Abstract
Chromosome number, meiotic behavior and pollen fertility were determined for six species of the genus Chrysolaena H.Rob. (Vernonieae, Asteraceae). All the species had a base chromosome number of x = 10, with somatic numbers that ranged between 2n = 20 and 2n = 60. The chromosome number of Chrysolaena obovata (Less.) Dematt. (2n = 4x = 40) and a new cytotype for C. lithospermifolia (Hieron.) H.Rob. (2n = 4x = 40) are reported for the first time. Normal meiotic behavior was observed for the diploid species C. platensis (Spreng.) H. Rob., C. propinqua (Hieron.) H. Rob. and C. flexuosa (Sims) H. Rob. However, the polyploid taxa C. obovata, C. lithospermifolia (tetraploid) and C. cognata (Less.) Dematt. (hexaploid) showed many meiotic irregularities such as multivalent formation, chromosomes outside plate, early segregation of chromosomes, laggards and bridges in anaphase and telophase. Pollen fertility was high in all species, however, including in the polyploids. Polyploidy is more common in Chrysolaena than in any other New World Vernonieae so far reported and appears to be an important factor in the evolution of this genus.
Introduction
The Vernonieae Cass. is one of the largest tribes of Compositae (Asteraceae), with about 1700 species distributed throughout the tropical regions of Asia, Africa and America. It is also considered to be one of the most complex tribes in the family from both biological and taxonomic viewpoints (Keeley et al. Citation2007). Most species have been traditionally included in the huge core genus Vernonia Schreb, traditionally ascribed ca. 1000 species. However, in a series of studies on the New World Vernonieae, Robinson (Citation1999) greatly reduced the size of the genus and now most species of Vernonia are restricted to eastern North America. South American species previously in Vernonia were placed in new genera, one of which is the genus Chrysolaena H. Rob. (Subtribe Vernoniinae).
The genus Chrysolaena differs from other New World Vernonieae by the presence of sericeous or velutinous indumentum on the leaves, anthers with glandular apical appendages, styles without a basal node and cypselas with glands (Robinson 1988). The genus can also be separated from the other American genera by pollen morphology, and especially by the base chromosome number of x = 10 (Dematteis Citation2002). This chromosome number is very common in the Old World Vernonieae, but is mostly absent in American species (Dematteis Citation1997). As presently delimited, Chrysolaena comprises 18 species geographically concentrated in southern Brazil and northern Argentina, with some species extending to Uruguay, Paraguay, Bolivia and Peru (Dematteis Citation2009). The distinctions among Chrysolaena species are relatively clear; however, they vary considerably in chromosome number due to polyploidization (Ruas et al. Citation1991; Dematteis Citation1997, Citation2007; Oliveira et al. Citation2007).
Chromosome studies reveal that chromosome numbers range from 2n = 20 to 2n = 80 and there is variation among species in cytotypes as well as ploidy levels (Ruas et al. Citation1991; Dematteis Citation1997). For example, in C. platensis (Spreng.) H. Rob. there are diploid (2n = 20), tetraploid (2n = 40) and octoploid (2n = 80) populations (Dematteis Citation1997). Likewise, Chrysolaena flexuosa (Sims) H. Rob. has both diploid and tetraploid cytotypes, while C. sceptrum (Chodat) Dematt. includes hexaploid and octoploid populations (Dematteis Citation2009). The most extreme chromosome variation is found in C. cognata (Less.) Dematt., with diploid, tetraploid, hexaploid and even odd-number polyploids, such as 2n = 5x = 50, which would be the result of hybridization between different cytotypes (Dematteis Citation2002). Chromosome number, meiotic behavior and pollen fertility of six species of Chrysolaena were examined in this study. The results are discussed in relation to the taxonomy and evolution of the genus.
Materials and methods
Material was obtained from natural populations in different localities within Argentina, Bolivia and Uruguay (Table ). Voucher specimens are deposited in the herbarium of Instituto de Botánica del Nordeste (CTES).
Table 1. Examined specimens and somatic chromosome numbers (2n) observed in six species of Chrysolaena.
Meiosis was studied in young inflorescences fixed in lactic acid–ethanol (1:5) and refrigerated until examined. Pollen mother cells were macerated and squashed using 2% acetocarmine. Permanent slides were prepared using Euparal. Photographs were taken through a Zeiss Axioplan microscope with a Cannon Power Shot A640 camera.
