Abstract
We present here a characterization of Erythrina crista-galli L. (syn: Erythrina lamifolia Jacq.) seedlings, obtained from a plant from the Botanical Garden of Pisa University. This plant produces seeds that, during germination, have shown two different seedling phenotypes: normal (NT, 75%) and “albino” types (AT, 25%). Albino seedlings survive only 8–9 weeks and their growth is dramatically reduced when compared with wild type seedlings. Biochemical investigations have shown that albino seedlings completely lack chlorophyll and carotenoids and also soluble sugar levels are lower than in the normal type. We have also conducted sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) experiments and silver staining analysis on different protein extracts from shoots and leaves of both phenotypes, and demonstrated strong differences in protein patterns. The almost total absence of putative small and large RuBisCo bands in albino seedlings should be emphasized. We have also microspectrophotometrically determined the DNA content of this species, as it was lacking in the literature, by means of the Feulgen method. The recorded chromosome count of the species is confirmed to be 2n = 42, a number characteristic of the genus Erythrina. No variation in Feulgen absorption and in chromosome number is detectable between NT and AT seedlings. To better characterize this species and the two phenotypes, the complete nucleotide sequences of the internal transcribed spacer regions (ITS1 and ITS2) and the 5.8S rDNA have been also reported and compared with analogous sequences of some woody Leguminosae present in the European Molecular Biology Laboratory (EMBL) database. The results of this initial report lead us to conclude that albinism, in our system, has probably occurred as a result of natural mutation, and should be connected to genetic factors rather than to environmental conditions.
Introduction
The genus Erythrina (Leguminosae) is distributed in tropical and subtropical regions of the world and has been used in traditional medicine for the treatment of various diseases, especially microbial infections (Mitscher et al. Citation1987; Pillay et al. Citation2001). These plants are known to be a rich source of bioactive alkaloids (Ghosal et al. Citation1971; Furukawa et al. Citation1976; Barakat et al. Citation1977; Cordell Citation1981) and flavonoids, mainly isoflavones, pterocarpans, flavanones and isoflavanones (Chacha et al. Citation2005). Some of these flavonoids have been found to display a variety of biological properties, such as antimicrobial (Mitscher, Okwute, Gollapudi, Drake et al. Citation1988; Mitscher, Okwute, Gollapudi & Keshavarz-Shokri Citation1988; Chacha et al. Citation2005; Yenesew et al. Citation2005), anti-HIV (Mckee et al. Citation1997), antibacterial (Tanaka et al. Citation2002, Citation2004; Sato et al. Citation2002; Rukachaisirikul et al. Citation2007), anti-inflammatory (Njamen et al. Citation2003, Citation2004) and anti-plasmodial activities (Andayi et al. Citation2006 and references therein).
In this paper we present physiological, cytological and molecular data on the seedlings derived from an Erythrina crista-galli L. plant living in the Botanical Garden of Pisa University. It has previously been observed that this plant always produces seeds giving two different seedling phenotypes: normal type (NT) and albino (AT), 75% and 25% respectively.
Albinism is a consequence of partial or complete loss of chlorophyll pigments and of incomplete differentiation of chloroplast membranes, and is typical of higher plants (Kumari et al. Citation2009 and references therein). Studies have shown that in Nicotiana tabacum L. the chlorophyll absence in albino plants is due to some punctiform mutations or deletions in the plastidial genome (Avini et al. Citation1989; Fluhr & Cséplo Citation1986; Svab & Malinga Citation1986). The albino phenotype can also be due to mutation of many different nuclear loci as shown in Fagopyrum esculentum Moench. by Ohnishi (Citation1982), in Rhizophora mangle L by Klekowski & Godfrey (Citation1989) and in rye by Ballesteros et al. (Citation2009).
This type of recessive mutation normally leads to plastidial ribosome disappearance (Bradbeer et al. Citation1979), although the real mechanism responsible for this phenomenon is still unclear (Feierabend & Berberich Citation1991).
