night) has been observed worldwide [Citation14]. Research from South African sulphide flotation plants revealed that seasonal variations were responsible for the loss of millions of US dollars every year [Citation14,Citation66]. The acuity of the seasonality problem correlates well with territories characterised by significant seasonal temperature fluctuations. For example, the Uchalinskaja Cu–Zn plant in the Russian Urals has introduced thermal treatment of the pulp in winter to improve zinc concentrate grade and recovery which, however, requires treatment of the pulp with steam at 140–150°C to increase the pulp temperature to 25–30°C [Citation124]. Zinc flotation without pulp heating has higher seasonally induced risks. For example, the Chinese Fankou Zn–Pb plant reported a seasonal drop in both Zn recovery and grade by 0.66% and 0.4% respectively at lower temperature slurries (22–28°C), when compared to the results at 31–39°C [Citation125]. Historical data from a German zinc operation in Meggen with pulp temperatures similar to the ones in the Fankou plant in winter, indicated a greater inter-seasonal contrast in concentrate grades [Citation126], attributed to increased winter losses triggered pH fluctuations.
Other historical operating data on differential polymetallic flotation showed that zinc–lead separation may be facilitated by low temperatures. Thus, Base Metals Mining Corp (USA) reported better separation efficiency in the lead cleaner at 16°C resulting from low sphalerite flotability in cold pulps. For related reasons, zinc cleaners on the same plant were reported to be heated up to 32°C. Bolivian Potosi Mine historical data has also indicated increased winter zinc losses, where the zinc concentrate was found to have higher concentrations of iron minerals during the cold season [Citation127]. Investigations at Brunswick Mine concentrator (Canada) also reported similar results: a strong dependence of zinc grade and recovery on pulp temperature was confirmed by a series of tests (), where cold pulps were found to enhance pyrite flotation instead of sphalerite [Citation128].
Figure 7. Laboratory results of zinc rougher flotation, adapted from Ref. [Citation128].
![Figure 7. Laboratory results of zinc rougher flotation, adapted from Ref. [Citation128].](/cms/asset/62e641d3-35cc-4667-aa4c-2ce3125bc672/ycmq_a_2127788_f0007_oc.jpg)
Under significantly colder winter conditions the operations may experience more drastic performance fluctuations. For example, many pyrite flotation plants in Tibet and Yunnan (China) were reported to experience winter shutdowns due to a significant decrease in production output [Citation32]. Even for naturally hydrophobic molybdenite, cold temperatures in winter may cause a decrease in recovery. As Zhao noted with an example of molybdenite operation in Northeastern China, there was an inter-seasonal drop in molybdenite recovery by 1–2% when cold and hot seasons were compared [Citation129]. Boliden Cu–Pb–Zn plants in Sweden also experienced winter recovery issues, which were particularly acute at temperatures below 12°C. A winter decline for the Boliden plants could be explained by poorer sphalerite depression in the copper-lead circuit and less efficient copper activation in the zinc circuit [Citation130]. Isshiki has also found for Zn/Pb/Cu flotation systems that the grades of these base metals increase in the respective concentrates as the temperature rises [Citation131].
Flotation performance of sulphide ores also declines if the temperature is too high. For example, it has been observed at the Neves-Corvo zinc plant (Portugal) that the highest losses were recorded during traditionally hot summers with slurry temperatures after milling reaching above 50°C [Citation132]. Fernandes also mentioned that the hottest days were usually accompanied by sphalerite recovery drop at the plant () [Citation132].
Figure 8. Zinc recovery at the Neves-Corvo zinc plant in relation to the daily temperature, adapted from Ref. [Citation132].
![Figure 8. Zinc recovery at the Neves-Corvo zinc plant in relation to the daily temperature, adapted from Ref. [Citation132].](/cms/asset/22820ff3-b431-4b03-989c-fc773addd5c2/ycmq_a_2127788_f0008_oc.jpg)
Another example of decreased performance in summer is the processing of polymetallic ores of Hudson Bay Mining and Smelting (HBMS) in Canada, where recovery degradation was amplified after the plant transitioned to recycled water. The fluctuations at HBMS facilities demonstrated correlations with temperature, conductivity, Eh, as well as thiosulphate and calcium concentration in the pulp [Citation28]. A decrease in performance in hot and cold conditions suggests that there is an optimal temperature range for sulphide flotation that maximises a plant performance.
2.1. Collector efficiency
The sensitivity of flotation with xanthates to temperature changes was found to increase significantly with decreasing collector dosage [Citation28]. Moreover, for the example of Almalukskaja, Zyryanovskaja, and Belousovskaja plants in Central Asia [Citation133] it has been shown that maximum copper, lead and zinc recovery was achieved when the ratio between dosed and required (to maximise recovery) concentration of xanthate is close to 1. Several mechanisms were suggested to explain the fall in recovery from xanthate excess in the pulp, namely partial desorption of copper activating ions from sphalerite surface by xanthates and an increase in slimes production as a result of xanthates reaction with dissolved metal ions in the pulp. Reductions in temperature decrease xanthate demand, which will increase the ratio between dosed and required xanthates concentration from around 1 at 15°C to above 2 at 10°C [Citation133]. He et al. studied the effect of temperature on galena flotation using NaBX as a collector and MIBC as a frother, showing that at a low temperature (5°C) the recovery was reduced by 7% compared to that at 20°C. FTIR results suggested that the chemicals adsorbed on the mineral surface were not affected by temperature. Zeta potential and XPS analysis suggested that lower temperatures would reduce the surface oxidation of galena, which could reduce the number of adsorption sites and, therefore, reduce the adsorption of NaBX [Citation104].
Another important temperature-driven aspect of xanthate adsorption is an analysis of a Krafft point or a temperature at which collector solubility reaches a level of critical micelle concentration. Tests with alkyl xanthates series revealed that with an increase in the hydrocarbon chain, the Krafft point increases as well [Citation134,Citation135].
Research on the flotation of monomineralic samples of chalcopyrite, pentlandite, and pyrrhotite at 10°C, 20°C, 50°C and 70°C observed an increased demand for xanthates with increased temperature [Citation136]. In the case of pyrrhotite flotation, an increase in pulp temperature from 10°C to 50°C led to an increasing butyl xanthate consumption by 10% at pH 10, 36% at pH 11, and 49% at pH 12. For chalcopyrite, collector consumption increased even more drastically: 64% at pH 11.5 and 73% at pH 12.5. Pentlandite showed the largest temperature-induced changes in collector consumption (177% at pH 10.5 and 166% at pH 11.5) [Citation136].
To achieve efficient sulphide flotation, both types of collector adsorption forms should be present on the surface: physisorbed dixanthogen and chemisorbed xanthates [Citation137]. The presence of both types of adsorption masks the polar bonds of a surface more effectively, increasing the chances of a liquid film rupture between a particle and a bubble, as well as a more stable bubble-particle aggregate. Dixanthogen physisorption is believed to derive from the partial oxidation of xanthates by involving superficially adsorbed oxygen taking an electron from the conductivity zone. The reaction is catalysed by the presence of copper ions on a sulphidic surface [Citation138]. The temperature has the potential to change the availability of both ingredients: oxygen and copper [Citation90,Citation139]. Xanthate adsorption and oxidation on a mineral surface may be summarised by .
Figure 9. Interaction scheme of xanthates with sulphide surface in aerated pulp, adapted from Ref. [Citation140], where Ev is the location of the energy level of the valence band ‘ceiling’, and Ec is the location of the energy level of the conductivity band ‘bottom’.
![Figure 9. Interaction scheme of xanthates with sulphide surface in aerated pulp, adapted from Ref. [Citation140], where Ev is the location of the energy level of the valence band ‘ceiling’, and Ec is the location of the energy level of the conductivity band ‘bottom’.](/cms/asset/c5cefd4d-0611-4763-beab-7e76333bdb7f/ycmq_a_2127788_f0009_oc.jpg)
For dixanthogen adsorption on pyrite, it has been shown that enthalpy decreases from approximately 250 kJ mol−1 at pH 4.5 to approximately 105 kJ mol−1 at pH 12 [Citation141]. The morphology of dixanthogen adsorption is another important parameter. In a laboratory investigation of xanthate adsorption on chalcopyrite, hydrophobicity increased with temperature owing to the low melting point of dixanthogen (30°C), which caused the irregular shape of dixanthogen aggregates leading to a larger surface coverage at elevated temperatures [Citation142]. Conversely, excessive increases in temperature (up to 65°C) in iron-containing pulps induce a 20-fold increase in dixanthogen dissolution from the surface [Citation143].
Xanthate adsorption on a mineral surface also requires certain potential values. Surface potential determines the probability of collector adsorption and it depends on numerous factors including initial mineral hydrophobicity, size of non-polar collector radical, concentration and structure of a collector, etc. [Citation144]. Xanthate adsorption on sulphide minerals increases with temperature, which has been shown with pyrite [Citation32] and chalcopyrite [Citation145] in different temperature ranges ().
Figure 10. (a) Butyl xanthate adsorption on pyritic surface, adapted from Ref. [Citation32]; (b) change of butyl xanthate concentration in the pulp (Ct/C0) with time during adsorption onto chalcopyrite, adapted from Ref. [Citation145].
![Figure 10. (a) Butyl xanthate adsorption on pyritic surface, adapted from Ref. [Citation32]; (b) change of butyl xanthate concentration in the pulp (Ct/C0) with time during adsorption onto chalcopyrite, adapted from Ref. [Citation145].](/cms/asset/763a5db7-5518-4ef9-898f-8453afa012f9/ycmq_a_2127788_f0010_oc.jpg)
Xanthate oxidation to dixanthogen () occurs for most sulphide minerals at potential values around +0.2 V, while the desorption of the dixanthogen layer was found between +0.5 and +0.8 V. Formation of chemisorbed xanthate species occurs at negative potential values (−0.2 to −0.1 V). There are some exceptions, for example, it is thermodynamically impossible to produce dixanthogen on non-activated sphalerite surfaces [Citation137].
2.2. Flotation modifiers
Copper activation is a method commonly used in sulphide flotation for minerals such as sphalerite, pyrrhotite, pyrite, among others [Citation146]. The aim of copper activation is to lower the Fermi level of a mineral surface with a broad forbidden zone (e.g. 3.7 eV for sphalerite), which allows xanthate to oxidise and form dixanthogen on a mineral surface [Citation137]. From a temperature perspective, it has been determined that the limiting concentration (a value of concentration before precipitation) of aqueous copper in solution increases with pulp temperature. The limiting concentration has been shown to be a good indicator of conditions with the highest sphalerite recovery during collectorless flotation [Citation86]. Aqueous copper stability borders shift to lower pH values and higher copper concentrations as temperature rises. Lower temperatures result in poorer copper dissolution, which decreases the amount of aqueous metal species available for sphalerite activation, as indicated in .
Figure 11. Areas of pH and concentration stabilities of copper species at different temperatures, adapted from Ref. [Citation86].
![Figure 11. Areas of pH and concentration stabilities of copper species at different temperatures, adapted from Ref. [Citation86].](/cms/asset/1a3045b1-b413-4d4d-898e-57bfc5bdf93e/ycmq_a_2127788_f0011_oc.jpg)
Increasing temperature from 5°C to 20°C has led to an increase in Zn recovery of approximately 15% and a 5-fold improvement in sphalerite flotation kinetics. Albrecht et al. attributed the improved results at higher temperatures to the acceleration of the multi-step copper activation process, with copper–zinc displacement not being affected, and copper deposition on the zinc surface being temperature sensitive [Citation86]. Copper activation could be described by Equations (21)–(25) [Citation86,Citation147,Citation148]:
(21)
(21)
(22)
(22)
(23)
(23)
(24)
(24)
(25)
(25)
A critical temperature of 10°C has been established by Manouchehri et al. for the collectorless flotation of copper-activated sphalerite. Below this temperature, a drastic decrease in recovery was observed [Citation89]. To compensate for this loss in recovery, increasing the copper dosage was found to be effective. Tests on the Garpenberg ore (Sweden) revealed that by decreasing pulp temperature during activation and during flotation, the grade and recovery values were impacted, with temperature during conditioning (activation) having a slightly higher impact on recovery [Citation89].
Copper hydroxide precipitation on the mineral surface plays an important role in copper activation (Equation (26)). It has been shown to be a temperature-dependent parameter [Citation139]: aqueous copper concentration () in solution was found to be approximately 3 times larger at 4.2°C than at 23.6°C under neutral pH conditions. To achieve an efficient copper activation, a temperature-dependent ratio between copper and zinc ions in the solution should be favourable (which means a predominance of copper ions), as per Equation (26) [Citation125]:
(26)
(26)
It has been suggested that the optimal temperature range for copper activation from the perspective of Zn grade and recovery using sodium butyl xanthate as collector is between 25°C and 40°C, but not higher than 50°C [Citation125].
Lime is a low-cost modifier widely used in sulphide flotation. It is an important reagent for iron sulphide depression [Citation149]. Changing concentrations of dissolved oxygen due to temperature fluctuations might have an impact on pH and lime consumption at a plant. In research conducted on the ore from the Neves-Corvo mine (Portugal), it was noted that the required lime dosage for pH adjustment increased with temperature [Citation132]. One suggested reason was the reduced solubility of lime at elevated temperatures – according to chemical handbook data, it has a negative linear relationship with temperature: from 0.14 g L−1 at 40°C to approximately 0.17 g L−1 at 10°C [Citation150].
Lime also serves as a source of calcium ions in the flotation pulp. In the case of galena flotation, some studies recorded a depressive effect (up to 15% Pb loss) when the calcium ion concentration was below 0.5 g L−1, with a more pronounced effect at concentrations higher than 0.5 g L−1 (up to 30% Pb loss). The depressive effect of calcium could be linked to the formation of hydrophilic calcium compounds on the sulphide surface [Citation151]. Ikumapayi and Rao performed flotation tests with Renström Zn ore (New Boliden, Sweden), at simulated water qualities, based on Ca2+ and ion balances at different temperatures [Citation152]. It was found that Ca2+ consumption (due to adsorption on the mineral surface and/or precipitation as calcium carbonate) at 4°C was lower than at 11°C and 22°C, while the concentrate grade in pulps with low Ca2+ concentrations was showing a decreasing trend with temperature [Citation152]. In another example of sulphide flotation, Grano et al. investigated the Hilton concentrator (Australia) and proposed that galena depression originated from calcium thiosulphate and calcium sulphate precipitation on the lead mineral surface [Citation153]. The rate of such heterogeneous nucleation may be described by Equation (27) [Citation154]:
(27)
(27) where
is the heterogeneous nucleation rate (1 (cm3 s)−1), A is pre-exponential factor,
is geometrical shape factor,
is the molecular volume,
is interfacial energy (subscripts
,
, refer to liquid, solid and critical nucleus respectively),
is the Boltzmann constant (J K−1),
is the supersaturation states, and T is temperature in Kelvin.