Pollen fertility was estimated by staining with carmine-glycerin (1:1). Uniformly stained pollen grains were considered fertile while the non-stained grains were scored as sterile. Approximately 300–500 pollen grains were analyzed per species.
Results
The six species of Chrysolaena investigated here all had a base chromosome number of x = 10. There were also polyploids of differing ploidy levels (Table ). In Chrysolaena platensis (Figure A), C. propinqua (Figure E) and C. flexuosa (Figure H), all the populations were diploid with 2n = 20. On the other hand, Chrysolaena obovata (Figure J) and C. lithospermifolia were tetraploid with 2n = 40, while C. cognata was hexaploid with 2n = 60 (Figure N).
Figure 1 Meiotic behavior in Chrysolaena. (A–D) C. flexuosa: (A) diakinesis, 10II; (B) diakinesis, 9II + 2I (arrows); (C) off-plate chromosomes in metaphase I (arrows); (D) chromosome bridge with fragment in anaphase I. (E–G) C. platensis: (E) diakinesis, 9II + 2I (arrows); (F) diakinesis, 10II; (G) anaphase I with one bridge and fragment. (H–I) C. propinqua: (H) diakinesis, 10II; (I) bridge and fragment in anaphase I. (J–M) C. obovata: (J) diakinesis, 20II; (K) diakinesis, 18II + 1VI (arrows); (L) bridges and lagging chromosomes in anaphase I; (M) off-plate chromosome in metaphase II. (N–P) C. cognata: (N) diakinesis, 26II + 1VI + 2I (arrows); (O) bridges in anaphase I; (P) lagging chromosome in anaphase I. (Q–R) C. lithospermifolia: (Q) diakinesis, 5IV + 1III + 2II + 13I (arrows indicate tetravalent); (R) bridges and lagging chromosomes in anaphase II. Scale = 5 μm.
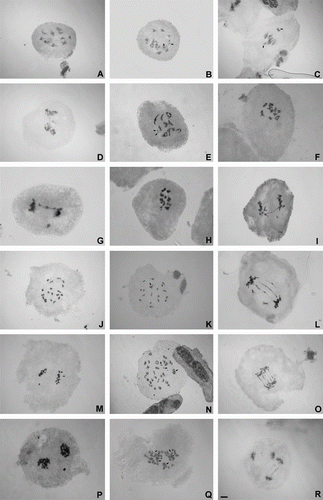
The meiotic behavior of the analyzed species is presented in . In Chrysolaena platensis, C. propinqua and C. flexuosa, due to their diploid condition, there was a preponderance of bivalents at diakinesis, but univalents were also observed in some cells (Figure B, E). Only in C. propinqua did 100% of the cells form bivalents at this stage (Figure H). In general, meiosis was normal in these three species, but some irregularities did occur. As seen in Figure C some chromosomes were off-plate and precocious chromosome segregation occurred during metaphase I along with lagging chromosomes and bridges in anaphase I (Figure D, G, I). For example, in C. flexuosa chromosome bridges (without fragments) were also observed at anaphase II in 16.66% of the cells.
Table 2. Number of pollen mother cells analyzed (N°) and percentage of meiotic irregularities found in six species of Chrysolaena.
Table 3. Meiotic configurations and pollen fertility (%) in six analyzed species of Chrysolaena.
In the tetraploid species, C. obovata, a high frequency of bivalents were observed at diakinesis (Figure J), along with occasional univalents and tetravalents (Figure K). At metaphase I off-plate univalents (Figure M) and early segregation of chromosomes were also observed while at anaphase I there were between one and four chromosome bridges per cell (with or without fragments) (Figure L). In C. lithospermifolia, on the other hand, at metaphase I there were off-plate chromosomes and in anaphase II and telophase II (Figure Q–R) there were also lagging chromosomes and bridges that, in some cases, occurred in the same cell (Figure R). Also both bivalents and tetravalents were observed (Figure Q) in addition to off-plate chromosomes in metaphase I and irregular second meiotic division, in both anaphase II and telophase II.