Several studies were conducted to analyze the gene expression pattern of albino plants in comparison to normal type. RuBisCO is the most abundant protein involved in photosynthetic processes driving CO2 into the biosphere. It is also one of the largest enzymes in nature, with a molecular mass of 560 kDa, a holoenzyme composed by eight large and eight small subunits (Spreitzer & Salvucci Citation2002). In land plants and green algae, the chloroplast rbcL gene encodes the large subunit, whereas a family of rbcS nuclear genes encodes nearly identical small subunits.
Results have shown that RuBisCO is not expressed, or is expressed at very low levels, in albino plants (Zubko & Day Citation1998; Ankele et al. Citation2005), suggesting a relationship between the absence of this protein and albinism.
We present here data on germination, growth rate, sugar levels, and protein patterns of leaf, shoot, and root derived from the two seedling types (NT or AT); moreover to better characterize this species and its mutant, nuclear DNA content, chromosome number and rDNA sequences are reported.
Materials and methods
Plant material
Seeds of Erythrina crista-galli L. were obtained from a tree living in the Botanical Garden of Pisa University. A total of 200 seeds were soaked for 12 h in water and than sowed in Petri dishes on a bed of sand saturated with distilled water. When root emergence was visible, seedlings were transferred into peat-based growing medium in 8 cm diameter pots. All the biochemical analyses were carried out in triplicate in 7-week-old plantlets. From germinating seeds, collected in three subsequent years, it was possible to observe that growing plantlets were distributed in two groups: NT (75%) and AT (25%). As control we used 200 seeds obtained from a plant living in the Botanical Garden of Corrientes (Argentina). All the seedlings derived from the Argentinean plant showed the normal phenotype.
Phenotypic characterization of plantlets
Leaf length was measured from petiole to the top of the organ while leaf width was measured in the larger portion of the leaf. Shoot and root length were measured from the residual cotyledons to the shoot apex or to the root tip respectively. The weight of each single organ was also measured. All the measurements were conducted in triplicate for normal as well as for albino types. All the phenotypic characterizations were carried out in 7-week-old plantlets.
Analysis of carbohydrates
Samples (0.1–1 g FW) were rapidly frozen in liquid nitrogen and ground to a powder, then sugars extracted as described by Tobias et al. (Citation1992) and assayed for glucose, fructose and sucrose content through coupled enzymatic assay methods (Guglielminetti et al. Citation1995). The efficiency of the method was tested by using known amounts of carbohydrates. Incubations of the samples and standards were carried out at 37°C for 30 min. The reaction mixtures (1 ml) were as follows. Glucose: 100 mM Tris-HCl, pH 7.6; 3 mM MgCl2; 2 mM ATP; 0.6 mM NADP; 1 unit Glc6P dehydrogenase; the A340 was recorded. Fructose was assayed as described for glucose plus the addiction of 2 units of PGI; the increase in A340 was recorded. Sucrose was first broken down using 85 units of invertase (in 15 mM Na-acetate, pH 4.6) and the resulting glucose and fructose were assayed as described above.
Recovery experiments evaluated losses taking place during the extraction procedures. Two experiments were performed for each metabolite by adding known amounts of authentic standards to the sample prior to the extraction. The concentration of the standards added were similar to those estimated to be present in the tissues in preliminary experiments. The recovery ranged between 93 and 108%.
Analysis of pigments
Pigments were extracted by incubating tissues (50–100 mg) in 1.5 ml 80% acetone for 1 week at 4°C in darkness. The absorbance of extracts was measured spectrophotometrically at 470.0, 663.2, and 646.8 nm. These absorbance values were used for calculation of chlorophyll a, chlorophyll b, and total carotenoids contents by means of formulae suggested by Lichtenthaler & Wellburn (Citation1983) and Wellburn (Citation1994).