For different minerals, there is a critical pH level which prevents particle-bubble attachment as a result of prevention of collector adsorption on the mineral surface [Citation155]. For galena, the critical pH was found around 9.7 at 35°C, which increased to 10.8 at 10°C [Citation34]. This means that under certain conditions galena flotation may well improve during the cold season.
Cyanides are effective depressants for sulphides. They are used extensively for the selective separation of base metals, mainly as a depressant for iron sulphides (e.g. pyrite), or sphalerite [Citation156]. Research conducted on zinc ore noted that the cyanide reagent scheme was temperature sensitive, as hydrogen cyanide gas (HCN) is more likely to form at elevated temperatures [Citation74]. The detrimental influence of cyanide at high temperatures has been also shown in flotation research of a polymetallic ore from the Belousovskaja plant in Kazakhstan. It was noted that the optimal plant temperature range was between 18°C and 20°C, as lower temperatures led to decreased mineral floatabilities, while higher temperatures sharply increased cyanide activity, which depressed some copper sulphides in addition to the targeted pyrite [Citation133].
Laboratory captive bubble tests [Citation34] have revealed that there is a general increase in the required cyanide concentration that prevents mineral-bubble contact with temperature. In the case of a pyritic surface, the required cyanide concentration appears to be in a power relationship with temperature up to 35°C. The tests were conducted at pH 9 with 150 mg L−1 of CuSO4·5H2O, and 25 mg L−1 of KEX (). After achieving a peak at around 40°C, the required cyanide concentration dropped by approximately 14% as the temperature reached 60°C. For pyrite and chalcopyrite at pH > 10 without activation, an increase in the temperature from 10°C to 35°C resulted in a decrease in critical cyanide concentration, almost a 7-fold drop for chalcopyrite (from 0.87 to 0.13 mg L−1) and 2 fold decrease for pyrite (from 0.08 to 0.04) [Citation34].
Figure 12. Critical cyanide concentrations in captive bubble tests for combinations of activator and collector at different pH and under varying temperature conditions: (a) chalcopyrite (25 mg L−1 KEX), (b) sphalerite (150 mg L−1 Cu-vitriol), (c) pyrite, adapted from Ref. [Citation34].
![Figure 12. Critical cyanide concentrations in captive bubble tests for combinations of activator and collector at different pH and under varying temperature conditions: (a) chalcopyrite (25 mg L−1 KEX), (b) sphalerite (150 mg L−1 Cu-vitriol), (c) pyrite, adapted from Ref. [Citation34].](/cms/asset/d3bd25e7-7137-4156-a75b-44d84bec6ab6/ycmq_a_2127788_f0012_oc.jpg)
Strathcona copper-nickel operations in Canada have also reported the temperature sensitivity of the cyanide scheme. Chalcopyrite and nickel minerals (pentlandite and millerite) separation was experiencing seasonal effects described by poor selectivity during the cold season (poor nickel depression). As an alternative, Glencore investigated dextrin as a substitute for sodium cyanide, which demonstrated better selectivity at cold temperatures in laboratory trials [Citation65].
Sulphide flotation in alkali media may generally be seen as a balance between two types of reagents: increasing hydrophobicity through the use of an activator (e.g. copper sulphate) and collector (e.g. xanthates) on one side; and depressant (e.g. cyanide) and pH modifier on the other side (increasing OH− groups tends to hydrophilize mineral surfaces). Temperature may be interpreted as an external factor shifting the equilibrium depending on mineral and reagent properties.
Wark and Cox [Citation34] noted that in the case of non-activated pyrite, activated and non-activated chalcopyrite, a temperature increase shifts the equilibrium towards the hydrophilic side, which was confirmed by decreased cyanide concentration required for mineral depression. For activated sphalerite, a different scenario was observed: higher temperatures led to an increased hydrophobicity. Increasing collector concentration, as reported by McCreedy and Honeywell [Citation157] retards the cyanide dissolution processes (depression), as shown in .
2.3. Mineral surface properties
The change in ore surface properties with temperature is mainly related to different oxidation rates and the change in oxidation mechanism. Increased oxidation processes require higher collector dosages. The required xanthate concentration is linked to mineral thermodynamic parameters through Equation (28) [Citation137]:
(28)
(28) where A is a coefficient, dependent on changes in thermodynamic characteristics of minerals as temperature rises, B is a coefficient characterising an incremental value of required xanthate concentration with pH increase [Citation137]. Temperature is a factor which controls thiosalt oxidation. Thiosulphates (
), trithionates (
), and intermediate products of sulphide minerals’ oxidation are considered important flotation parameters [Citation63].
As an example, it has been found for iron sulphides that B from Equation (28) does not change over the temperature range of 15–70°C, which is a consequence of the unchanging mechanism of mineral oxidation (predominant sulphur oxidation product remains ) [Citation137]. Thus, only an increase in parameter A was observed with temperature. Analysis of chalcopyrite revealed changes in both A and B as oxidation at ambient conditions to
was substituted by the formation of
with increasing temperature (up to 50°C) and pH (up to 13). Consequently, a shift from
to
led to increased xanthate demand [Citation136]. In the case of galena flotation, sulphate ions (
) were believed to decrease the recovery at cold pulps through surface coverage with sulphoxy complexes [Citation151]. Such complexes might be considered to be one of the reasons for galena’s 20% recovery drop in winter reported for the Belousovkaja plant (Kazakhstan) [Citation133]. Historical data from Brunswick Mine concentrator (Canada) also indicates seasonal metallurgical fluctuations correlating with thiosulphate concentrations in process water [Citation97].
In addition to thiosulphates, the concentration of dissolved metal cations rises when heating the pulp above room temperature: up to 3 times when the temperature reaches 65°C [Citation143]. In sphalerite flotation [Citation89], it has been demonstrated that an increase in pH and temperature creates favourable conditions for the stability of the zinc hydroxide complex on the mineral surface. In the Somincor plant (Portugal), significant (25%) seasonal fluctuations in zinc recovery were attributed to collector desorption from the mineral surface at pulp temperatures above 60°C, whereas sphalerite surfaces were found to be dominated by zinc hydroxide species. Research conducted by Orii [Citation158] resulted in similar flotation responses: the recovery of sphalerite gradually decreased with temperature up to 50°C. Moreover, at 80°C, floatabilities of chalcopyrite, pyrite, and sphalerite were found to be significantly reduced. Historical data from bornite flotation in the USA Magma concentrator revealed that too high a temperature after milling also caused drops in copper recovery as a result of overoxidation of the mineral [Citation127]. Kuroko concentrator (Japan) [Citation159] and Broken Hill concentrator (South Africa) [Citation122] attempted to benefit from the oxidation phenomenon: they applied differential oxidation by heating (60–70°C) for galena depression in copper-lead concentrate separation circuits. Kubota et al. reached similar conclusions: flotation of galena with n-BAF was found to be depressed when the temperature was increased from 20°C to 60°C. This was justified by increased hydrophilicity on the galena surface due to oxidation and desorption of collectors [Citation35].
2.4. Water quality
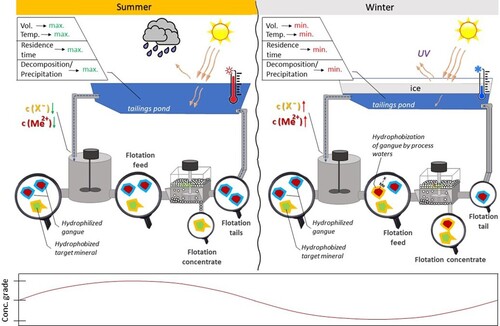
Considering that most flotation plants tend to recycle process water, its quality and influence on plant performances have been extensively studied and reviewed [Citation17,Citation160]. The seasonality of water quality may be associated with dissolved organic or inorganic matter. As an example, results of investigations at Clarabelle mill (Canada) claimed that the seasonal metallurgy phenomenon owed its existence to process water quality variation: summer seasons allowed longer residence time in ponds before water recycling to the plant; whereas winter process water had shorter residence times in the pond due to freezing and channelling phenomena. Eventually, summer process water contained less dissolved ions and organic matter compared to winter water. The main reason for process inefficiencies during the cold season was believed to be an increased concentration of gangue activating nickel ions in process water [Citation63]. Seasonal changes in flotation water quality could be derived from pH variations (impacting flotation kinetics), evaporation and precipitation rates (dissolved ion and colloidal species concentrations control), as well as varying combinations of the water streams in different seasons [Citation161].
At Kevitsa mill (Finland), seasonal variations of organics (xanthates) in process water led to poorer selectivity in Cu–Ni separation during winter. Higher xanthate concentrations in process waters were attributed to shorter residence times in ponds and the lack of UV-light (xanthate degradation accelerator) due to ice cover. Additionally, low water temperatures were believed to be unfavourable for xanthate degradation [Citation162]. Another study on the Kevitsa concentrator [Citation163] revealed that during winter months the content of impurities in process water was higher, related to the locking (freezing) of clean water into the tailings pond ice covering, and slowing down water purification processes, while the spring months (March and April) were characterised by melting of snow and the associated influx of fresh clean water, which brought improvements to the flotation process. Phosphorus content variations in the pulp were explained by varying Aerophine (copper collector) decomposition rates [Citation163]. Metal and sulphate content variations were also suggested to vary depending on bacterial activity in the water coming from an open pit. Plant water management brings variability to the process water composition as in summer months the water pumped from an open pit may be mixed with process waters [Citation162,Citation163]. It has also been shown that the cyclicity of sulphate concentration in the process waters of Kevitsa disappeared after summer 2017, as a result of warm winters in northern Finland which has triggered continuous bacteria oxidation of the ore, and explains the recently observed steady increase in the amount of sulphates and dissolved metals [Citation163].
An assessment of acid mine drainage (AMD) at closed sulphide mines in Finland has also demonstrated another impact that seasonal variations have on water quality. It has been found that the mineralogy of iron hydroxide precipitates in the tailings ponds changes from schwetmanite () after melting of snow in spring to goethite (
) during summer time. Moreover, seasonal variations in pH trigger fluctuations in the content of SO4, Al, As, Cu, and Zn in tailings water [Citation164]. Investigations into the seasonal phenomenon and water quality in relation to the bacterial activity observed in Calumet Pb–Zn tailings in Canada revealed that SO4 reduction by sulphide-reducing bacteria (SRB) was higher in summer than in spring owing to the higher temperature and organic carbon concentrations (impacted by an agricultural runoff). The activity of iron-reducing bacteria (FeRB) in the alkali tailings, however, was not hindered by the temperature changes under the investigated conditions [Citation165]. Dold indicated that fluctuating SO4 concentrations in the active tailings of copper-porphyry mines are closely related to gypsum solubility and weathering, which results in increased dissolved sulphate concentrations towards the end of the summer (evaporation effects) with some cases showing a general rising trend over time [Citation166]. At Iron Mountain (USA), another AMD site, the Zn to Cu ratio fluctuated as a result of the formation and dissolution of melanterite
during the dry and wet seasons respectively [Citation167].
Atmospheric precipitations are another external trigger, seen as bringing an influx of fresh water to the plant that improves source water quality. Rain brings fresh water, lowering the concentration of detrimental ions in the pulp such as calcium, magnesium, sulphates and carbonates among others [Citation168]. Precipitation and temperature effects have been summarised in .
The seasonality of flotation performance has been also found to be controlled by variations in dissolved organics (specifically humic acids), one example being molybdenite flotation, with molybdenite recovery being reduced by 3.5% [Citation169]. As the concentration of organics increases in the summer, low recovery is expected (vegetative period).
Janishevskaja et al. investigated seasonal variations at a Cu–Ni plant in Russia (JSC ‘Kolskaja GMK’), noting that residual concentrations of xanthates and Aeroflot in process waters played an important role in the saprotrophic bacteria growth. In particular, a seasonal drop in reagents concentration during summer was discovered to increase collector consumption during the warm months. Four different strains of Pseudomonas bacteria increased in number in the tailing ponds during the summer-fall season, observed as a ‘water bloom’, consuming flotation hydrocarbon reagents. Moreover, laboratory tests have shown that the bacteria reduced sulphide flotation kinetics [Citation16].
3. Oxide ores
3.1. Collector efficiency
Seasonal changes in oxide ores flotation are frequently associated with collector efficiency, particularly in terms of solubility and selectivity under low (winter) temperatures. Cationic collector efficiency at different temperatures is dependent on the dissolution (diffusion) rate and adsorption density, along with other factors. Collector adsorption density (Г) increase with temperature can be represented through an Arrhenius-type equation [Citation136,Citation170–172]:
(29)
(29) where Г is adsorption density (mol m−2), r is the collector’s polar group radius (m), C is the concentration of collector molecules (mol m−3), z is the valence of adsorbent, e0 is the elementary charge (1.602 × 10−19 coulombs), ΔE is mineral surface potential (V) in relation to a zero charge, n is the number of carbon atoms in a collector radical group, ΔWa is the energy (J) of association for each CH2 group, ΔWx is the energy (J) of non-electrostatic interaction between a polar group of a collector and a mineral surface in joule, k is the Boltzmann constant, and T is the temperature in Kelvin. Data from JSC ‘Mihajlovsky GOK’ in Russia shows seasonality-related issues in the reverse flotation of quartz when cleaning the magnetite concentrate, which are related to low temperatures: as the pulp temperature dropped below 10–12°C between November and March, there was a 2–3% decrease in grade and recovery of the final product [Citation173]. In the Lanping Pb–Zn oxide mine located in Hengduan Mountains (China), Zhang et al. reported that the flotation pulp, which used octadecyl amine as a collector, needed to be heated up to 25–30°C to achieve good zinc recovery [Citation174].
Kulkarni and Somasundaran [Citation175] conducted adsorption and flotation tests using oleic acid as a collector for haematite flotation. They demonstrated that temperature was important for facilitating molecular transport and increasing solubility of oleic acid by increasing the soluble oleate (R−) and acid-soap (R2H−) ions. The prevalence of ionic species ensures faster adsorption kinetics. A lower adsorption density, but with stronger bonding was achieved, compared to a regime dominated by oleic acid (RH) and associated non-solvated droplets adsorption. Consequently, higher temperatures provided better haematite recovery [Citation175]. Reverse flotation of quartz from haematite ores using RA-915 (fatty acid collector, composition not disclosed) was studied by Li and Hui, who found that iron recovery and grade in the cleaner stage were both reduced if the slurry temperature in the rougher stage was increased. When the cleaner stage temperature dropped from°C 30 to 22°C, the iron grade reduced by 3%. It was suggested that the flotation temperature should be kept between 30°C and 35°C, while the temperature difference between the rougher and cleaner stages should not be too large [Citation36].