Hexaploid individuals of C. cognata showed bivalents and many configurations having multivalents at diakinesis (Figure N). The meiotic behavior also revealed irregularities in meiosis I and II, such as univalents outside the plate, early segregation of chromosomes in metaphase I, lagging chromosomes (Figure P) and a variable number of bridges per cell in anaphase I and anaphase II (Figure O). The pollen fertility was relatively high in all species, between 89.27% and 98.04% (Table ). Chrysolaena cognata recorded the lowest percentage of fertility (89.27%), while C. platensis showed the highest (98.04%).
Discussion
The chromosome number 2n = 20 found in C. platensis, C. propinqua and C. flexuosa and the cytotype with 2n = 60 observed in C. cognata are consistent with the chromosome counts recorded in previous cytological studies (Angulo & Dematteis Citation2009; Dematteis Citation1997, Citation2002, Citation2009; Dematteis & Fernandez 2000). Chrysolaena obovata has not been previously studied and consequently this constitutes the first chromosome report for the species. In C. lithospermifolia (2n = 40) a new tetraploid cytotype was discovered for a population from Argentina. A previous study of this species showed a count of 2n = 20 (Dematteis Citation1998a), indicating that there are two different ploidy levels within C. lithospermifolia.
A similar result has been reported for C. platensis with diploid (2n = 20), tetraploid (2n = 40) and octoploid (2n = 80) cytotypes (Dematteis Citation1997). Chrysolaena flexuosa has also been reported to have diploid and tetraploid populations, and C. sceptrum was found to have both hexaploid and octoploid populations (Dematteis Citation2009). The greatest variation in chromosome number was observed in C. cognata where there are diploid, tetraploid and hexaploid cytotypes (Dematteis Citation2002). Difference in ploidy level did not prevent clear identification of species, however. There were some morphological differences: for example, the hexaploid plants were generally taller than those that were tetraploid, which were, in turn, taller than the diploids. This increase in size with increasing ploidy level is known as the gigas effect, and is characteristic of the polyploidization process (Stebbins Citation1971). The size differences were not uniform enough, however, to accurately predict ploidy level from morphology alone.
Meiotic counts ranged from 2n = 20 to 2n = 60 across species with regular meiotic behavior in the majority of cases. There was a prevalence of 10 II at diakinesis in the diploid species, C. platensis, C. propinqua and C. flexuosa. In the polyploids C. cognata, C. obovata and C. lithospermifolia a variable number of multivalents were observed, however, suggesting possible autopolyploidy. Chromosome numbers for C. sceptrum (Chodat) Dematt. (2n = 80), C. simplex (Less.) Dematt. (2n = 40) and C. verbascifolia (Less.) H.Rob. (2n = 20) also support a base number of x = 10 for Chrysolaena, as suggested previously (Dematteis Citation1998b, Citation2002; Salles de Mello et al. 2010). This is a common base number for several genera from the Old World, although polyploidy does not appear to be common (Jones Citation1979).
In the New World, Vernonanthura H. Rob. have counts of n = 17 and n = 34, while counts for Lessingianthus H. Rob. include n = 16, 32, 64 (Dematteis Citation2002; Angulo & Dematteis 2012), some of which likely represent polyploids. However, in Chrysolaena polyploidy occurs much more frequently than elsewhere in American genera. Additionally, a base number of x = 10 has been reported for a few other New World genera (i.e. Critoniopsis Sch. Bip. and Lepidaploa Cass.) but it is not common. Most of the New World groups show basic numbers that range between x = 14 and x = 17. In some cases, these numbers are specific for a genus or section, and are generally considered among the most valuable phylogenetic characters (Dematteis Citation2002).
The only American genera of Vernonieae with a base number lower than x = 13 are Acilepidopsis H. Rob. and Mesanthophora H. Rob., which present x = 9 and are not related to Chrysolaena (Dematteis & Robinson Citation1997; Dematteis & Salgado Citation2001). These two genera have triporate pollen grains, clearly differing from Chrysolaena, which always shows tricolporate grains (Keeley & Robinson Citation2009; Angulo & Dematteis Citation2010).
The chromosome numbers observed in this study, together with those reported previously for other American genera, indicate that polyploidy has been of notable importance in the evolution of the New World Vernonieae.
Acknowledgments
This work has been supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Secretaría General de Ciencia y Técnica of the Universidad Nacional del Nordeste, which are greatly appreciated.
References
- Angulo , MB and Dematteis , M . 2009 . Karyotype analysis in eight species of Vernonia (Vernonieae, Asteraceae) from South America . Caryologia , 62 ( 2 ) : 81 – 88 .