Protein extraction and separation
Seedling tissues were dissected and collected from 7-week-old plantlets and extracted in 50 mM Tris-HCl buffer (pH 7.6 containing 10 mM DTT and 10% glycerol). Protein quantification was performed according to Guglielminetti et al. (Citation1997). Equal amounts of protein (2μg) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 12.5% polyacrylamide gels following by conventional silver nitrate staining.
Nuclear DNA determination and chromosome number
Roots (1 cm long) from 10 germinating seeds of Erythrina crista-galli (NT and AT) were collected and fixed in ethanol–acetic acid (3:1, v/v) overnight. The apical portions were then squashed in a drop of 45% acetic acid after treatment with a 5% aqueous solution of pectinase (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C, according to Venora et al. (Citation2002). After removal of the cover slips by the dry ice method, the slides were air dried and then hydrolyzed in 5 N HCl at room temperature for 30 min, stained with Schiff’s reagent, and washed according to the method of Kotseruba et al. (Citation2000). Squashes of Vicia bithynica L. root tips were stained concurrently with the other slides and used as an internal standard. Feulgen absorptions were measured using a Leitz MPV3 integrating microdensitometer (Leitz, Wetzlar, Federal Republic of Germany); absorption measured in V. bithynica preparations was used to convert relative Feulgen arbitrary units into picograms of DNA, by assuming a 4C DNA content of 18.30 pg for V. bithynica (Frediani et al. Citation1992). For each of five different root meristems of the two phenotypes, 50 nuclei were scored. For the internal standard (Vicia bithynica) 20 nuclei were scored for three different root meristems. For chromosome number determination, 1 cm long roots of E. crista-galli were treated with 8-hydroxyquinoline for 2 h, then fixed, squashed, and stained according to Bitonti et al. (Citation1996). For each phenotype at least five metaphases for each of five seedlings were analyzed.
DNA extraction and sequencing
Nuclear DNA was extracted and purified from roots of germinating seeds of Erythrina crista-galli (NT and AT) using a DNeasyPlant Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol. Each phenotype was sampled twice. The entire region including internal transcribed spacer regions ITS1, 5.8S and ITS2 rDNA was amplified by standard polymerase chain reaction (PCR) using the primers ITS5 and ITS4 (White et al. Citation1990). Amplifications were carried out using the following parameters: 95°C for 5 min, followed by 35 cycles of 95°C for 1 min, 55°C for 2 min, 72°C for 2 min, and finally 72°C for 5 min. The PCR-amplified DNA fragments showed a single band when examined on agarose gel. The PCR products were sequenced directly for both strands by using the automated sequencer ABI Prism 310 (Applied Biosystems, Foster City, CA, USA). Internal transcribed spacer sequences were aligned by the Clustal 1.83 software with default values (Thompson et al. Citation1994) with analogous sequences of some woody Leguminosae, present in the European Molecular Biology Laboratory (EMBL) database: Tamarindus indica L. (accession number AB378735), Dalbergia sissoo Roxb. ex DC. (accession number AF189023), Ceratonia siliqua L. (accession number AJ245575, AJ245576) and Robinia pseudoacacia L. (accession number AF467495). Alignments were carried out as daughter processes of BioEdit (Hall Citation1999), which was also used for sequence editing and manipulation.
Statistical analyses
All computations were performed with R 2.13.2 (R Development Core Team Citation2011) and R package mgcv version 1.6-1.