Work by Cooke et al. on iron ore flotation showed that at pH 6, haematite recovery improves with temperature, while calcium-activated quartz recovery drops. Moreover, out of fatty acids such as linolenic, linoleic, oleic, elaidic, and stearic acids, the last one was found to be most heavily dependent on temperature. It was speculated that in such systems, temperature-assisted collector diffusion rate plays a decisive role in flotation performance through induction time control [Citation176]. Improvements to long chain fatty acid solubility and collection efficiency at low temperatures (down to 10°C) have been demonstrated through collector modification targeting α-carbon position with amino and hydroxyl groups [Citation177]. In general, improvements to flotation with fatty acids in cold pulps is achievable through mixtures with non-ionic reagents, or through collector modification (sulphonation, halogenation, nitration, etc.).
Pyrolusite flotation with oleic acid between 23°C and 60°C also indicated recovery improvements (from 40% to nearly 100%) [Citation178]. For ilmenite fatty acid flotation, it has been demonstrated that the temperature of the flotation stage is more important than the conditioning temperature. Ilmenite recovery increased with temperature, while the quality of the concentrate deteriorated owing to increased collector adsorption on some gangue (feldspars). It was also noted that collector physisorption on feldspars increased at pH 8, whereas at pH 9.5, such trends were not observed [Citation179].
3.2. Flotation modifiers and water quality
Depressants are also vital in improving the selectivity of oxide flotation. The depressing effect of starch at different temperatures was investigated in the context of quartz reverse flotation in Brazilian iron ore processing. Successful applications of starch in flotation requires gelatinisation by NaOH addition or increased temperature. For the regular non-modified cornstarch used in the work by Carlos Silva et al., it was shown that the minimum temperature allowing gelatinisation without NaOH addition was 65°C, indicating an increase in the depressive effect with increasing temperature [Citation180].
Within the frameworks of flotation modifiers and flotation temperature, a novel approach to haematite fines recovery is the application of thermo-responsive polymers (e.g. PNIPAM) [Citation37]. This is the next step of profiting from temperature changes in flotation, where PNIPAM is utilised as a multitasking reagent. According to Ng et al., the ore was first conditioned with PNIPAM at 20°C, which allowed haematite flocculation, with subsequent flotation at elevated pulp temperature (50°C) at which PNIPAM demonstrates hydrophobic properties and acts like collector. The PNIPAM results appear promising when compared to sodium oleate tests for +20 μm samples (see ).
Figure 14. Schematic representation of PNIPAM haematite flotation steps, with results compared with sodium oleate flotation, adapted from Ref. [Citation37].
![Figure 14. Schematic representation of PNIPAM haematite flotation steps, with results compared with sodium oleate flotation, adapted from Ref. [Citation37].](/cms/asset/5ed04387-3d36-49a1-8f5c-34d0e94f5a25/ycmq_a_2127788_f0014_oc.jpg)
For base metal oxide ores, sulphidation with subsequent xanthate flotation is often employed [Citation181]. As highlighted by Tyushkova et al., this process is also temperature dependent: large lead losses were registered when the process temperature decreased from 24°C to 15°C. Thus, conditioning at elevated temperatures was included in the flowsheet of the pre-production trials [Citation182]. In another example, smithsonite (a zinc carbonate mineral) sulphidation was enhanced by heating the pulp from 20°C to 60°C, which resulted in a 30% flotation recovery increase [Citation181].
Another potential reason for possible seasonal variation in oxide flotation lies in the process water quality. For example, Niobec (Canada) reported a mean 4% niobium (pyrochlore) recovery decrease in winter, which was related to a decrease in the preceding carbonate flotation efficiency (). A 4-fold increase in tall fatty acid consumption has been found during the cold season. An investigation of the discrepancy trigger revealed that process waters contained more phosphorous during winter as algae activity in cold seasons was suppressed [Citation62].
Figure 15. Seasonal variations of niobium recovery at Niobec facilities, adapted from Ref. [Citation62]: months with negative recovery shift have blue bars, with positive shift having red bars, months with close to zero shift (<1%) have yellow bars or no bars. The zero point is mean summer recovery.
![Figure 15. Seasonal variations of niobium recovery at Niobec facilities, adapted from Ref. [Citation62]: months with negative recovery shift have blue bars, with positive shift having red bars, months with close to zero shift (<1%) have yellow bars or no bars. The zero point is mean summer recovery.](/cms/asset/dd7e33a1-e24a-47a5-b2dd-17d90749e366/ycmq_a_2127788_f0015_oc.jpg)
4. Industrial minerals
Industrial minerals flotation is also sensitive to seasonal variations. It involves collectors of anionic and cationic nature, with temperature-dependent efficiency, which requires the development of new reagents, utilisation of reagent mixtures, and/or maintaining acceptable pulp temperatures. For example, Cao et al. studied the use of benzohydroxamic acid (BHA) to remove iron from a potassium feldspar ore. It was found that BHA could enhance the collecting performance of oleic acid at low temperature. With 1.2 kg t−1 oleic acid, Fe grade in the reverse flotation concentrate increased from 0.67% to 0.93%. However, the Fe recovery decreased from 83.61% to 48.47% when the temperature decreased from 45°C to 15°C. This was attributed to the poor solubility and collecting ability of oleic acid at low temperature. If BHA is mixed with oleic acid (coded as Yb105 mixed collector), the temperature influence on collecting capacity was minimal, and a concentrate with a higher iron recovery and a lower iron grade could be obtained at the same temperature. The better collecting effect of BHA was explained by the formation of a more stable chelate between the acid and the iron. The –C(O)NHOH group could chelate with metal ions to form stable chelate, and the π–π conjugated bond in the benzene ring can enhance the stability of chelate. A stable O–O chelate pentacyclic compound could be obtained through the interaction between the bidentate in benzohydroxamic acid and the iron ions [Citation38]. Flotation experience of other common industrial minerals is given in the following sub-sections.
4.1. Sulphates and carbonates
Flotation of magnesium and calcium carbonates is strongly dependent on mineral dissolution. Thus, in laboratory experiments of magnesite flotation from dolomite, it has been shown that the largest selectivity was achieved at 15°C. In the presence of oleic acid, dissolution of cations from the surface of magnesite was maximum at 25°C, while for dolomite it was at 15°C at pH 11. Consequently, the largest ratio between dissolved cations from dolomite and magnesite was found at 15°C, which resulted in more metal cation adsorption sites on magnesite compared to dolomite [Citation183]. Further research into magnesite and dolomite dissolution at different temperatures by Li et al. identified that dissolution generally decreases for both minerals with increasing pH (up to 11). A decrease in the mineral solubility was demonstrated to be beneficial for flotation with dodecylamine (DDA) as a collector. When the pH was greater than 11 (i.e. at 12) the solubility increased drastically. This was explained by the formation of Mg(OH)2 which is more stable than MgCO3 [Citation39].
In alunite flotation, oleic acid as a collector shows improvements as the temperature increases, allowing for reagent savings. In the temperature range from 20°C to 80°C, the largest savings of collector were observed by increasing the temperature from 35°C to 50°C. Above 50°C, an increase in temperature did not yield further substantial improvements in reagent savings [Citation184]. In addition to collector efficiency, sulphate and carbonate flotation practice relies heavily on depressants. Celestite laboratory flotation tests revealed that at low concentrations of depressant in a quebracho-oleic acid-celestite system at neutral to mildly alkali pH conditions, a temperature increase shifts the equilibrium towards surface hydrophobization; however, high collector dosages masked the temperature effects [Citation185]. In tests on calcite, which is a common gangue in celestite ores, flotation recovery was maximum at high collector dosages and with increased temperature: from below 10% at 20°C to approximately 95% at 50°C. The depressing effect of the quebracho on calcite during the tests increased with temperature in the same tested ranges: the recovery of calcite dropped from 95% to 5–10% [Citation185].
In order to improve selectivity, magnesite processing plants apply and test different combinations of depressants, carboxymethyl cellulose (CMC) being a common one [Citation40,Citation41]. Unlike most magnesite collectors, CMC solubility is considered good at both cold and warm temperatures. However, when considering a plant operation with a recycled water system, the seasonal triggering of biodegradation of the chemical should be taken into account [Citation186].
A study on Nigerian baryte flotation by Ofor and Nwoko using oleate showed decreased reagent adsorption density at temperatures above 40°C, which was accompanied by a decrease in recovery. The authors explained this through changes in the adsorption mechanism and dissociation of chemisorbed collector from the baryte surface [Citation187].
4.2. Fluorite
Laboratory tests by Li et al. on a fluorite ore from Hebei (China) revealed that an increase in temperature from 13°C to 27°C considerably improved flotation performance (10% recovery increase), which was accompanied by a slight grade decrease of 0.8% (). A similar effect was found in tests which varied the oleic acid collector dosage. To achieve optimal flotation performance, it was suggested to decrease collector dosage when the temperature rises [Citation188]. In Chinese fluorite flotation plants, it is common to heat up the pulp to 40°C to improve the flotation performance of oleic acid through the improved dispersing conditions [Citation43]. Consequently, some fluorite mines in northern China were reported to experience significant energy costs in winter [Citation189]. Laboratory scale tests demonstrated that by using a GY-2 collector at 10°C, it was possible to achieve similar flotation results as with oleic acid at 35°C [Citation43].
Figure 16. Effect of flotation temperature on fluorite grade and recovery at pH 9 with oleic acid, adapted from [Citation188].
![Figure 16. Effect of flotation temperature on fluorite grade and recovery at pH 9 with oleic acid, adapted from [Citation188].](/cms/asset/a841a50f-8e6a-4766-80be-7a73da9a3559/ycmq_a_2127788_f0016_oc.jpg)
At JSC ‘Jaroslavsky GOK’ in the Russian Far East, decreasing ore quality and low winter temperatures challenged the sustainable production of fluorite through flotation, which was addressed by the addition of organic thermoregulatory modifiers. It was found that the application of oxyethylated compounds allowed fluorite flotation at 12°C [Citation190]. Moreover, laboratory pre-emulsification trials of fatty acids of tall oils were found to be efficient. For example, pre-emulsification with oxyethylated fatty acids showed the potential to exclude costly pulp heating in winter for the Mongolian Bor-Undur GOK flotation plant [Citation191].
Cold winter conditions were also blamed for the decrease in efficiency of industrial trials on Jaroslavsky GOK plant with mixed collectors (Asparal F and fatty acids of tall oils) [Citation44]. Consequently, degradation of selectivity associated with cold winter temperatures were addressed by introducing a more selective and temperature resistant mixture of collectors and activators: sodium fluorite, a mixture of diphosphonic acids and tetra-sodium salt of modified aspartic acid [Citation192].
Chen et al. also noted that due to the low solubility and poor dispersity of oleates under low temperatures, good fluorite flotation performance could be achieved only by heating the pulp to above 30°C, or by using new reagent systems [Citation46]. They investigated the use of petroleum sodium sulphonate in fluorite flotation rather than conventional sodium oleate systems. A sulphonate collector (PSK-13) was investigated at different temperatures with Na2CO3 as pH modifier and Na2SiO3 as a depressant. PSK-13 is an anionic surfactant, mainly composed of sulphonates (37%) with a general structure RSO3Na, where R is an alkyl group. Closed circuit flotation studies showed that the CaF2 grade gradually increased by decreasing the temperature: 97.9% (30°C), 97.8% (20°C), 98.3% (10°C), and 98.6% (5°C). In the same tests the recovery dropped with the temperature: 88.1% (30°C), 84.4% (20°C), 79.5% (10°C), and 75% (5°C). The decreased recovery might be due to lower adsorption of PSK-13 on fluorite. Adsorption tests indicated that when the temperature was below 15°C the adsorption of oleate on fluorite decreased rapidly, whereas the adsorption of PSK-13 decreased slowly with the temperature drop in the ranges of 5–30°C. Generally, the adsorption of PSK-13 was significantly higher than that of oleate, which explained the better efficiency [Citation46].
Corpas-Martínez et al. also demonstrated that at temperatures below 30°C, oleates and oleic acid lead to a significant decrease in fluorite recovery (). They suggested utilising new collectors (DP-I and DP-II), which consist of a mixture of oleic, linoleic and rosin acids. These mixed collectors did not demonstrate a sharp decrease in recovery below 30°C [Citation47].
Figure 17. Fluorite recovery as a function of pulp temperature and collector type, adapted from Ref. [Citation47].
![Figure 17. Fluorite recovery as a function of pulp temperature and collector type, adapted from Ref. [Citation47].](/cms/asset/1181c453-c47c-4cd6-95df-14d2171f8d31/ycmq_a_2127788_f0017_oc.jpg)
Another attempt to improve oleic acid flotation of fluorite at low temperatures using ‘boosters’ was by Zhou and Lu [Citation48]. At 10°C, the tested boosters improved recovery, especially B700 and B724. When the oleic acid concentration was 10−5 mol L−1, just 1% of a booster recovered almost all of the fluorite. With the presence of 1% B710 or 1% B724, a temperature decrease from 25°C to 5°C led to less than a 5% drop in recovery. Compared to saponified oleic acid and sonicated oleic acid, oleic acid mixed with boosters could reduce the collector dosage by approximately 50% in low-temperature flotation conditions [Citation48]. Deng et al. also studied methods of improving fluorite flotation with oleic acid at temperatures below 15°C, which was considered a threshold for effective oleic acid flotation. Tests at 8–9°C confirmed that applying only sonication as an emulsification method was not sufficient. They developed an approach of adding 20–30% diesel, 10% sodium dodecyl sulphate, coupled with sonication, which yielded high fluorite recovery and grade (85.3% and 98.4% respectively) [Citation193]. Reasonable fluorite recoveries at low pulp temperatures were also obtained using the EV-1 collector synthesised from EvodiaeFructus oil. Pulp temperature still significantly affected flotation results: at 6.5°C CaF2 recovery was around 80%, while at 30°C it reached almost 97% [Citation49]
4.3. Phosphates
Phosphorus minerals’ flotation is also challenged by low efficiency and low thermal robustness of collectors, especially when it concerns separation from silicate gangue. However, the separation of phosphorite from silicates in a Peruvian ore has shown to be insensitive to temperatures between 25°C and 40°C [Citation194]. Possible methods of dealing with efficiency issues are: mixing reagents, use of boosters, and modifying the properties of fatty acids and soaps. For example, to improve flotation, some alternative collectors (e.g. cottonseed oil) which have better miscibility at room temperature have been suggested [Citation195]. Furthermore, Li et al. investigated the impact of boosters on phosphate rock flotation with soapstock of cottonseed oil as collector. It was found that anionic and non-ionic surfactants could be used as low-temperature boosters. At 7–12°C, the addition of 5% of sodium dodecyl sulphate (SDS) to the soapstock resulted in 30.7% recovery increase. Tests with only soapstock suffered from larger temperature impacts compared to the tests with a booster [Citation51].