- Angulo , MB and Dematteis , M. 2010 . Pollen morphology of the South American genus Lessingianthus (Vernonieae, Asteraceae) and its taxonomic implications . Grana , 49 ( 1 ) : 12 – 25 .
- Angulo , MB and Dematteis , M . 2012 . Cytotaxonomy of some species of the South American genus Lessingianthus (Asteraceae, Vernonieae) . Pl Syst Evol ,
- Dematteis , M . 1997 . Cromosomas en Vernonia platensis y especies afines (Asteraceae) . Bonplandia , 9 ( 3–4 ) : 259 – 264 .
- Dematteis , M . 1998a . Chromosome studies on Vernonia flexuosa and V. lithospermifolia . Compositae Newsl , 32 : 10 – 16 .
- Dematteis , M . 1998b . Karyotype analysis in some Vernonia species (Asteraceae) from South America . Caryologia , 51 ( 3–4 ) : 279 – 288 .
- Dematteis , M . 2002 . Cytotaxonomic analysis of South American species of Vernonia (Vernonieae: Asteraceae) . Bot J Linn Soc , 139 ( 4 ) : 401 – 408 .
- Dematteis , M . 2007 . Taxonomic notes on the genus Chrysolaena (Vernonieae, Asteraceae) including a new species endemic to Paraguay . Ann Bot Fenn , 44 : 56 – 64 .
- Dematteis , M . 2009 . Revisión taxonómica del género sudamericano Chrysolaena (Vernonieae, Asteraceae) . Bol Soc Argent Bot , 44 ( 1–2 ) : 103 – 170 .
- Dematteis , M and Fernández , A . 2000 . Chromosome studies on nine South American species of Vernonia (Vernonieae, Asteraceae) . Caryologia , 53 ( 1 ) : 55 – 61 .
- Dematteis , M and Robinson , H . 1997 . Chromosome studies and taxonomic considerations in Acilepidopsis (Vernonieae, Asteraceae) . Phytologia , 83 : 366 – 370 .
- Dematteis , M and Salgado , CR . 2001 . Pollen morphology and chromosome number of Vernonia rojasii (Vernonieae, Asteraceae) . Compositae Newsl , 36 : 69 – 76 .
- Jones , SB . 1979 . Chromosome numbers of Vernonieae (Compositae) . Bull Torrey Bot Club , 106 : 79 – 84 .
- Keeley , SC , Forsman , ZH and Chan , R . 2007 . A phylogeny of the “evil tribe” (Vernonieae: Compositae) reveals Old/New World long distance dispersal: support from separate and combined congruent datasets (trnL-F, ndhF, ITS) . Molec Phylog Evol , 44 : 89 – 103 .
- Keeley , SC and Robinson , H . 2009 . “ Vernonieae ” . In Systematics, evolution and biogeography of Compositae , Edited by: Funk , VA , Susanna , A , Stuessy , T and Bayer , RJ . 439 – 469 . Vienna : International Association for Plant Taxonomists .
- Oliveira , VM , Forni-Martins , ER and Semir , J . 2007 . Cytotaxonomic studies in six species of Vernonia (Asteraceae, Vernonieae) . Caryologia , 60 ( 1–2 ) : 7 – 47 .
- Robinson H. 1988. Studies in the Lepidaploa complex (Vernonieae: Asteraceae). V. The new genus Chrysolaena. Proc Biol Soc Wash. 100(4):952–958.
- Robinson , H . 1999 . Generic and subtribal classification of American Vernonieae . Smithsonian Contrib Bot , 89 : 1 – 116 .
- Ruas , PM , Ruas , CF , Vieira , AOS , Matzenbacher , NI and Martins , NS . 1991 . Cytogenetics of genus Vernonia Schreber (Compositae) . Cytologia , 56 : 239 – 247 .
- Salles de Melo , MRC , de Lucena , RM , Semir , J , de Carvalho , R , Araújo Pereira , RC and Benko-Iseppon , AM . 2010 . Karyological features and cytotaxonomy of the tribe Vernonieae (Asteraceae) . Pl Syst Evol , 285 : 189 – 199 .
- Stebbins , GL . 1971 . Chromosomal evolution in higher plants , London : Arnold .