Results
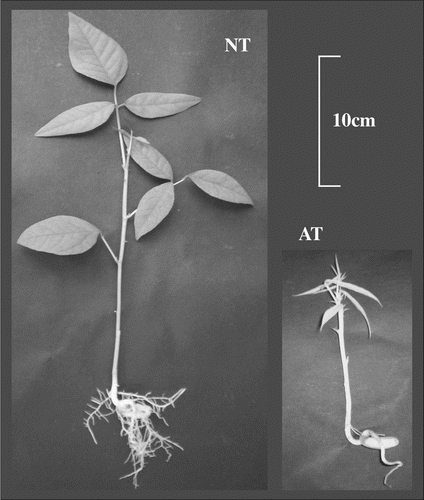
Figure shows a representative sample of the two 7-week-old Erythrina crista-galli phenotypes. Phenotypic data characterizing the two samples are reported in Table . NT plants present several large and green leaves, a high shoot and a long root. Conversely, albino plants show some small white leaves, and also the length of shoots is strongly reduced in comparison to NT while roots do not show significant differences. The differences are more evident when comparing organ weights. NT leaves are about 10 times heavier than AT samples. Also roots and shoots are heavier in NT than in AT (3/4 times and 2 times, respectively).
Table 1 Phenotypic data of 7-week-old Erythrina crista-galli plants.
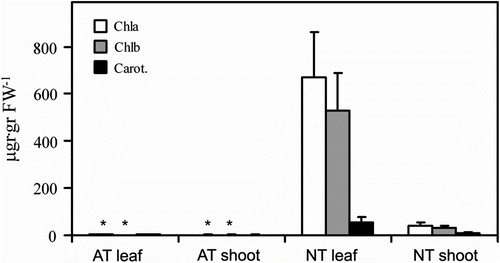
Figure illustrates data on pigment content. The levels of chlorophylls a and b, and carotenoids are reported for leaf or shoot samples from both AT and NT plants. It is evident that all the pigments are almost absent in leaves and shoots of albino plants, while high levels of chlorophylls a and b and a moderate level of carotenoids are detectable in NT leaves. Low levels of all three pigments are also present in the NT shoots. The chlorophyll differences are statistically significant, while differences in carotenoids do not show significance between NT and AT shoot and leaf.
Figure 2 Pigment content in 7-week-old Erythrina crista-galli shoot and leaf. Chla = chlorophyll a; Chlb = chlorophyll b; Carot. = carotenoids; AT = albino type; NT = normal type. ∗ indicates AT data are significantly different to NT data.
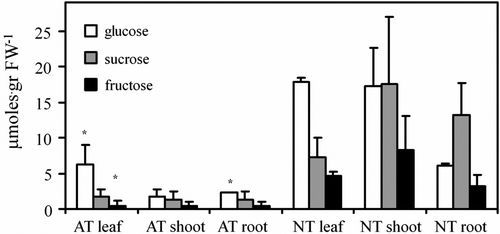
Data on soluble sugar levels (glucose, fructose and sucrose) in the different Erythrina crista-galli organs after 7 weeks of germination are reported in Figure . In general sugar amounts are lower in the AT organs than in NT ones. Glucose is the most abundant sugar in all the albino samples while sucrose represents the most abundant sugar in all the NT samples with the exception of leaves, where glucose is higher.
Figure 3 Soluble sugars content in 7-week-old Erythrina crista-galli shoot, root and leaf. AT = albino type; NT = normal type. ∗ indicates AT data are significantly different to NT data.
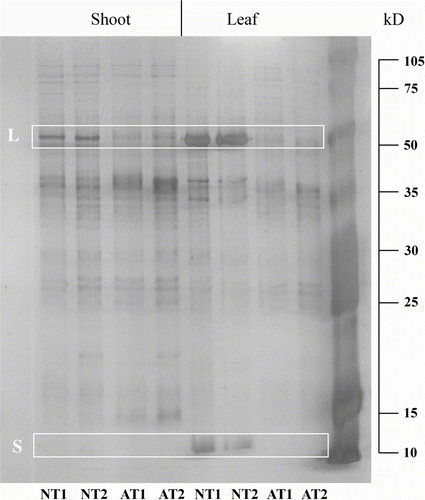
Concerning protein separation after SDS-PAGE, AT and NT protein patterns obtained from leaf and shoot extracts after silver staining are shown in Figure . In shoots the large RuBisCO subunit (the band of about 55 kD) is strongly expressed in NT samples while only a weak band is present in AT samples at the same molecular weight both in AT1 and in AT2. In leaf extracts the intensity of the same band increases dramatically while no signal is detectable for the AT sample. In addition the small RuBisCO subunit (a band of about 12 kD) is evident exclusively in the NT leaves. It is also worth noting, in both organs, several bands of about 35 kD differentially expressed depending on the plant type.