In other research, Zhang and Li studied low temperature (13–15°C) phosphate ore flotation using cotton soap as main collector, mixed with different surfactants. It was found that cotton soap mixed with 10% SDS and a polar organic compound (exact composition unknown) could achieve the highest P2O5 recovery (89.9%) among all studied chemicals. This boosting mechanism was explained by lowering the surface tension of the polar organic compound. Adding SDS and this polar organic compound could decrease the surface tension and therefore improve the solubility and dispersibility of cotton soap in water [Citation196].
Another collector system has been suggested by Zheng et al., who synthesised a new apatite flotation collector (EV-1) out of evodiae fructus oil using saponification, sulphation, and amidation. Compared to the traditionally used commercial collector (also a mixture of organic compounds), EV-1 gave 73.4% P2O5 recovery at 5°C, versus 64.6% recovery using a commercial alternative. At 12°C, the recoveries for traditional and EV-1 were 74.9% and 86.5% respectively. The reason for EV-1’s better performance at low temperature was attributed to the content of fatty acids and their low melting points (as shown in ). For instance, the content of C17H29COONa and C17H29CONH2 (which have melting temperature as low as −11°C) is 10.9% in EV-1, compared to only 1.9% in the traditional collector. Consequently, the solubility and flotation performance at low temperatures were improved [Citation50].
Figure 18. Comparison of the composition of a commercial fatty acid collector and EV-1, plotted using data from Ref. [Citation50].
![Figure 18. Comparison of the composition of a commercial fatty acid collector and EV-1, plotted using data from Ref. [Citation50].](/cms/asset/66de17c6-7da4-4d16-a5f1-33931578ac6e/ycmq_a_2127788_f0018_oc.jpg)
Recovery of phosphorus minerals has challenges under conditions with significantly lower temperatures, especially in the Arctic regions. Thus, at the JSC ‘Apatity’ plant in Russia, it has been demonstrated that winter water temperature (10°C after mixing with recycled water) was below the ideal temperature for apatite flotation (16–20°C) under the used reagent scheme (mixture of different tall oils with sulphonate) [Citation197]. This resulted in poor selectivity, increased consumption of reagents, decreased recovery, and excessive frothing. Consequently, the plant was forced to heat the pulp to maintain plant operations during the 8-month-long Arctic winter. The possibility of operating without heating has been demonstrated at a laboratory scale using different combinations of oils and resin acids [Citation197,Citation198]. Located in the Arctic Circle, the Swedish iron company LKAB used Atrac collector for apatite reverse flotation. In laboratory tests, it was found that apatite flotation kinetics, even when using this non-conventional collector, was subject to variations with temperature in the range of 10–30°C [Citation52,Citation53]. In addition to temperature, apatite flotation is also sensitive to water quality, specifically to the presence of such ions as Ca2+, , and
[Citation199]. Thus, research on Siilinjärvi concentrator (Finland) sampling data revealed seasonal and daily variations in pH and the above-mentioned ions, potentially contributing to fluctuations in flotation efficiency [Citation199].
To overcome low-temperature issues, Xu studied new collectors at 8–9°C for phosphate flotation. In the case study of PA-808A collector at Xinpu (China) phosphate flotation plant, under low temperatures (7–9°C), low mass pull and low recoveries were observed in a cleaner stage. Xu tested PA-900B collector and concluded that it could be used as a phosphate flotation collector, without pulp heating throughout the year provided that there are seasonal adjustments to the reagent dosage [Citation54].
4.4. Salts
Unlike most other ore types, sylvite flotation generally degrades at elevated temperatures. The first reason is an increased adsorption of the amine collector to slime particles [Citation200]. Seasonal summer drops in potash flotation recovery from Solikamsk ores (JSC ‘Sylvinite’ plant) in Russia were reported when the pulp temperature reached 35–37°C [Citation201]. Lower sylvite recoveries were also reported at JSC ‘Belaruskali’ in Belarus during the summer months with the temperature reaching 36–39°С. Lower recoveries were explained by increased adsorption of amine collector on NaCl and clay minerals [Citation202]. Aliferova, who investigated sylvinite flotation of JSC ‘Sylvinite’ (Russia), reported weak amine adsorption on coarse sylvite particles, revealed through higher losses of the coarse fraction in summer (pulp temperatures between 32°С and 37°С) compared to the flotation performance in winter (pulp temperature between 20°С and 25°С) [Citation201]. So, for larger particles amines with longer hydrocarbon chains should be used, which is realised through collector mixtures [Citation200]. Aliferova noted out that amine collector mixtures possess 2–3 times lower viscosity compared to individual collectors [Citation201]. By increasing the temperature above 25°C, it has been reported that the solubility of shorter chain amines increases, disturbing the mixture balance and decreasing sylvite recovery [Citation200]. Consequently, longer chain amines are generally used in summer (recommended above 32°C), while shorter chain amines are preferred in winter (recommended below 15°C) [Citation203].
Another possible reason for lower flotation performance during summer is connected with ores extracted from deep mines leading to higher brine temperatures, causing increased KCl surface hydration and a decrease in amine adsorption [Citation200]. Additionally, the solubility of magnesium salts also has a depressing effect on sylvite, which increases with temperature [Citation204]. A concentration of magnesium ions in the brine greater than 4% has the potential to negatively impact sylvite flotation though increased viscosity [Citation205], even though increased magnesium concentrations would theoretically decrease sylvite dissolution by rising KCl dissolution temperature (, and Equation (30)).
(30)
(30) where S is total salinity (NaCl + KCl, in wt-%), T is the dissolution temperature of sylvite, M is MgCl2 concentration in wt-%, while a, b and c are constants. Regarding NaCl flotation, Abu-Hamatteh and Al-Amr investigated carnallite reverse flotation at different temperatures [Citation207]. A sharp decrease in NaCl removal efficiency (related to the halite recovery to froth) was observed when the temperature increased from 20°C to 50°C [Citation207], which might be related to increased mineral dissolution.
Figure 19. Solubility surfaces for (a) halite and (b) sylvite as a function of MgCl2 and temperature (in a system with NaCl:KCl ratio of 1:1), adapted from Ref. [Citation206].
![Figure 19. Solubility surfaces for (a) halite and (b) sylvite as a function of MgCl2 and temperature (in a system with NaCl:KCl ratio of 1:1), adapted from Ref. [Citation206].](/cms/asset/53bbb62f-af34-4e1a-94ba-e68ea06254d0/ycmq_a_2127788_f0019_oc.jpg)
Cold temperatures have also been reported to degrade potash recovery, such as in Saskatchewan, Canada. Essilfie attributed the negative impact on flotation at cold temperatures to lower transport kinetics of sylvite as a result of increased viscosity and associated lower bubble velocities and bubble-particle transport [Citation203]. Another mechanism related to viscosity issues is that at too low a temperature, there is an increase in viscosity which triggers sylvite surface hydration by increased contact with magnesium ions coming from slimes [Citation200]. Some laboratory tests have also demonstrated a decreased desliming efficiency by dropping the temperature from 45°C to approximately 15°C [Citation208]. Thus, optimal potash flotation was reported to be at approximately 21°C [Citation200]. Essilfie suggested 20–32°C as the optimal sylvite flotation temperature, at temperatures below 15°C, an increased viscosity would degrade flotation through poor amine dispersion [Citation203]. Wang et al. found that poor collector efficiency in cold pulps was the main reason behind the Chinese Qinghai Avic Resources Ltd plant’s annual scheduled shutdowns from November to March, as well as some other plants in the Chaerhan area with winter temperatures below 10°C [Citation42]. A suggested solution is to improve amine dispersion through the application of a mixed amine collector scheme [Citation209]. Another Chinese producer, Qinghai Salt Lake Group Co., mitigated the negative winter impact by using reverse flotation technology, floating NaCl from carnallite with a more temperature robust DDM collector [Citation42].
5. Critical minerals
Critical minerals are the source of raw materials which are essential for the booming industrial sectors. Their supply and economic importance are below an established, arbitrary, safety threshold [Citation210]. Exponential growth in renewable energy, energy storage systems, electronics, electric cars, and construction steel sectors require rare earth (RE), tungsten, carbon, and lithium minerals among others.
5.1. RE minerals
Flotation of RE minerals and associated gangue is highly influenced by temperature variations. Using the Mountain Pass mine (USA) as an example, bastnaesite flotation from calcite and barite is a system consisting of semi-soluble minerals. This semi-solubility amplifies flotation variability with temperature. Improvement in flotation selectivity though surface cleaning and selective collector adsorption was facilitated on the plant through pulp heating. It has been found that temperature has no effect on depression using lignin sulphonate, while collector adsorption was the most optimal at approximately 70°C for oleic acid and 85°C for tests with alkyl hydroxamic acid [Citation211–213]. High pulp temperatures were also applied in a series of industrial-scale tests conducted on the RE concentrator at 60–65°C [Citation214].
For the largest RE mine in China, Bayan Obo, it has been confirmed on an industrial scale that bastnaesite grade and recovery increase in the temperature range of 25–75°C, with more than 80% of the growth observed by increasing the temperature up to 50°C [Citation215]. Further investigations revealed a decrease in collector adsorption density with temperature, which was, however, accompanied by a larger amount of characteristic (–C(=O)N–) bonds indicating stronger adsorption [Citation215]. Similar conclusions of the greater difference in adsorption-free energies between gangue minerals and bastnaesite have been demonstrated in experiments on the Mountain Pass ore [Citation211]. It has been suggested that the easily detachable physisorbed collector layer becomes chemisorbed with a temperature increase, promoting the growth of chelate complexes [Citation215]. Using L102 and L108 collectors, for the example of the tailings from the Baotou (China) concentrator, a possible optimal operation at lower temperatures (35–45°C) has been demonstrated [Citation216]. Research conducted on Wei Shan (China) ore [Citation217] and the middlings of the Baotou mineral processing plant [Citation218] came to a similar conclusion indicating an optimal temperature range being 40–43°C. It has been demonstrated that the selectivity index is poor at pulp temperatures below 32°C, and above 44°C, and the poor efficiency at a higher temperature was associated with collector decomposition [Citation217]. Studies on mixed collector systems on ores from Sichuan and Hubei RE mines were assumed to be more robust in relation to normal temperature fluctuations [Citation24].
Sodium metasilicate acts as a typical depressant in RE mineral flotation systems. Its solubility and dissolution rate increase substantially with temperature [Citation219]. Pradip and Fuerstenau investigated monazite flotation from rutile-zircon mixtures, revealing that with a temperature rise in systems with oleic acid or potassium octyl hydroxamate collectors accompanied by sodium metasilicate or sodium sulphide depressants there is a clear trend of improved selectivity [Citation211]. Tests with the commercial hydroxamic acid-based Flotinor V3759 collector demonstrated a selectivity loss with increased temperature, as the collector showed an increase in collection effectiveness with temperature, which increased greater than the depressive ability of sodium metasilicate and sodium sulphide [Citation56]. Another commercial collector from the Flotinor series, Flotinor V3579, doubled the amount adsorbed on bastnaesite surfaces as the temperature increased from 25°C to 80°C [Citation55]. Moreover, in the flotation of bastnaesite and xenotime, it was found that the free surface energy of the hydroxamate collector increases with temperature, providing a rise in the recovery of floated minerals [Citation55,Citation220].
5.2. Tungsten minerals
Cold winters can also cause problems for different scheelite flotation plants [Citation221]. Samatova et al. investigated the Primorskaja plant in Russia; it was noted that a temperature drop leads to poorer collector solubility and an increased concentration of micelles with a shortage of ionic species for adsorption on mineral surfaces [Citation222]. To expand the ‘window’ of operable collector dosages under conditions of lowering of critical micelle concentration with temperature, mixed chemistry may be applied, e.g. mixtures of fatty acids with non-polar surfactants (polyoxyethylene ether) [Citation30]. Thus, Chen et al. investigated the effect of the addition of a non-ionic polyoxyethylene ether (JFC-5) to scheelite flotation with sodium oleate (NaOl). It was found that at 10°C, the recovery of scheelite could be increased from 22% to 85% in the presence of JFC-5 with a mass ratio of 20% at pH 10. This was attributed to the reduced electrostatic repulsion between the oleate ions by JFC-5, which resulted in enhanced NaOl adsorption on scheelite surface [Citation57].
Seasonality effects may also be attributed to the water quality and associated changes in chemistry. For example, the Luanchuan flotation plant (China) was experiencing a 5–8% recovery drop in winter, associated with fluctuations of silica glass in process waters. It was found that winter process water contained more than 500 mg L−1 Si, which created an excess of reagent in the system and depressed the scheelite [Citation223,Citation224]. As a solution to a seasonal (from late autumn to early spring) degradation of the scheelite concentration index at a Hunan copper-tungsten separation plant, similar to the Primorskaja plant, a special mixture of collectors that exhibits resistance to low temperatures (down to 10°C) has been suggested, where the ratio of the reagents is adjustable depending on pulp temperature [Citation225]. Some laboratory investigations of scheelite flotation determined that at a temperature of 10°C mineral recovery was improved by a sodium oleate – TX15 (a collector based on octaphenyl polyoxyethyienes) mixture at a ratio of 5:1 [Citation27]. In another study by Zhu et al. oleate collector efficiency for cold pulps was enhanced by adding MOA-9 (lauryl alcohol polyoxyethylene ether), where a synergistic effect with the non-ionic reagent was explained by the screening of electrostatic repulsions between the polar anionic groups [Citation30].
Adsorption and flotation studies by Meng et al. at elevated temperatures conducted on wolframite fines using sodium oleate demonstrated that higher pulp temperatures improved ionisation of oleate species intensifying chemisorption and flotation performance [Citation226].
5.3. Coal
As summarised by Bhattacharya and Pascoe, temperature fluctuations in coal flotation have a climatic origin but may be also related to seasonal changes in water blending strategies [Citation9]. Non-polar reagents are the principal collectors of naturally hydrophobic minerals, including coal, and it has been reported that their strength increases with a viscosity [Citation227]. It is suggested that in coal flotation, reagent efficiency is dependent on viscosity and dispersibility. Generally, as non-polar reagent viscosity rises, the temperature required to maintain the same level of dispersibility also increases. When the collector dispersion level is too high and the non-polar reagent droplets size is less than 5 μm, there is insufficient kinetic energy for droplet-particle collisions. Therefore, an excessive temperature rise may decrease coal flotation efficiency [Citation228]. Moreover, from adsorption studies with different hydrocarbons, it was found that the temperature-induced viscosity decrease correlates with contact angle values, implying a decrease in reagent adhesion to minerals [Citation227].
Temperature also controls coal surface oxidation, which impacts recovery. Research by Gayle et al. on a low volatile bituminous coal and an alcohol-type frother demonstrated a decrease in recovery due to increased coal surface oxidation because of increased conditioning time at room temperature. Applying a mixture of kerosene and pine oil translated into consistent recovery values as a result of being more robust towards coal oxidation [Citation229].
