Abstract
Platycodon grandiflorus is an important medicinal plant in China and its root has been used as medicine or food for centuries. Polyploids are usually valuable because they exhibit increased biomass and contain effective medicinal compounds. Polyploidy induction was successfully achieved in this plant in a previous study by apical shoot tip treatment of young seedlings for 72 h using semi-solid 0.05% colchicine. Polyploidy was further confirmed by meiosis behavior of pollen mother cells in this study. The meiosis of diploid controls is regular, with normal chromosome pairing, and diploids were identified with 2n = 2x = 18 chromosomes at metaphaseI, nine bivalents at diakinesis and 9–9 separation at meiosis I, then differentiating into tetrads that subsequently give rise to mature pollen grains. Induced tetraploids were cytogenetically distinguished from diploids by the occurrence of 36 chromosomes at diakinesis with different combinations of univalents, bivalents, trivalents, quadrivalents, and multivalents at metaphase I and insynchronous chromosome segregation at anaphase II. Other abnormalities were observed at anaphase I in induced tetraploids as laggard chromosomes and some chromosome bridges. The univalents, unmatched chromosomes and lagging chromosomes meant that induced tetraploids were a meiotically unstable species with low pollen viability; this gives further proof of their polyploidy. Therefore, it is helpful to confirm the ploidy level by studying the meiosis of pollen mother cells.
Introduction
Platycodon grandiflorus “sentimental blue” belongs to the Campanulaceae flowering plant family. It is a native plant to China, Japan, Korea and East Siberia, and is also known as Japanese bellflower, Chinese bellflower or balloon flower. This species is used in traditional Chinese medicine; the root (Radix Platycodi) is used extensively as an anti-inflammatory in the treatment of coughs and colds. In some areas of China the plant and its root, either dried or fresh, is a popular ingredient in salads and traditional cuisine. Recent studies show that platycodins are one of the essential functional components in Platycodi Radix in terms of the inhibition of pancreatic lipase (Zhao & Kim Citation2004), cholesterol lowering, and anti-obesity effects (Zhao et al. Citation2006).
Polyploidy has played a major role in the evolution of many eukaryotes (Soltis & Soltis Citation1999) and most angiosperms (approximately 70%) have polyploidy during their evolution process (Masterson Citation1994). Polyploid plants are often larger, increasing their commercial interest and attracting agricultural production (Elrad & Unal Citation2010). Polyploid medicinal plants are usually more valuable because they exhibit increased biomass and content of effective compounds (Gao et al. Citation1996).
Meiosis is a premise of sexual reproduction and behavior in meiosis is dependent on the chromosomes and genes (Swanson et al. 1981). In all sexually reproducing organisms, meiosis is a complex process that helps to maintain a constant chromosomal number from generation to generation, and ensures the operation of Mendel’s laws of heredity (Singh Citation1993; Wendel Citation2000). So it is helpful to confirm the ploidy level by studying the meiosis of pollen mother cells.
The objective of this work is to check the chromosome ploidy level of induced tetraploids during meiosis of pollen mother cells (PMCs), to offer cytogenetic evidence for obtained tetraploids through meiotic behavior.
Materials and methods
Plant materials
Platycodon grandiflorus is a species of perennial flowering plant of the family Campanulaceae. Diploid P. grandiflorus came from Natural Medicinal Plant Garden in Shanxi province, China. Tetraploids were successfully obtained in a previous study (Wu et al. Citation2011) by apical shoot tip treatment of young seedlings for 72 h using semi-solid 0.05% colchicine. The first generation, M1, was always used as material for meiosis examination in our study, but it is also feasible to use M2 and M3 for meiosis examination.
Meiotic studies
Flower buds in different development stages were collected from diploid controls and obtained tetraploids, and fixed between 8.00 and 11.00 am in freshly prepared Carnoy’s solution (ethanol: acetic acid = 3:1) for 24 h, after which they were transferred to 70% alcohol and stored at 4°C for future study. Meiotic analyses were carried out on suitable size flower buds and after washing the fixed buds in distilled water, anthers were squashed on slides in Carbol fuchsin solution. Photographs were taken from freshly prepared slides using an Olympus BX60 microscope with automatic camera. Size and sterility of pollen grains from randomly selected anthers were also studied following staining with Carbol fuchsin solution. Meiosis was studied using a minimum of 30 PMCs.