Figure 4 Protein patterns of AT and NT leaf and shoot extracts (12.5% SDS-PAGE and silver staining). NT = normal type; AT = albino type. All samples were loaded in duplicate (1 or 2). L = putative RuBisCO large sub-unit; S = putative RuBisCO small sub-unit.
As regards DNA analysis, the Feulgen absorptions, determined in prophase nuclei of root tips using Vicia bithynica preparations as an internal standard, are comparable within the two samples; and the nuclear DNA content is 7.98 ± 0.02 pg/4C nucleus. This determination is the first reported in the literature for this species. The 2n chromosome number of E. crista-galli was 42 in both the phenotypes (Figure ). This basic chromosome count of n = 21 is typical of the genus (Atchinson Citation1947). Both chromosome number and DNA content are higher than other reported data concerning some key woody Leguminosae (Table ).
Figure 5 Metaphase plate from normal type (NT) and albino type (AT) obtained from cytogenetic analysis of Erythrina crista-galli root meristem.
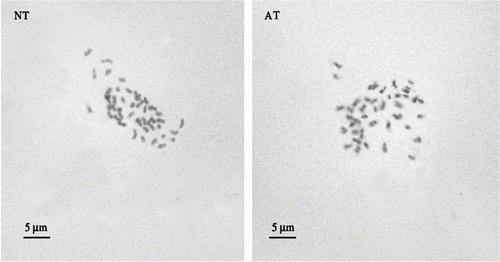
Table 2 Chromosome number, mean nuclear DNA content and accession number of rDNA sequences in E. crista-galli and related species.
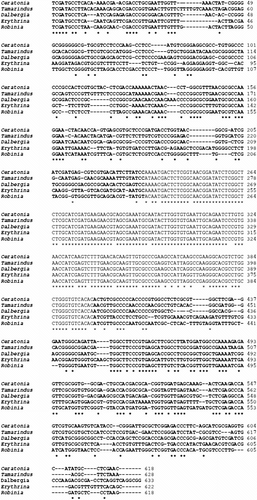
The nucleotide sequences of ITS1, 5.8S and ITS2 rDNA (Figure ) are available in the EMBL database, with the accession number FN825780; no relationships between albinism and ITS sequences have been found since they are identical in NT and AT. The ITS1 region is 224 bp long with 50.4% GC content. The ITS2 region is 233 bp long and has 50.7% GC content. The 5.8S rDNA is 164 bp long with 51.8% of GC content. In Figure the obtained sequences are compared to analogous sequences of some woody Leguminosae, present in the EMBL database (accession numbers reported in Table ). A low homology is present if we compare ITS1 and ITS2 sequences; conversely we have an high level of homology when analyzing the 5.8S rDNA alignments: sequence identity was 90.24% for Ceratonia siliqua, 96.30% for Robinia pseudoacacia, 95.09% for Dalbergia sissoo and 94.44% for Tamarindus indica.
Discussion
Albinism in Erythrina crista-galli, as in all higher plants, can be determined by one or by a concomitance of two or more factors including genotype, environment, meiotic abnormalities, hormonal imbalance, nuclear–plastid genome incompatibility, deletion in plastid DNA, mutation in genes responsible for chlorophyll biogenesis and/or functioning of photosynthetic apparatus (Kumari et al. Citation2009 and references therein). For these reasons, in this study we have compared Erythrina crista-galli AT lethal mutants with NT for several morphological and physiological traits and our results can be considered a first step in the analysis of the possible factors that can concur to albinism phenotype in this system.