Another mechanism contributing to seasonal variations suggested for coal flotation plants in northern China was the formation of nanobubbles on mineral surfaces in winter. For nano-bubbles formation, a temperature gradient is required, which could be obtained when a slurry is transported from the cold outside to the warm interior part of the process plant [Citation230]. Laboratory tests confirmed an improvement in coal flotation efficiency when nano-bubbles were formed on the surface [Citation231].
Frother properties and efficiency also play a critical role in coal flotation. Froth volume was reduced by half when the temperature dropped from 25°C to 10°C when pine oil was used [Citation232], and a more stable froth was produced. Higher solubility and lower media viscosity at elevated temperatures provide better efficiency of air dispersion, reflected through finer bubbles and greater recovery [Citation9].
There is also a hypothesis explaining the decrease of coal recovery at temperatures above 40°C, observed in tests with Barnesboro (USA) coal plant rejects, through the frothing coefficient. The frothing coefficient is believed to be in direct relationship with coal recovery [Citation233], as can be seen from Equation (31):
(31)
(31) where
is the froth coefficient,
is the froth volume (cm3), and
is the froth stability in seconds. The frothing coefficient is seen to decrease for elevated temperatures, leading to lower coal recoveries [Citation233]. Investigations into laboratory coal flotation accompanied with contact angle measurements at different temperatures concluded that coal flotation variation is heavily dependent on physical factors rather than chemical [Citation234].
Flotation studies with coal samples taken from the Karbomet Mining Coal Washing Plant in Turkey also confirmed the existence of an optimal flotation temperature range between 25°C and 50°C, low recovery at higher temperatures was explained by decreased froth stability and increased bubble burst-rate [Citation235]. Bailey and Whelan [Citation236] obtained a slightly different optimal flotation temperature range (20–40°C) for weakly hydrophobic coals, while for strongly hydrophobic coal flotation, the impact of temperature was insignificant [Citation9]. Similar results were also obtained in laboratory flotation trials with anthracite, graphite and coking coal, where the flotation rate constant was found to have a non-linear behaviour with temperature (modelled with a second degree polynomial) [Citation82].
5.4. Lithium minerals
Spodumene (LiAl(SiO3)2) is reported to be floated using anionic collectors, known to be sensitive to temperature variations. Laboratory testing of ore from Vilatuxe (Spain) by Menendez et al. revealed that an increase in temperature above 33°C resulted in higher recoveries, with an abrupt selectivity loss observed above 50°C. Consequently, the best results for the ore under the investigated reagent scheme were achieved at 15°C [Citation237] (). Xu et al. found that when only oleic acid was used as a collector for spodumene and feldspar flotation, there was a dramatic decrease in recovery for both minerals below 30°C, while a mixture of oleic acid with dodecyl trimethyl ammonium chloride reduced the negative impact of temperature drop for spodumene, with no significant impact on the recovery profile of feldspar [Citation238], providing a greater degree of freedom for selective separation.
Figure 20. Recovery and grade of lithium concentrate at different temperatures of the flotation pulp, adapted from Ref. [Citation237].
![Figure 20. Recovery and grade of lithium concentrate at different temperatures of the flotation pulp, adapted from Ref. [Citation237].](/cms/asset/e3ed8f82-13e1-4027-aba1-b4cdeac6182d/ycmq_a_2127788_f0020_oc.jpg)
Recovery of another lithium mineral, lepidolite, from Yichun Tantalum Niobium Mine (China) tailings was accomplished with coco amine, which resulted in significant winter losses. Poor concentrate quality and recovery were associated with the low crystallization point (17°C) of coco amine. To address such poor efficiency, it was recommended to apply a more complex reagent scheme involving primary alkyl amine, mixed alkyl alcohols, and alkali metal salts [Citation239].
6. Challenges and perspectives
For most minerals, there is an optimal temperature range that maximises the flotation performance. Seasonal variations could result in increased energy costs for pulp heating, decreased mineral recovery, and even shutdowns. Many sources reported the most feasible method of dealing with seasonal variations is through modifications of the reagent regime. For fatty acid flotation, most efforts have been on collector modification, while in sulphide flotation, it is generally agreed that xanthates are quite resistant to temperature fluctuations. In sulphide flotation, most seasonal discrepancies reported were related to polymetallic and zinc flotation systems, where copper activation was often considered the main trigger of low performance in winter. It is observed in the reviewed research that not only temperature but also water quality is an important factor causing recovery seasonality issues. Extreme pulp temperature conditions are seen to depress sulphides flotation by promoting surface oxidation and collector desorption at high temperatures, and by reducing reaction kinetics, increasing viscosity, and reagent efficiency at low temperatures. Non-sulphidic flotation systems demonstrated increased collector and mineral solubilities at elevated temperatures, which were more sensitive to winter temperature drops. Different sources reported optimal conditioning for flotation to be close to room temperature for most ore-types ().
Table 2. Summary of some reported optimal flotation temperatures for different minerals.
Temperatures above 40–50°C, depending on the ore and operating parameters, may indicate an upper limit of optimal operating conditions, while 10–12°C is seen as a lower optimal temperature limit. A rough generalisation could be made by stating that temperature-driven changes are more impactful on non-sulphide ores where the most common issues are increased pulp viscosity and poor reagent dispersion. Sulphide flotation systems are more temperature resistant; however, for the case of polymetallic ores and the application of several modifiers, significant changes in flotation performance could also be observed. Water quality variation is also a factor that is seen as important for both discussed flotation systems.
Different dissolution rates, precipitation and adsorption of ions in the pulp driven by altered interactions and properties of water, mineral surfaces, reagents, and air suggests expanding the concept of the flotation system as a ‘flotation triangle’ previously developed by Kawatra [Citation241] to a multi-domain ‘flotation rectangle’, as seen in .
Figure 21. A concept of ‘flotation rectangular’ demonstrates seasonal triggers sphere of influence and flotation system components interactions.
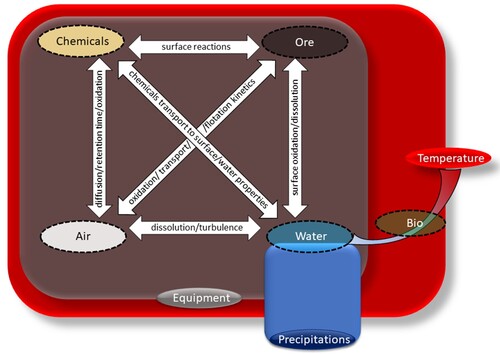
As depicted in , the temperature has the potential to trigger changes in all 4 major flotation components, by altering interactions between them, and impacting the efficiency of the equipment domain, hosting above mentioned interactions. Liquid precipitations are seen as another external trigger, that supplies fresh water to a system, predominantly impacting the water component in flotation. Increased precipitations, as a rule, lead to improvements in water quality, especially in the plants with a high rate of process water recirculation, ultimately improving flotation performance. A biological sub-trigger is activated by temperature and precipitations. It also has a strong impact on the water component and its interactions. As for the chemicals domain, because different reagents have varying power at different temperatures, plants operating at starvation regimes, or at lower bounds of chemical dosages, have higher risks to be shifted to sub-optimal areas as a result of a seasonal shift in reagent efficiency. Recirculation of process waters complicates even more the seasonally based choice of optimal reagent dosage. The air domain has been expressed through seasonal bubble size deviations, nano-bubbles, and dissolved gas contents, which are all important parameters to consider when analysing seasonal discrepancies in flotation.
Such a comprehensive understanding of flotation components’ response to external climate-induced triggers is of great importance, as the era of black-box flotation models is consigned to history when considering the new challenges that lie ahead of the industry, such as even larger fluctuations in weather conditions triggered by climate change, continuous deterioration of ore quality, scarcity of freshwater resources, increased environmental restrictions, and increased demand on minerals and metals. To successfully cope with the above-mentioned problems flotation process should be more deeply investigated which would assist in more accurate production forecasts, developing flexible flotation solutions, and understanding of the required properties for new flotation reagents. The investigations of new chemistries are one of the most important directions for sustainable flotation development, especially in view of the development of temperature-robust chemicals. Additionally, implementation of the artificial intelligence in the decision-making process at the plants would be another important step toward linking flotation performance with weather forecasts on the way to developing sustainable flotation production.
7. Conclusions
Seasonality in flotation performance has been reported on numerous plants processing different ore types. Since flotation is a physicochemical process, there are physical and chemical origins of seasonality. The triggers of seasonal changes are temperature and water quality (which depends on precipitations and bio-activity among other factors). The poor efficiency of most non-sulphide ores under cold conditions has been linked to collector solubility and dispersibility. In sulphide systems, the process of activation and depression are seen as the most vulnerable, despite some information given on reduced collector adsorption at lower temperatures. Too high a temperature also has a negative impact on flotation, mostly related to surface oxidation or dissolution processes. Water quality under conditions of recycled water supply design on a plant is seen to impact all types of flotation and should be the first point to check when seasonal patterns are revealed on a plant. The scope of future work addressing seasonal variations on flotation plants should encompass modelling the mechanisms deteriorating flotation performance on the level of individual flotation components. The development of such models would allow us to computationally reveal the most impactful factors and develop diagnostic algorithms and flexible economic solutions to seasonal process fluctuations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Fuerstenau M, Jameson G, Yoon R. Froth flotation: a century of innovation. Littleton (CO): Society for Minning, Metallurgy, and Exploration; 2007.
- Brito-Parada PR, Neethling SJ, Cilliers JJ. CFD study of liquid drainage in flotation foams. In: Computer aided chemical engineering. Elsevier; 2012. p. 1143–1147. DOI:10.1016/B978-0-444-59520-1.50087-7
- Wang H, Zhang Y, Wang C. Surface modification and selective flotation of waste plastics for effective recycling—a review. Sep Purif Technol. 2019;226:75–94. DOI:10.1016/j.seppur.2019.05.052
- Yarar B. Flotation. In: Kirk-Othmer encyclopedia of chemical technology. Wiley; 2000. DOI:10.1002/0471238961.0612152025011801.a01
- Kyzas GZ, Matis KA. Flotation in water and wastewater treatment. Processes. 2018;6:116. DOI:10.3390/pr6080116
- Hughes J, Cowper-heays K, Olesson E, et al. Impacts and implications of climate change on wastewater systems: a New Zealand perspective. Clim Risk Manag. 2020:100262. DOI:10.1016/j.crm.2020.100262
- Choung J, Walker J, Xu Z, et al. Effect of temperature on the stability of froth formed in the recycle process water of oil sands extraction. Can J Chem Eng. 2008;82:801–806. DOI:10.1002/cjce.5450820419
- Schramm LL, Stasiuk EN, Yarranton H, et al. Temperature effects from the conditioning and flotation of bitumen from oil sands in terms of oil recovery and physical properties. J Can Pet Technol. 2003;42. DOI:10.2118/03-08-05
- Bhattacharya S, Pascoe RD. Effect of temperature on coal flotation performance – a review. Miner Process Extr Metall Rev. 2005;26:31–61. DOI:10.1080/08827500490477586
- Wang C, Wang H, Liu Y. Separation of polyethylene terephthalate from municipal waste plastics by froth flotation for recycling industry. Waste Manag. 2015;35:42–47. DOI:10.1016/j.wasman.2014.09.025
- Kökkılıç O, Mohammadi-jam S, Marion C, et al. Separation of plastic wastes using froth flotation – an overview. Adv Colloid Interface Sci. 2022:89. DOI:10.1016/j.cis.2022.102769
- Downing JA. The effects of temperature and pH in flotation deinking of ultraviolet-cured inks. Pap Eng Sr Theses. 1984:124.
- El-khalek MAA. Performance of different surfactants in deinking flotation process. J Ore Dress. 2011;13:15–21.
- Lin IJ. The effect of seasonal variations in temperature on the performance of mineral processing plants. Miner Eng. 1989;2:47–54. DOI:10.1016/0892-6875(89)90064-2
- Mikhlin Y, Karacharov A, Vorobyev S, et al. Towards understanding the role of surface gas nanostructures: effect of temperature difference pretreatment on wetting and flotation of sulfide minerals and Pb-Zn ore. Nanomaterials. 2020;10:1–12. DOI:10.3390/nano10071362
- Janishevskaja E, Gershenkop A, Evdokimova G, et al. Изучение развития и функционирования микроорганизмов в процессе флотации сульфидных медно-никелевых руд на обогатительной фабрике АО“Кольская ГМК” [Investigation of microorganisms in the process of Cu-Ni flotation at Kolskaja plant]. In: VI всероссийская Научная Конференция Экологические Проблемы Северных Регионов и Пути Их Решения. Apatity: Kola Science Center of the Russian Academy of Sciences; 2016. p. 308–311. Russian.
- Liu W, Moran CJ, Vink S. A review of the effect of water quality on flotation. Miner Eng. 2013;53:91–100. DOI:10.1016/j.mineng.2013.07.011
- Delevingne L, Glazener W, Grégoir L, et al. Climate risk and decarbonization: what every mining CEO needs to know; 2020. [cited 2022 Sep 11]. Available from: https://www.mckinsey.com/business-functions/sustainability/our-insights/climate-risk-and-decarbonization-what-every-mining-ceo-needs-to-know
- Jowitt SM, Mudd GM, Thompson JFH. Future availability of non-renewable metal resources and the influence of environmental, social, and governance conflicts on metal production. Commun Earth Environ. 2020;1:1–8. DOI:10.1038/s43247-020-0011-0
- MarketsandMarkets Research Private Ltd. Froth flotation equipment market by machine type (cell-to-cell flotation, and free-flow flotation), component, application (mineral & ore processing, wastewater treatment, and paper recycling), region – global forecast to 2025; 2020. Available from: https://www.marketsandmarkets.com/Market-Reports/froth-flotation-equipment-market-186447862.html
- Global Market Insights. Flotation reagents market size, industry analysis report, regional outlook, application development potential, price trends, competitive market share & forecast, 2022–2028; 2021. Available from: https://www.gminsights.com/industry-analysis/flotation-reagents-market
- Nikolaev AA, Konyrova A, Goryachev BE. Wetting of sphalerite, chalcopyrite and pyrite in treatment with sulfhydryl collectors in saltish and Sea water. J Min Sci. 2020;56:654–662. DOI:10.1134/S1062739120046946
- Pecina-Trevino ET, Uribe-Salas A, Nava-Alonso F, et al. On the sodium-diisobutyl dithiophosphinate (aerophine 3418A) interaction with activated and unactivated galena and pyrite. Int J Miner Process. 2003;71:201–217. DOI:10.1016/S0301-7516(03)00059-0
- An D. Improved flotation of bastnaesite and chalcopyrite [PhD thesis]. Department of Mining and Geological Engineering, University of Arizona; 2017.