Results and discussion
Meiotic behavior of diploid controls
During the meiotic analyses, only normal stages were observed in control cells, including interphase, leptotene, zygotene, pachytene, diplotene, diakinesis, metaphase I, anaphase I, telophase I, prophase II, metaphase II and telophase II (Figure ). Results show that the basic chromosome number in the genus Platycodon is x = 9. Diploid controls were also confirmed with nine bivalents in diakinesis (Figure (F, G)) and 2n = 2x = 18 chromosomes configurations by metaphase I (Figure (H–J)). Equal and regular separation of 9–9 chromosomes at anaphase-I was encountered in all PMCs of diploid controls (Figure (K)).
Figure 1 Meiosis behavior of PMCs in diploid controls of P. grandiflorus. (A) interphase; (B) leptotene; (C) zygotene; (D) pachytene; (E) diplotene; (F, G) diakinesis; (H–J) metaphase I; (K) anaphase I; (L) telophase I; (M) prophase; (N) metaphase II; (O) anaphase II; (P) telophase II (tetrad); (Q, R) mature and viable pollen grains. Bar = 5 μm.
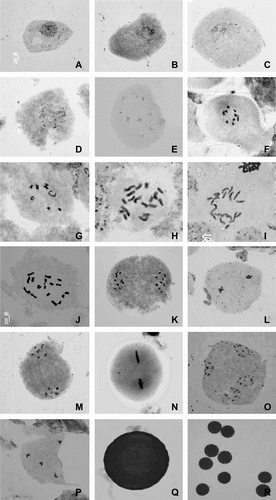
At diplotene stage, most bivalents presented a cruciform configuration (Figure ). At diakinesis (Figure (F, G)), chromosomes became further condensed and appeared like short sticks. Bivalents appeared in a cruciform configuration or presented an “O”, “V” form, due to the fact that chiasmata had moved to the end of the chromosome arms. In metaphase I, the homologous chromosomes come together and match up (synapsis) in pairs (tetrads or bivalents) and the chromosomes appeared as 9 normal paired bivalents (Figure (H–J)). At anaphase I, after the 18 chromosomes had moved to opposite poles, nine chromosomes could be seen clearly at each pole (Figure (K)). At anaphase II, when the centromere of each chromosome split, the chromatids separated, resulting in four daughter cells, each with an 1n chromosome number clearly visible as n = x = 9 (Figures ). Most microsporocytes nuclei successively undergo meiosis I and II, and generate four haploid nuclei, then differentiate into tetrads that subsequently give rise to mature pollen grains (Figure (Q, R)).
Meiotic behavior of induced tetraploids
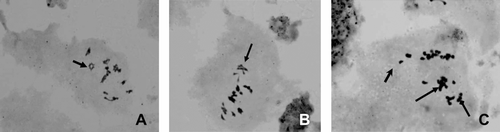
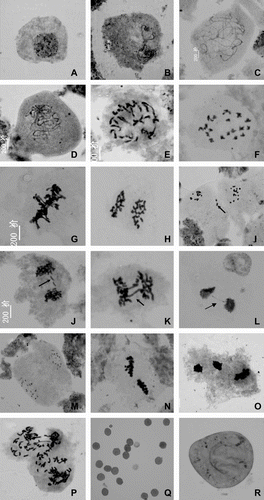
Observations were made on chromosome morphology and behavior during meiosis of PMCs for induced tetraploids. The steps of induced tetraploidy in meiosis are similar to diploid controls, but are much more complex (). At diakinesis, bivalents appeared as a cruciform configuration of 18 bivalents (Figure (F)). During metaphase I, the paired homologous chromosomes lined up in the central plane of the cell (Figure ). The chromosome configurations are formulated as various kinds of synapsis, e.g. univalent, bivalent, trivalent, quadrivalent and multivalent, but the main types are bivalent and quadrivalent (Figure ). In anaphase I (Figure ), the homologous chromosomes separate, and one of each pair travels to each of the two poles of the cell. The most common meiotic abnormalities observed in tetraploids were laggard chromosomes and some irregular chromosome bridges in telophase I (Figure (I–L)). Abnormalities observed in telophase II are the insynchronous segregation of bivalents. Some segregated earlier, and others later (Figure ). In this study, we only chose plants in which all meiosis cells are tetraploids. However, in some induced plants, most PMCs are tetraploids, whereas others are polyploids; these are called chimeras (Figure ). Some chimeras and the proportion of normal and abnormal cells in these chimeras will be further studied in later work.