One point of interest in the study of potential factors affecting albinism in plants is the control of ploidy level variations, which often depend on meiotic irregularities and abnormalities (Kim et al. Citation2003; Kumari et al. Citation2009 and references therein). To determine ploidy levels in our two systems we counted the chromosome number and estimated possible changes in nuclear DNA content by Feulgen absorption. This is a simple but accurate procedure that reflects real values in the nuclear DNA content, since the analyzed nuclei were chosen in the same post-synthetic conditions from tissues in the same developmental stages and all squashes were stained concurrently with V. bithynica root meristems, used as an internal standard. It is worth nothing that our determination of DNA content provides the first data regarding E. crista-galli and our values are in line with the determinations of Ohri et al. (Citation2004) on Erythrina genus. No differences in chromosome number and in the mean Feulgen absorption between NT and AT type were detectable and these results are an indication that the albino phenotype, at least in our system, seems not to be related to difference in the chromosome number and/or in genome size due to variation of repetitive sequences that are cytophotometrically detectable. Obviously the method we have used does not allow recognition of small differences in DNA content due to changes and/or deletions in specific sequences of the nuclear genome.
Our studies focused as well on some morpho-physiological traits of albinism in plants. Phenotypic data are considerably different between NT and AT plants, the latter showing typical features of albinism, e.g. a strong reduction both in the growth level and in the organ weight, a reduced number of leaves and the typical white color. The reported lack of chlorophyll production (Borner & Sears Citation1986) is confirmed for our albino samples and also carotenoid level in albino tissues is very low. As a consequence the severe lack of pigment observed in E. crista-galli AT necessarily damages photosynthetic process and blocks plant growth causing plant death.
We have also analyzed RuBisCO levels in leaf and shoot tissues. Both large and small subunits of RuBisCO are almost absent in albino tissues, as reported by Zubko & Day (Citation1998) for Nicotiana tabacum, some Brassica species and Arabidopsis thaliana L. (ecotype Columbia) and by Ankele et al. (Citation2005) for cereals. It is to underline the presence of a weak band corresponding to the large subunity of RuBisCO, coming from protein extracts belonging only to AT shoots and completely lacking in AT leaves. Taking into account that the two enzyme subunities of RuBisCO are encoded in two different genomes, nuclear and chloroplast genome expression may differently affect albinism in our system and this imbalance may vary depending on the plant organ. As a consequence of chlorophyll and RuBisCO absence, albino seedlings cannot grow autotrophically and are quickly subjected to sugar starvation that becomes lethal at 8–9 weeks. In contrast, in Oryza sativa it is possible to obtain a greenable albino mutant, which is able to survive temporarily as an albino until the 3-leaf-stage, in defense against the adverse environmental conditions (i.e. low temperatures); after that the mutant is able to begin photosynthetic metabolism, turning gradually green and completing its lifecycle (Xia et al. Citation2006).
In our system we have not observed albinism reversion phenomena; however, the very low level of sucrose (the normal soluble sugar transport form in plants) in AT plantlets could be explained by additional inefficient sugar translocation due to a general carbohydrate metabolism imbalance.
To better characterize E. crista-galli DNA, we have also determined the nucleotide sequences of a region of the ribosomal cistrons, i.e. the internal transcribed spacer regions (ITS1 and ITS2), separated by the 5.8S ribosomal RNA gene. ITS sequences have been used extensively to characterize a wide range of organisms, including fungi and plants, and have successfully been used to determine phylogenetic relationships among the taxa. The ITS regions of E. crista-galli present a very low homology with analogous regions of some woody Leguminosae and no relationship is detectable, as expected, between ITS sequences and the albino phenotype, since such sequences are identical in NT and AT samples.
The 5.8S rDNA sequence of E. crista-galli, if compared with the analogous regions of the above reported species, shows high level of sequence identity, ranging from 90.24% in Ceratonia siliqua to 96.30% in Robinia pseudoacacia, and confirming the idea that 5.8S rDNA, inside the rDNA sequences, is highly conserved.