- Li F, Zeng X. 攀西地区氟碳 矿速矿工艺研究 [Beneficiation technology for bastnaesite in Panxi area. J Shanghai Second Polytech Univ. 1999;1:1–7. Chinese.
- Smith G, Smadi R. Surfactant compositions containing alkoxylated amines. US patent no. 6,617,303 B1. 2003.
- Chen C, Zhu H, Sun W, et al. Synergetic effect of the mixed anionic/non-ionic collectors in low temperature flotation of scheelite. Minerals. 2017;7. DOI:10.3390/min7060087
- Levanaho J, Hoover K, Spence C, et al. Effect of pulp temperature on copper and gold collectors at Hudson Bay Mining and Smelting. In: 37th Annu. Meet. Can. Miner. Process.; 2005. p. 481–496.
- Marais P. Some practical considerations in the design and operation of a plant for the differential flotation of mixed sulphides, especially copper and zinc. J South African Inst Min Metall. 1980;80:385–394.
- Zhu H, Qin W, Chen C, et al. Interactions between sodium oleate and polyoxyethylene ether and the application in the low-temperature flotation of scheelite at 283 K. J Surfactants Deterg. 2016;19:1289–1295. DOI:10.1007/s11743-016-1864-1
- Makhotla N, Hearn S, Boskovic S. The Huntsman approach to flotation frothers. In: 8th South African Base Met. Conf. Livingstone: Southern African Institute of Mining and Metallurgy; 2015. p. 129–140.
- Liu X, Li Z, Li Z, et al. 黄铁矿低温浮选试验及机理分析 [Study and mechanism analysis on the flotation of pyrite in low temperature. Conserv Util Miner Resour. 2019;4. Chinese. DOI:10.13779/j.cnki
- Celanese Corp. MIBC: methyl isobutyl carbinol. Dallas; 2013.
- Wark W, Cox A. Principles of flotation, VI – influence of temperature on effect of copper sulfate, alkalies and sodium cyanide on adsorption of xanthates at mineral surfaces. Australasian Institute of Mining and Metallurgy; 1938.
- Kubota T, Yoshida M, Hashimoto S, et al. 黄銅鉱および方鉛鉱の浮選分離における温度の影響に関する基礎研究 [Fundamental study on the effect of pulp temperature in copper-lead bulk differential flotation]. 日本鉱業会誌 [J Japan Min Assoc]. 1974;90:641. Japanese.
- Li C, Hui Y. 安徽李楼铁矿强磁选尾矿反浮选温度试验 [Temperature test of reverse flotation for high intensity magnetic separation tailings of Anhui Lilou iron ore]. 现代矿业 [Mod Min]. 2013;534:112–113. Chinese.
- Ng WS, Sonsie R, Forbes E, et al. Flocculation/flotation of hematite fines with anionic temperature-responsive polymer acting as a selective flocculant and collector. Miner Eng. 2015;77:64–71. DOI:10.1016/j.mineng.2015.02.013
- Cao Z, Qiu P, Wang S, et al. Benzohydroxamic acid to improve iron removal from potash feldspar ores. J Cent South Univ. 2018;25:2190–2198. DOI:10.1007/s11771-018-3907-4
- Li Q, Yin WZ, Ma YQ, et al. 含镁碳酸盐矿物溶解度 模拟计算及对浮选过程的影响 [Simulation and effects on flotation of solubility of magnesium carbonate minerals]. J Northeast Univ Nat Sci. 2011;32:1348–1351. Chinese.
- Baranovskii N. Flotation beneficiation of Savinsk magnesites. Refractories. 1980;21:349–352.
- Baranovskii N. Flotation beneficiation of Semibratsk magnesite. Refractories. 1968;8:740–741.
- Wang X, Miller JD, Cheng F, et al. Potash flotation practice for carnallite resources in the Qinghai Province, PRC. Miner Eng. 2014;66:33–39. DOI:10.1016/j.mineng.2014.04.012
- Zhang Y. 萤石低温浮选捕收剂的研究 [Collectors for low temperature flotation of fluorite: a study]. Min Metall Eng. 1995;15:25–27. Chinese.
- Samatova L, Kienko L, Shestovets V, et al. Промышленное внедрение низкотемпературной флотации флюорита с применением Аспарала Ф и собирательных смесей на его основе в комплексе с модификатором [Industrial testing of low-temperature fluorite flotation with asparal F mixtures]. Горный Информационно-Аналитический Бюллетень [Min Informational Anal Bull]. 2007;12:308–318. Russian.
- Zhylev A, Pihovkin L, Rykin V, et al. Смазачно-охлаждающая жидкость для механической обработки металлов [Cutting fluid for metal machining]. USSR patent no. SU777053A1. 1979. Russian.
- Chen H, Ren Z, Gao H, et al. 石油磺酸钠低温浮选石英型萤石的试验研究 [Experimental study on low-temperature flotation of quartz-type fluorite with petroleum sodium sulfonate]. Conserv Util Miner Resour. 2020;40:135–139. Chinese.
- Corpas-Martínez JR, Pérez A, Navarro-Domínguez R, et al. Testing of new collectors for concentration of fluorite by flotation in pneumatic (modified hallimond tube) and mechanical cells. Minerals. 2020;10. DOI:10.3390/min10050482
- Zhou Q, Lu S. 萤石浮选增效剂及其应用 [Fatty acid booster and its application in fluorite flotation]. 化工矿山技术 [Chem Min Technol]. 1995;24:22–25. Chinese.
- Jong K, Paek I, Kim Y, et al. Flotation mechanism of a novel synthesized collector from Evodiaefructus onto fluorite surfaces. Miner Eng. 2020;146:106017. DOI:10.1016/j.mineng.2019.106017
- Zheng G, Sun T, Kou J, et al. 新型捕收剂EV-1的合成及其在磷矿选矿中的应用 [Synthesis of collector (EV-1) and its application in flotation of apatite ores]. 化工矿物与加工 [IM P]. 2016;2:4–8. Chinese.
- Li D, Leng X, Qin F. 宜昌磷矿低温捕收剂研究 [Low temperature collector for Yichang phosphate rock]. 化工矿物与加工 [IM P]. 2010;10:1–4. Chinese.
- Su F, Hanumantha Rao K, Forssberg KSE, et al. The influence of temperature on the kinetics of apatite flotation from magnetite fines. Int J Miner Process. 1998;54:131–145. DOI:10.1016/S0301-7516(98)00021-0
- Smolko-Schvarzmayr N, Klingberg A, Nordberg H. Use of branched alcohols and alkoxylates thereof as secondary collectors. US patent no. 2017/0252753 A1. 2017.
- Xu J. 新浦磷矿低温捕收剂的选择 [Selection of collectors in Xinpu phosphate mine at lower temperature]. 化工矿物与加工 [IM P]. 2005;12:21–23, 26. Chinese.
- Anderson CD. Improved understanding of rare earth surface chemistry and its application to froth flotation [PhD thesis]. Metallurgical and Materials Engineering, Colorado School of Mines; 2015.
- Pavez O, Peres AEC. Effect of sodium metasilicate and sodium sulphide on the floatability of monazite-zircon-rutile with oleate and hydroxamates. Miner Eng. 1993;6:69–78. DOI:10.1016/0892-6875(93)90164-I
- Chen C, ling Zhu H, qing Qin W, et al. Improving collecting performance of sodium oleate using a polyoxyethylene ether in scheelite flotation. J Cent South Univ. 2018;25:2971–2978. DOI:10.1007/s11771-018-3967-5
- Wills B, Finch J. Wills’ mineral processing technology; 2016. DOI:10.1115/DSCC2013-3715
- The Mining Association of Canada. The State of Canada’s mining industry; 2019. p. 104. Available from: https://mining.ca/documents/facts-and-figures-2019/
- Calvo G, Mudd G, Valero A, et al. Decreasing ore grades in global metallic mining: a theoretical issue or a global reality? Resources. 2016;5:1–14. DOI:10.3390/resources5040036
- Rötzer N, Schmidt M. Decreasing metal ore grades – is the fear of resource depletion justified? Resources. 2018;7. DOI:10.3390/resources7040088
- Marois J-S, Downey D, Matton G, et al. Resolving detrimental seasonal effect on the flotation processes at Niobec. In: 50th Annu. Camadian Miner. Process. Conf.; 2018. p. 106–118.
- Xu M, Wilson S. Investigation of seasonal metallurgical shift at Inco’s Clarabelle mill. Miner Eng. 2000;13:1207–1218. DOI:10.1016/s0892-6875(00)00105-9
- Nesset JE, Gomez CO, Finch JA, et al. The use of gas dispersion measurements to improve flotation performance. In: 34th Annu. Meet. Can. Miner. Process.; 2002. p. 361–383.
- Malafarina L, Deredin C. Sudbury integrated nickel operations Strathcona mill [PowerPoint slides]. 53rd Annual Canadian Mineral Processors’ Conference; 2021.
- O’Connor CT, Dunne RC, de Sousa AMRB. Effect of temperature on the flotation of pyrite. J South African Inst Min Metall. 1984;84:389–394.
- Lin IJ, Chapman R. The effect of seasonal variations in temperature on the performance of JCI mineral plants. JCI Internal Report; 1982.
- Natural Resources Canada. The Canadian minerals and metals plan, Toronto; 2019. Available from: https://www.nrcan.gc.ca/sites/www.nrcan.gc.ca/files/CMMP/CMMP_The_Plan-EN.pdf
- Lafitte G. Spoiling Tibet: China and resource nationalism on the roof of the world. London: Zed Books; 2013.
- Gouvernment du Quebec. Plan Nord. Building Northern Quebec together. The project of a generation. Québec: Ministère des ressources naturelles et de la faune; 2011. Available from: https://numerique.banq.qc.ca/patrimoine/details/52327/2420759?docpos=19
- Boyd R, Bjerkgård T, Nordahl B, et al. Mineral resources in the Arctic. Trondheim: Geological Survey of Norway; 2016.
- Mekis É, Vincent LA, Shephard MW, et al. Observed trends in severe weather conditions based on Humidex, Wind Chill, and Heavy Rainfall Events in Canada for 1953–2012. Atmos Ocean. 2015;53:383–397. DOI:10.1080/07055900.2015.1086970
- Dinçer İ, Zamfirescu C. Appendix B thermophysical properties of water; 2015. DOI:10.1002/9781118534892.app2
- C. V. Umipig, E.E. Israel, G.G. Hutalle, S.R. Williams, Canatuan Cu/Zn flotation metallurgy – dealing with zinc pre-activation. In: 44tn Annu. Meet. Can. Miner. Process.; 2012. p. 187–195.
- Yianatos J. Industrial flotation process modelling, a chemical engineering approach. In: Mohanty JN, Biswal SK, Reddy PSR , et al., editors. Role of chemical engineering in processing of minerals and materials. Bhubaneswar: Allied; 2003. p. 34–45.
- van’t Hoff JH. Die Rolle des osmotischen Druckes in der Analogie zwischen Lösungen und Gasen [The role of osmotic pressure in the analogy between solutions and gases]. Zeitschrift Für Phys Chemie [J Phys Chem]. 1887. German. DOI:10.1515/zpch-1887-0151
- Rao SR. Surface chemistry of froth flotation. Springer; 2004. DOI:10.1007/978-1-4757-4302-9
- Cook M, Last A. Fluorite flotation II. Bull. Univ. Utah. Bulletin No. 47. 40; 1950.
- Arrhenius S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte [About the heat of dissociation and the influence of temperature on the degree of dissociation of the electrolytes]. Zeitschrift Für Phys Chemie [J Phys Chem]. 1889. German. DOI:10.1515/zpch-1889-0408
- Mingulina E, Maslennikova G, Korovin N, et al. Курс общей химии [General chemistry course]. Moscow: Vysshaya Shkola; 1990. Russian.
- Xu L, Hu Y, Wu H, et al. Surface crystal chemistry of spodumene with different size fractions and implications for flotation. Sep Purif Technol. 2016;169:33–42. DOI:10.1016/j.seppur.2016.06.005
- Drzymala J, Kliszowska K, Ratajczak T. Theoretical and experimental aspects of influence of temperature on kinetics of carbonaceous materials froth flotation. Miner Process Extr Metall Rev. 2021;00:1–5. DOI:10.1080/08827508.2021.1883012.
- Du B, Zhang Z, Grubner S, et al. Temperature-dependent estimation of Gibbs energies using an updated group-contribution method. Biophys J. 2018;114:2691–2702. DOI:10.1016/j.bpj.2018.04.030
- Marsden D. The effect of pH value, temperature and density on the kinematic viscosity of some South African gold mine slurries. J South African Inst Min Metall. 1962;62:391–398.
- Kawatra S, Eisele T, Zhang D, et al. Effects of temperature on hydrocyclone efficiency. Int J Miner Process. 1988;23:205–210.
- Albrecht T, Addai-Mensah J, Fornasiero D. Critical copper concentration in sphalerite flotation: effect of temperature and collector. Int J Miner Process. 2016;146:15–22. DOI:10.1016/j.minpro.2015.11.010
- Dassey A, Theegala C. Optimizing the air dissolution parameters in an unpacked dissolved air flotation system. Water (Basel). 2012;4:1–11. DOI:10.3390/w4010001
- Faust SD, Aly OM. Chemistry of water treatment. 2nd ed. Boca Raton (FL): Taylor & Francis; 1998.
- Manouchehri HR, Johansson B, Ikumapayi F. Effect of temperature in flotation of Zn from massive sulfide ores. In: 26th Int. Miner. Process. Congr. IMPC 2012 Innov. Process. Sustain. Growth – Conf. Proc.; 2012. p. 3227–3238.
- Radtke DB, White AF, Davis JV, et al. Dissolved oxygen. In: U.S. Geol. Surv. TWRI B. 9; 1998. p. 1–27.
- Zhang W. Evaluation of effect of viscosity changes on bubble size in a mechanical flotation cell. Trans Nonferrous Met Soc China Engl Ed. 2014;24:2964–2968. DOI:10.1016/S1003-6326(14)63432-4
- Kaye GWC, Laby TH. Tables of physical and chemical constants. London: Longmans; 1962.
- Gray P, Bowyer G, Castle J, et al. Sulphide deposits—their origin and processing. London: Institution of Mining and Metallurgy; 1990. DOI:10.1007/978-94-009-0809-3
- Schulze HJ. Hydrodynamics of bubble-mineral particle collisions. Miner Process Extr Metall Rev. 1989;5:43–76. DOI:10.1080/08827508908952644
- Cho Y. Effect of flotation frothers on bubble size and foam stability [Master’s thesis]. Mining and Mineral Process Engineering, The University of British Columbia; 2001.
- Leite M. Liberation by size reduction, consequences and improvements on flotation kinetics. In: Mavros P, Matis K, editors. Innovations in flotation technology. Thessaloniki: Springer; 1992. p. 149–170.