Pollen sterility for induced tetraploids
During PMC meiosis in almost all earlier tetraploids studied, some chromosome stickiness, chromosome bridges and laggard chromosomes were observed during metaphase, anaphase I and telophase I (Fadaei et al. Citation2010; Li et al. Citation2010; Talukdar Citation2010), resulting in irregular segregation and contributing to production of unbalanced cells and consequently irregular meiotic products (Damasceno et al. Citation2010). Pairing disturbances in the induced tetraploids were also manifested in different combinations of multivalents, bivalents and univalents at diakinesis and metaphase-I in an earlier study (Talukdar Citation2010). The univalents, unmatched chromosomes and lagging chromosomes in S. przewalskii and S. brevilabra provide evidence that they are polyploids (Meng et al. Citation2009). These meiotic irregularities, through the formation of unbalanced gametes, have been blamed for pollen sterility and reduced seed yield in tetraploid plants (Talukdar Citation2010). These data also suggest that induced autotetraploids are meiotic unstable species with low pollen viability (Damasceno et al. Citation2010). In this study, we also observed inviable pollen grains (Figure (Q, R)) in induced tetraploids, and need to improve their fertility in future research.
Figure 2 Meiosis behavior of PMCs in induced tetraploid of P. grandiflorus. (A) interphase; (B) leptotene; (C) zygotene; (D) pachytene; (E) diplotene; (F) diakinesis; (G) metaphase I; (H) anaphase I; (I–J) telophase I (arrow showing laggard chromosome); (K–L) telophase I (arrow showing chromosome bridge); (M) prophase II; (N) metaphase II; (O) insynchronous chromosome segregation at telophase II; (P) polyploidy cell (chimeras); (Q–R) inviable pollen grains. Bar = 5 μm.
Meiosis effect on plant evolution
Meiosis is a process of cell division in which the number of chromosomes in certain cells is halved during gamete formation. In the sexual life cycle, there is an alternation of diploid and haploid generations. It involves two chromosome separations but only one chromosome replication. The three unique features of meiosis are synapsis, homologous recombination, and reduction division. Meiosis is controlled by hundreds of genes, both those shared with mitosis and specifically meiotic ones (Bogdanov 2003). The halved chromosomal number and the occurrence of genetic recombination are the main features in diploidy and in the evolutionary success of sexual reproduction in eukaryotes (Miller & Venable Citation2000; Mercier et al. Citation2001). Non-reduced gametes can be identified by their meiotic configuration during chromosomal pairing and can allow the detection and accurate determination of chromosomal rearrangements (Dias et al. Citation2005). Genetic diversity is the raw material of evolution, the fuel that drives it and determines its potential directions. The evolutionary significance of meiosis is that it generates large amounts of recombination, rapidly reshuffling gene combinations, producing variability upon which evolutionary processes can act. Genetic variation for recombination in meiosis can spread in very large populations under a strictly multiplicative-fitness, deleterious-allele model (Gessler & Xu Citation1999). Meiotic studies allow the analysis of a large variety of chromosomal alterations, such as polyploidy, which is very common in plants (Otto & Whitton Citation2000). Such chromosomes structural changes of quadrivalent formation in metaphase of meiosis-I in polyploidy may increase the amount of genetic variability in the gametes leading to new genotypes which may be useful to adverse environmental conditions (Fadaei et al. 2010). So it would be our further study for tetraploid other characterization and the desirable traits of these tetraploid plants would be explored for our breeding program.
In conclusion, the present work identified diploid controls by 2n = 2x = 18 chromosomes at metaphase-I and nine bivalents at diakinesis. The meiosis is regular, with normal chromosome pairing. Abnormalities were observed in different meiotic stages in induced tetraploids as laggard chromosomes, chromosome bridges and insynchronous chromosome segregation. The univalents, unmatched chromosomes and lagging chromosomes in induced plants provide further evidence that they are polyploids.