- Wills BA, Finch JA. Froth flotation. In: Wills’ mineral processing technology; 2016. p. 265–380. DOI:10.1016/b978-0-08-097053-0.00012-1
- Tongass National Forest. Quartz Hill molybdenum project mine development: final environmental impact statement; 1989. DOI:10.1017/CBO9781107415324.004
- Sutherland KL, Wark IW. Principles of flotation. Melbourne: Australasian Institute of Mining and Metallurgy; 1955.
- Drzymala J. Arrheniusan activation energy of separation for different parameters regulating the process. Physicochem Probl Miner Process. 2018;54:1152–1158. DOI:10.5277/ppmp18146
- Laskowski JS. Thermodynamic and kinetic flotation criteria. Miner Process Extr Metall Rev. 1989;5:25–41. DOI:10.1080/08827508908952643
- Lazarov D, Alexandrova L, Nishkov I. Effect of temperature on the kinetics of froth flotation. Miner Eng. 1994;7:503–509. DOI:10.1016/0892-6875(94)90163-5
- Scheludko A, Toshev BV, Bojadjiev DT. Attachment of particles to a liquid surface (capillary theory of flotation). J Chem Soc Faraday Trans 1 Phys Chem Condens Phases. 1976;72:2815–2828. DOI:10.1039/F19767202815
- He H, He T, Wang X, et al. 矿浆温度对方铅矿浮选效果的影响及机理研究 [Study on the effect and mechanism of pulp temperature on galena flotation]. Conserv Util Miner Resour. 2020;40:88. Chinese.
- Princen H, Kiss A. Rheology of foams and highly concentrated emulsions. J Colloid Interface Sci. 1989;128:176–187.
- Wang L, Li C. A brief review of pulp and froth rheology in mineral flotation. J Chem. 2020. DOI:10.1155/2020/3894542
- Li C, Runge K, Shi F, et al. Effect of flotation froth properties on froth rheology. Powder Technol. 2016;294:55–65. DOI:10.1016/j.powtec.2016.02.018
- Shi FN, Zheng XF. The rheology of flotation froths. Int J Miner Process. 2003;69:115–128. DOI:10.1016/S0301-7516(02)00120-5
- Silverman D, Roseveare WE. An equation relating viscosity and surface tension. J Am Chem Soc. 1932;54:4460. DOI:10.1021/ja01350a505
- Shaw DJ. Introduction to colloid and surface chemistry. 4th ed Oxford: Elsevier Science; 1992.
- Yarar B, Kaona J. The critical surface tension of wetting of sulfide minerals. SME-AIME Annu. Meet.; Atlanta; 1983.
- Yörük S, Smith GW, Finch JA. Critical surface tension of wetting and flotation separation of hydrophobic solids. Sep Sci Technol. 1987;22:1527–1546. DOI:10.1080/01496398708058416
- Fox HW, Zisman WA. The spreading of liquids on low energy surfaces. I. Polytetrafluoroethylene. J Colloid Sci. 1950;5. DOI:10.1016/0095-8522(50)90044-4
- Guancheng J. Evaluation methods and influencing factors of gas wettability; 2018. doi:10.1016/b978-0-12-815150-1.00002-x
- Gayle J, Smelley A. Effects of temperature variations on contact angles for coal and related substances. Washington (DC): U.S. Bureau of Mines; 1960.
- An D, Zhang J. A study of temperature effect on the xanthate’s performance during chalcopyrite flotation. Miner Eng. 2020;10. DOI:10.3390/min10050426
- Peng Y, Grano S. Effect of iron contamination from grinding media on the flotation of sulphide minerals of different particle size. Int J Miner Process. 2010;97:1–6. DOI:10.1016/j.minpro.2010.07.003.
- Rao M, Natarajan K. Electrochemical aspects of grinding media-mineral interaction on sulphide flotation. Bull Mater Sci. 1988;10:411–422.
- Long T, Chen Y, Shi J, et al. Effect of grinding media on the flotation of copper-activated marmatite. Physicochem Probl Miner Process. 2020;56:229–237. DOI:10.37190/ppmp19100
- Dunne RC, Kawatra KS, Young CA. SME mineral processing and extractive metallurgy handbook. Englewood (CO): Society for Minning, Metallurgy, and Exploration; 2019.
- Glembotsky V, Klassen V. Флотационные методы обогащения [Flotation methods of mineral processing]. Moscow: Izd. Nedra; 1981. Russian.
- Twidle TR, Engelbrecht PC. Developments in the flotation of copper at Black Mountain. J South African Inst Min Metall. 1984;84:164–178.
- Plaksa N. Совмещение пропарки и селективной флотации медно-молибденовых концентратов [Combination of low-temperature steaming and selective flotation of copper-molybdenum concentrates]. Tsvetnye Met. 1970;43:79–82. Russian.
- Arustamjan A. Влияние температуры пульпы на показатели цинковой флотации [Pulp temperature influence on zinc flotation performance]. Zap Gorn Instituta [J Min Inst]. 2004;159:140–141. Russian.
- Zheng L, Du Z, Liu Y. 凡口矿高碱介质中闪锌矿浮选特性研究 [The study on flotation properties of sphalerite in high alkalinity medium in Fankou lead-zinc mine]. Min Metall Eng. 2005;25:37–40. Chinese.
- Weise K, Schmitz J, Wollmann G. Das Mineral- Anreicherungsverfahren Flotation (Ein Überblick) [The flotation mineral concentration process: an overview]. Karlsruhe: Kernforschungszentrum Karlsruhe GmbH; 1978. German.
- Taggart A. Handbook of mineral dressing. New York: Wiley; 1945.
- Roberts J, Deredin C, Paulin J. Process improvement update at Brunswick mine. In: 40th Annu. Meet. Can. Miner. Process.; 2008. p. 27–49.
- Zhao M. 温度对辉钼矿浮选的影响及其改善途径 [Effect of temperature on molybdenite flotation and improvement approaches]. 国外金属矿选矿 [Guowai Jinshukuang Xuankuang]. 1991;22:86–89. Chinese.
- Albrecht T, Fornasiero D, Addai-Mensah J. Effect of water temperature on sphalerite flotation. 27th ACSSSC; Roseworthy; 2010.
- Isshiki J. 余市選鉱場におけるパルプ温度の浮選成績におよぼす影響 [EfPulp temperature produce effects on flotation at Yoichi Mill]. 日本鉱業会誌 [J Japan Min Assoc]. 1961;77:724–728. Japanese.
- Fernandes I. Influence of temperature on sulphide ore flotation applied to the rougher regrind circuit of the zinc flotation plant of Neves-Corvo mine [PhD thesis]. Civil Engineering and Georesources, University of Lisbon; 2016.
- Abramov A. Технология переработки и обогащения руд цветных металлов [Technology for processing and concentration of non-ferrous metal ores]. 2nd ed. Moscow: Moscow State Mining University; 2005. Russian.
- Hamilton IC, Woods R. Surfactant properties of alkyl xanthates. Int J Miner Process. 1986;17:113–120. DOI:10.1016/0301-7516(86)90049-9
- Klevens HB. Structure and aggregation in dilate solution of surface active agents. J Am Oil Chem Soc. 1953;30:74–80. DOI:10.1007/BF02635002
- Abramov A. Флотация: реагенты собиратели [Flotation: collector reagents]. 8th ed. Moscow: Izd. Gornaja kniga; 2012. Russian.
- Abramov A. Флотация: Сульфидные минералы [Flotation: sulfide minerals]. 8th ed. Moscow: Gornaja Kniga; 2013. Russian.
- Pomianowski A, Leja J. Spectrophotometric study of xanthate and dixanthogen solutions. Can J Chem. 1963;41:2219–2230. DOI:10.1139/v63-322
- Hidmi L, Edwards M. Role of temperature and pH in Cu(OH)2 solubility. Environ Sci Technol. 1999;33:2607–2610. DOI:10.1021/es981121q
- Avdohin V. Физико-химические основы оптимизации флотации сульфидов [Physicochemical foundations of sulfide flotation optimization]. In: Symp. Mod. Min. Educ. Sci. Ind. Moscow: Sholokhov Moscow State University; 1996. p. 3–8. Russian.
- Haung HH, Miller JD. Kinetics and thermochemistry of amyl xanthate adsorption by pyrite and marcasite. Int J Miner Process. 1978;5:241–266.
- An D, Zhang J. A study of temperature effect on the xanthate’s performance during chalcopyrite flotation. Miner Eng. 2020;10. DOI:10.3390/min10050426
- Bocharov V. Интенсивные методы рудо- и пульпоподготовки при комплексной переработке сульфидных руд цветных металлов [Intensive methods of ore and pulp conditioning during complex processing of base metal sulphidic ores]. In: Симпозиум Современное Горное Дело Образование, Наука, Промышленность [Symp. Mod. Min. Educ. Sci. Ind.]; 1996. p. 40–45. Russian.
- Abramov A. Флотация: физико-химическое моделирование процессов [Flotation: physical-chemical modelling of processes]. 6th ed. Moscow: Izd.: Gornaja kniga; 2010. Russian.
- Mhonde N, Schreithofer N, Corin K, et al. Assessing the combined effect of water temperature and complex water matrices on xanthate adsorption using multiple linear regression. Minerals. 2020;10:1–18. DOI:10.3390/min10090733
- Bulatovic SM. Chemistry, theory and practice: flotation of sulfide ores; 2007. DOI:10.1016/B978-0-444-53082-0.00023-8
- Ryan L, Norris R. Chemistry coursebook. 2nd ed. Cambridge: Cambridge University Press; 2014.
- Reactions of some transition metal ions cobalt, presentation. Knockhardy; 2015. p. 1–8. Available from: http://www.knockhardy.org.uk/sci_htm_files/08tm2.pdf
- Zanin M, Lambert H, du Plessis CA. Lime use and functionality in sulphide mineral flotation: a review. Miner Eng. 2019;143. DOI:10.1016/j.mineng.2019.105922
- Rabinovich V, Havin Z. Краткий химический справочник [Brief chemical handbook]. Leningrad: Izd. Himija; 1978. Russian.
- Ikumapayi FK. Flotation chemistry of complex sulphide ores [Licentiate thesis]. Department of Chemical Engineering and Geosciences, Lulea University of Technology; 2010.
- Ikumapayi F, Rao K. Recycling process water in complex sulfide ore flotation: effect of calcium and sulfate on sulfide minerals recovery. Miner Process Extr Metall Rev. 2015;36:45–64. DOI:10.1080/08827508.2013.868346
- Grano SR, Lauder DW, Johnson NW, et al. An investigation of galena recovery problems in the Hilton concentrator of Mount Isa Mines Limited, Australia. Miner Eng. 1997;10:1139–1163. DOI:10.1016/s0892-6875(97)00100-3
- Wu W, Nancollas GH. Interfacial free energies and crystallization in aqueous media. J Colloid Interface Sci. 1996;182:365–373. DOI:10.1006/jcis.1996.0475
- Wark W, Cox A. Principles of flotation: an experimental study of the effect of xanthates on contact angles at mineral surfaces. Trans Am Inst Min Metall Eng. 1932;102, Tech. Pub. 461.
- Guo B, Peng Y, Espinosa-Gomez R. Cyanide chemistry and its effect on mineral flotation. Miner Eng. 2014;66:25–32. DOI:10.1016/j.mineng.2014.06.010
- McCreedy HH, Honeywell WR. Effect of xanthate in cyanidation. Can Min J. 1966;87:66–69.
- Orii M. 温 水 浮 選 に つ い て [On warm pulp flotation]. Resour Process Soc Japan. 1961;15:6–17. Japanese.
- Lukio Co. Mineral processing of complex sulfide ore (Kuroko), Tokyo; 2005. Available from: http://mric.jogmec.go.jp/public/report/1988-04/No2_eng.pdf
- Rao SR, Finch JA. A review of water re-use in flotation. Miner Eng. 1989;2:65–85. DOI:10.1016/0892-6875(89)90066-6
- Levay G, Smart RSC, Skinner WM. The impact of water quality on flotation performance. J South African Inst Min Metall. 2001;101:69–75.
- Muzinda I, Schreithofer N. Water quality effects on flotation: impacts and control of residual xanthates. Miner Eng. 2018;125:34–41. DOI:10.1016/j.mineng.2018.03.032
- Le TMK, Schreithofer N, Dahl O. Dissolution test protocol for estimating water quality changes in minerals processing plants operating with closed water circulation. Minerals. 2020;10:653. DOI:10.3390/min10080653
- Kumpulainen S, Carlson L, Räisänen ML. Seasonal variations of ochreous precipitates in mine effluents in Finland. Appl Geochemistry. 2007;22:760–777. DOI:10.1016/j.apgeochem.2006.12.016
- Praharaj T, Fortin D. Seasonal variations of microbial sulfate and iron reduction in alkaline Pb-Zn mine tailings (Ontario, Canada). Appl Geochemistry. 2008;23:3728–3740. DOI:10.1016/j.apgeochem.2008.09.008
- Dold B. Evolution of acid mine drainage formation in sulphidic mine tailings. Minerals. 2014;4:621–641. DOI:10.3390/min4030621
- Alpers CN, Nordstrom DK, Thompson JM. Seasonal variations of Zn/Cu Ratios in acid mine water from Iron Mountain, California; 1993. p. 324–344. DOI:10.1021/bk-1994-0550.ch022
- Pryor EJ. Mineral processing. New York: Elsevier Applied Science; 1965. DOI:10.1007/978-94-010-2941-4
- Zhang J, Wei Z. Multi-scale investigation of applying secondary effluent in sulfde flotation. In: Water Miner. Process. – Proc. 1st Int. Symp.; Seattle; 2012. p. 279–290.
- Fuerstenau M, Miller J, Kuhn M. Chemistry of flotation. New York: Society of Mining Engineers of the American Institute of Mining, Metallurgical and Petroleum Engineers; 1985.
- Schubert H, Schneider V. О роли ассоциации аполярных групп при адсорбции собирателя [On the role of non-polar groups during collector adsorption]. In: VIII Int. Miner. Process. Congr. Leningrad (St. Petersburg): Izd. Instituta Mechanobr; 1969. p. 315–524. Russian.
- Mitrofanov S. Селективная флотация [Selective flotation]. Moscow: Nerda; 1967. Russian.
- Ignatova T, Shelepov E. Влияние температуры жидкой фазы пульпы на катионную доводку магнетитовых концентратов ОАО “Михайловский ГОК” [An influence of temperature of liquid phase of the pulp on cationic cleaner flotation of magnetite concentrates]. Min Informational Anal Bull. 2011;4:237–240. Russian.
- Zhang X, Shao G, Wu P, et al. 氧化铅锌矿石低温浮选工艺研究 [Study on the flotation of lead-zinc oxide ore at low temperature]. Min Metall. 2003;12:21. Chinese.
- Kulkarni RD, Somasundaran P. Flotation chemistry of hematite/oleate system. Colloids Surf. 1980;1:387–405. DOI:10.1016/0166-6622(80)80025-4
- Cooke S, Iwasaki I, Choi HS. Effect of temperature on soap flotation of iron ore. Trans AIME. 1960;217:76–83. DOI:10.2118/65-04-09
- Guo W, Han Y, Zhu Y, et al. Effect of amide group on the flotation performance of lauric acid. Appl Surf Sci. 2020;505. DOI:10.1016/j.apsusc.2019.144627
- Parrent MD. Separation of pyrolusite and hematite by froth flotation [Master’s thesis]. Department of Chemical and Materials Engineering, University of Alberta; 2012.
- Parkins E. Effect of temperature on the conditioning and flotation of an ilmenite ore [PhD thesis]. London University; 1975.
- Carlos Silva A, Maria Schons Silva E, Emérito P, et al. Temperature influence in cornstarch gelatinization for froth flotation. Rem Int Eng J. 2017;70:231–235. DOI:10.1590/0370-44672016700085
- Cai J, Liu D, Shen P, et al. Effects of heating-sulfidation on the formation of zinc sulfide species on smithsonite surfaces and its response to flotation. Miner Eng. 2021;169:106956. DOI:10.1016/j.mineng.2021.106956
- Tyushkova NI, Marasanova LV, Gorenkov NL. Research and developing of the technologies of oxidized Pb-Zn ores treatment. In: 26th Int. Miner. Process. Congr. IMPC 2012 Innov. Process. Sustain. Growth – Conf. Proc.; 2012. p. 5551–5555.
- Yin W, Sun H, Tang Y, et al. Effect of pulp temperature on separation of magnesite from dolomite in sodium oleate flotation system. Physicochem Probl Miner Process. 2019;55:1049–1058. DOI:10.5277/ppmp19027
- Miller J, Ackerman J. Bench scale flotation of alunite ore with oleic acid. In: Fine Part. Symp. New York: AIME; 1980. p. 832–852. DOI:10.1525/9780520959743-042
- De Castro FHB, Borrego AG. The influence of temperature during flotation of celestite and calcite with sodium oleate and quebracho. Int J Miner Process. 1996;46:35–52. DOI:10.1016/0301-7516(95)00059-3
- Batelaan JG, Van Ginkel CG, Balk F. Carboxymethylcellulose (CMC). Handb Environ Chem. 1992;3:329–336. DOI:10.1007/978-3-540-47108-0_11
- Ofor O, Nwoko C. Oleate flotation of a Nigerian baryte: the relation between flotation recovery and adsorption density at varying oleate concentrations, pH, and temperatures. J Colloid Interface Sci. 1997;186:225–233. DOI:10.1006/jcis.1996.4501
- Li S, Gong D, Zhang W, et al. Improving fluorite flotation under low temperature and neutral pH conditions. Surf Rev Lett. 2020;27. DOI:10.1142/S0218625X19501877
- Taguta J, Teme KC, Ngobeni P. The role of gangue mineralogy on flowsheet development in fluorite processing. Minerals. 2020;10. DOI:10.3390/min10030237
- Kienko L, Samatova L, Voronova O, et al. К проблеме снижения температуры флотации при обогащении карбонатно-флюоритовых руд [Addressing a problem of lowering a temperature of flotation during processing of carbonate-fluorite ores]. Fiz Probl Razrab Polezn Iskop. 2010;3:97–104. Russian.
- Vigdergauz V, Trofimova E, Sarkisova L, et al. Низкотемпературная флотация флюорита эмульсией карбоксильного собирателя [Low-temperature fluorite flotation with carboxyl collector emulsion]. Горный Информационно-Аналитический Бюллетень. 2000;2:235–238. Russian.
- Kienko L. Разработка эффективной технологии обогащения карбонатно-флюоритовых руд Вознесенского рудного района [Development of an efficient processing technology for carbonate-fluorite ores of the Voznesensky ore field] [PhD thesis]. Transbaikal State University; 2008. Russian.
- Deng J, Zhu Y, Hu F, et al. 油酸低温下浮选萤石的研究 [Study on fluorite flotation with oleic acid at low temperature]. 化工矿山技术 [Chem Min Technol]. 1993;22:24–26. Chinese.
- De Oliveira Baldoino R, Martins M, Rodrigues MVT, et al. Influence of temperature, water quality and collector type on flotation performance of a peruvian phosphate ore. In: 26th Int. Miner. Process. Congr. IMPC 2012 Innov. Process. Sustain. Growth – Conf. Proc.; 2012. p. 327–335.
- Ruan Y, Zhang Z, Luo H, et al. Ambient temperature flotation of sedimentary phosphate ore using cottonseed oil as a collector. Minerals. 2017;7:1–14. DOI:10.3390/min7050065
- Zhang Y, Li D. 几种表面活性剂在磷矿低温浮选中的应用 [Application of surfactants in low temperature flotation of phosphate ore]. 化工矿物与加工 [IM P]. 2007;4:8–9. Chinese.
- Ivanova V, Mitrofanova G, Perunkova T. Флотация апатито-нефелиновой руды жирокислотным собирателем на оборотной воде в условиях холодной пульпы [Flotation of apatite-nefeline ore with fatty acid collector and recycled waters under the conditions of cold pulp]. In: Gorn. Delo v Arktike. Apatity: Tipografia Ivan Fedorov; 2005. p. 294–299. Russian.
- Ivanova V, Mitrofanova G. О роли смоляных кислот при флотации апатита талловыми маслами в условиях водооборота [About the role of resin acids during apatite flotation with tall oils and recycled water]. Вестник МГТУ. 2009;12:583–587. Russian.
- Stén P, Parvinen P, Miettinen M, et al. On-line analysis of flotation process waters at Siilinjärvi (Finland) apatite concentrating plant. Miner Eng. 2003;16:229–236. DOI:10.1016/S0892-6875(03)00013-X
- Oliazadeh M, Aghamirian M, Grammatikopoulos T, et al. An overview of potash flotation. In: 44th Annu. Meet. Can. Miner. Process.; 2012. p. 431–442.
- Aliferova S. Активация процессов флотации шламов и сильвина при обогащении калийных руд [Activation of flotation processes for slimes and sylvite during potassium salt processing] [PhD thesis]. Ural State University; 2007. Russian.
- Turko M, Dormeshkin O, Miskov E, et al. Флотация сильвина из калийных руд при повышенных температурах [Flotation of sylvite from potash ores at elevated temperatures]. Труды БГТУ Химия и Технология Неорганических Материалов и Веществ. 2014;3:71–77. Russian.
- Essilfie E. Modification of amine collectors for low temperature flotation of potash [Master’s thesis]. Department of Mechanical Engineering, University of Saskatchewan; 2014.
- Mehri A, Haghani M, Mozaffari E. Flotation of potash for carnallite resources in Khur playa of Iran using Jameson flotation cell. J Environ Anal Chem. 2019;6:2–9.
- Perucca CF. Testing and evaluation of modifying reagents in potash flotation [Master’s thesis]. Department of Mining and Minerals Processing, University of British Columbia; 2000.
- Bodnar R, Vityk M, Hryn J, et al. Phase Equilibria in the system H2O-NaCl-KCl-MgCl2 relevant to salt cake processing. In: 126th Annu. Meet. Miner. Met. Mater. Soc.; Orlando; 1997. p. 1–7.
- Abu-Hamatteh ZSH, Al-Amr AM. Carnallite froth flotation optimization and cell efficiency in the arab potash company, dead Sea, Jordan. Miner Process Extr Metall Rev. 2008;29:232–257. DOI:10.1080/08827500801997894
- Vahrushev V. Повышение эффективности процессов обесшламливания и выщелачивания в технологии получения хлорида калия из сильвинитовых руд Верхнекамского месторождения [Improving sylvinite desliming and leaching] [PhD thesis]. Kazan State Technological University; 2014. Russian.
- Cheng FQ, Xue N, Cao B, et al. Experimental study on mixed amine collector for KCl flotation at low temperature. In: XXVI IMPC; New Delhi; 2012. p. 888–898.
- European Commission. Study on the review of the list of Critical raw materials – final report, 2020. DOI:10.2873/11619
- Pradip, Fuerstenau DW. The role of inorganic and organic reagents in the flotation separation of rare-earth ores. Int J Miner Process. 1991;32:1–22. DOI:10.1016/0301-7516(91)90016-C
- Pradip. The surface properties and flotation of rare-earth minerals [PhD thesis]. University of California; 1981.
- Schriner D. Advanced beneficiation of bastnaesite ore through centrifugal concentration and froth flotation [Master’s thesis]. Metallurgical and Materials Engineering, Colorado School of Mines; 2016.
- Yang G. 稀土浮选工艺流程优化试验 [Possibility of improving flow sheet of rare earth flotation beneficiation]. Chinese Rare Earths. 2005;26:25–26. Chinese. DOI:10.16533/j.cnki.15-1099/tf.2005.01.019
- Li M, Gao K, Zhang D, et al. The influence of temperature on rare earth flotation with naphthyl hydroxamic acid. J Rare Earths. 2018;36:99–107. DOI:10.1016/j.jre.2017.07.004
- Xu J, Li F, Zeng X. 从尾矿中回收氟碳铈矿和独居石的浮选研究 [Flotation of bastnaesite and monazite in dressing plant tailing]. Chinese Rare Earths. 2006;27:67–72. Chinese.
- Zeng X, Li F. 微山稀土矿选矿工艺研究 [Beneficiation technology for Wei Shan rare earth mine]. J Shanghai Second Polytech Univ. 1991;2:46–52. Chinese. DOI:10.19570/j.cnki.jsspu.1991.02.007
- Ren H, Hu Y. 用水杨羟肟酸捕收剂从强磁中矿中选取高品位稀土精矿的研究 [Study on the selection of high grade rare-earth concentrates from high-intensity separation middlings with salicylhydroxamic acid as the collector]. Met Mine. 1996;245(11):20–22.
- Metso. Sodium metasilicate: a soluble silicate for institutional and industrial cleaning; 2009. DOI:10.1007/978-3-642-41714-6_194967
- Zhang Y. Froth flotation of xenotime [Master’s thesis]. Metallurgical and Materials Engineering, Colorado School of Mines; 2016.
- Kupka N, Rudolph M. Froth flotation of scheelite – a review. Int J Min Sci Technol. 2018;28:373–384. DOI:10.1016/j.ijmst.2017.12.001
- Samatova L, Shepeta E, Kondratjev S. Изучение флотационных свойств собирателя FX-6 при обогащении шеелит-сульфидных руд [Investigation of flotational properties of FX-6 collector during processing of scheelite-sulfide ores]. Физико-Технические Проблемы Разработки Полезных Ископаемых [Phys Tech Probl Miner Dev]. 2015;2:156–160. Russian.
- Kang J, Chen C, Sun W, et al. A significant improvement of scheelite recovery using recycled flotation wastewater treated by hydrometallurgical waste acid. J Clean Prod. 2017;151:419–426. DOI:10.1016/j.jclepro.2017.03.073
- Kang J, Hu Y, Sun W, et al. Utilisation of FGD gypsum for silicate removal from scheelite flotation wastewater. Chem Eng J. 2018;341:272–279. DOI:10.1016/j.cej.2018.02.043
- Li J, Sun X, Zhang D, et al. 某铜钨矿中白钨矿的低温浮选效果优化 [Optimization on low temperature floatation of scheelite from copper-tungsten ore]. Met Mine. 2014;9:56–59. Chinese.
- Meng Q, Feng Q, Ou L. Effect of temperature on floatability and adsorption behavior of fine wolframite with sodium oleate. J Cent South Univ. 2018;25:1582–1589. DOI:10.1007/s11771-018-3850-4
- Glembotsky V, Dmitrieva G, Sorokin M. Аполярные реагенты и их действие при флотации [Non-polar reagents and their application in flotation]. Moscow: Nayka; 1968. Russian.
- Klassen V, Plaksin I. О механизме действия некоторых реагентов и аэрации пульпы при флотации каменных углей [About mechanism of action of some reagents during aeration in hard coal flotation]. Izv Akad Nauk SSSR Otd Teh Nauk. 1954;3. Russian.
- Gayle J, Eddy W, Shotts R. Laboratory investigation of the effect of oxidation on coal flotation (bureau of mines report of investigations). Washington (DC): U.S. Dept. of the Interior, Bureau of Mines; 1965.
- Li C, Xu M, Xing Y, et al. Efficient separation of fine coal assisted by surface nanobubbles. Sep Purif Technol. 2020;249:117163. DOI:10.1016/j.seppur.2020.117163
- Chang G, Xing Y, Zhang F, et al. Effect of nanobubbles on the flotation performance of oxidized coal. ACS Omega. 2020;5:20283–20290. DOI:10.1021/acsomega.0c02154
- Sun SC. Frothing characteristics of pine oils in flotation. Trans Am Inst Min Metall Eng. 1952;193:65–71.
- Sun S, Chao T. Hypothesis for the effect of temperature on coal flotation. SME Annu. Meet. Am. Inst. Mining, Metall. Pet. Eng.; New York; 1966.
- Gayle J, Eddy W. Laboratory investigation of the effect of temperature on coal flotation (bureau of mines report of investigations). Washington (DC): U.S. Dept. of the Interior, Bureau of Mines; 1961.
- Hacifazlioğlu H, Gerdan GH. Taşkömürü Tozları Flotasyonunda Sıcaklığın Etkisi [Effect of temperature on fine hard coal flotation. Adıyaman Üniversitesi Mühendislik Bilim Derg. 2016;5:1–8. Turkish.
- Bailey R, Whelan PF. The influence of pulp temperature on the froth flotation of four British fine coals. J Inst Fuel. 1953;25:304–307.
- Menéndez M, Vidal A, Toraño J, et al. Optimisation of spodumene flotation. Eur J Miner Process Environ Prot. 2004;4:130–135.
- Xu L, Hu Y, Tian J, et al. Selective flotation separation of spodumene from feldspar using new mixed anionic/cationic collectors. Miner Eng. 2016;89:84–92. DOI:10.1016/j.mineng.2016.01.013
- Liu G, Liu Z, Liu Y, et al. 一种钽铌尾矿浮选分离锂云母精矿的方法 [A method for concentrating lepidolite from tantalum-niobium tailings through flotation]. Chinese patent no. CN102151616A. 2011. Chinese. Available from: https://patents.google.com/patent/CN102151616A/zh
- Ikumapayi F, Makitalo M, Johansson B, et al. Recycling process water in sulfide flotation, part A: effect of calcium and sulfate on sphalerite recovery. Miner Metall Process. 2012;29:183–191. DOI:10.1007/bf03402455
- Kawatra SK. Froth flotation – fundamental principles. In: Min. Eng. Handb. Houghton: Michigan Technological University; 2009. p. 30.