Acknowledgments
This research was supported by National Natural Science Foundation of China (No. 31171599), and Research Project Supported by Shanxi Scholarship Council of China (2012-052), Postdoctoral Foundation of Shanxi Agricultural University (No. 2008001) and Natural Science Foundation of Shanxi Province (No. 2009011040-2).
References
- Bogdanov , YF . 2003 . Variation and Evolution of Meiosis . Russian Journal of Genetics , 39 ( 4 ) : 363 – 381 .
- Damasceno , J , Corrêa , P , Pereira , TNS , Neto , MF and Pereira , MG . 2010 . Meiotic behavior of Carica papaya and Vasconcellea monoica . Caryologia , 63 ( 3 ) : 229 – 236 .
- Dias , BA , Viccini , LF and Shirlei , MRP . 2005 . Meiotic analysis of two putative polyploid species of Verbenaceae from Brazil . Caryologia , 58 ( 4 ) : 315 – 319 .
- Elrad , T and Unal , M . 2010 . Production of colchicine induced tetraploids in Vicia villosa roth . Caryologia , 63 ( 3 ) : 292 – 303 .
- Fadaei , F , Sheidai , M and Asadi , M . 2010 . Cytological study of the genus Arenaria L. (Caryophyllaceae) . Caryologia , 63 ( 2 ) : 149 – 156 .
- Gao , SL , Zhu , DN , Cai , ZH and Xu , DR . 1996 . Autotetraploid plants from colchicine-treated bud culture of Salvia miltior-rhiza Bge . Plant Cell, Tiss Org , 47 ( 1 ) : 73 – 77 .
- Gessler , DDG and Xu , SZ . 1999 . On the evolution of recombination and meiosis . Genet Res Camb , 73 : 119 – 131 .
- Li , Z , Chen , S , Chen , F , Fang , WM , Li , J and Wang , HB . 2010 . Karyotype and meiotic analysis of five species in the genus Artemisia . Caryologia , 63 ( 4 ) : 382 – 390 .
- Masterson , J . 1994 . Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms . Science , 264 : 421 – 423 .
- Meng , W , Zhang , L , Ding , CB , Yang , RW , Zhou , YH , Yang , ZJ and Yin , ZQ . 2009 . Meiotic observations of eight taxa in the genus Salvia . Caryologia , 62 ( 4 ) : 334 – 340 .
- Mercier , R , Grelon , M , Vezon , D , Horlow , C and Pelletier , G . 2001 . How to characterize meiotic functions in plants? . Biochimie , 83 : 1023 – 1028 .
- Miller , JS and Venable , DL . 2000 . Polyploidy and the evolution of gender dimorphism in plants . Science , 289 : 2335 – 2338 .
- Otto , SP and Whitton , J . 2000 . Polyploid incidence and evolution . Annu Rev Genet , 34 : 401 – 437 .
- Singh , RJ . 1993 . Plant cytogenetics , Boca Raton , FL : CRC Press . 391 p
- Soltis , DE and Soltis , PS . 1999 . Polyploidy: recurrent formation and genome evolution . Trends Ecol Evol , 14 : 348 – 352 .
- Swanson CP, Merz T, Young WJ. 1981. The Chromosome in Division, Inheritance and Evolution. Cytogenetics, Englewood Cliffs, NJ: Prentice-Hal1. 239 p.
- Talukdar , D . 2010 . Cytogenetic characterization of induced autotetraploids in grass pea (Lathyrus sativus L.) . Caryologia , 63 ( 1 ) : 62 – 72 .
- Wendel , JF . 2000 . Genome evolution in polyploids . Plant Mol Biol , 42 : 225 – 249 .
- Wu , YX , Yang , FH , Zhao , XM and Yang , W.D. 2011 . Identification of tetraploid mutants of Platycodon grandiflorus by colchicine induction . Caryologia , 64 ( 3 ) : 343 – 349 .
- Zhao , HL , Cho , KH , Ha , YW , Jeong , TS , Lee , WS and Kim , YS . 2006 . Cholesterol – lowering effect of platycodin D in hyper cholesterolemic ICR mice . Eur J Pharm , 537 : 166 – 173 .
- Zhao , HL and Kim , YS . 2004 . Determination of the kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1, 2-diglyceride-based colorimetric assay . Arch Pharm Res , 27 : 1048 – 1052 .