In conclusion, albinism in our system has probably occurred as a result of natural mutation, and should be connected to genetic factors rather than to environmental conditions. The occurrence of AT (25%) versus NT (75%), taking place year by year, suggests that these two phenotypes are probably linked to a recessive trait governed by one gene with two alleles. Control of albinism by a single gene has been previously described in several taxa, including Leguminosae (Barwale & Widholm Citation1987; Neuffer et al. Citation1997; Shreekumari & Abraham Citation2005; Gettys & Wofford Citation2007). Further multidisciplinary studies are in progress to better characterize this system, aiming to clarify this highly genotype-dependent NT/AT ratio in E. crista-galli.
References
- Andayi , AW , Yenesew , A , Derese , S , Midiwo , JO , Gitu , PM , Jondiko , O.JI , Akala , H , Liyala , P , Wangui , J , Waters , NC , Heydenreich , M and Peter , MG . 2006 . Anti-plasmodial flavonoids from Erythrina sacleuxii . Planta Med , 72 : 187 – 189 .
- Ankele , E , Heberle-Bors , E , Pfosser , MF and Hofinger , BJ . 2005 . Searching for mechanisms leading to albino plant formation in cereals . Acta Physiol Plant , 27 : 651 – 665 .
- Atchinson , E . 1947 . Studies in the Leguminosae. 1. Chromosome number in Erythrina L . Am J Bot , 34 : 407 – 414 .
- Avini , A , Edelman , M , Rachailovich , I , Aviv , D and Fluhr , R . 1989 . A point mutation in the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase affects holoenzime assembly in Nicotiana tabacum . EMBO J , 8 : 1915 – 1918 .
- Ballesteros , I , Linacero , R and Vázquez , AM . 2009 . Mitochondrial DNA amplification in albino plants of rye (Secale cereale L.) regenerated in vitro . Plant Sci , 176 : 722 – 728 .
- Barakat , I , Jackson , AH and Abdulla , MI . 1977 . Further studies of Erythrina alkaloids . Lloydia , 40 : 471 – 475 .
- Barwale , UB and Widholm , JM . 1987 . Somaclonal variation in plants regenerated from cultures of soybean . Plant Cell Rep , 6 : 365 – 368 .
- Batlle I, Tous J 1997. Carob tree. Ceratonia siliqua L. Promoting the conservation and use of underutilized and neglected crops. 17. Rome, Italy: Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute.
- Bitonti , MB , Cozza , R , Wang , G , Ruffini Castiglione , M , Mazzuca , S , Castiglione , S , Sala , F and Innocenti , AM . 1996 . Nuclear and genomic changes in floating and submerged buds and leaves of heterophyllus waterchestnut (Trapa natans) . Physiol Plantarum , 97 : 21 – 27 .
- Borner , T and Sears , BB . 1986 . Plastome mutants . Plant Mol Biol , 4 : 69 – 92 .
- Bradbeer , JW , Atkinson , YE , Borner , T and Hagemann , R . 1979 . Cytoplasmic synthesis of plastid polypeptides may be controlled by plastid-synthesised RNA . Nature. , 279 : 816 – 817 .
- Ceccarelli , M , Morosi , L and Cionini , PG . 1998 . Chromocenter association in plant cell nuclei: determinants, functional significance, and evolutionary implications . Genome. , 41 : 96 – 103 .
- Chacha , M , Bojase-Moleta , G and Majinda , RRT . 2005 . Antimicrobial and radical scavenging flavonoids from the stem wood of Erythrina latissima . Phytochemistry. , 66 : 99 – 104 .
- Cordell , GA . 1981 . Introduction to Alkaloids: A Biogenetic Approach , New York (NY) : John Wiley & Sons .
- Domenech-Sanchez A, Hernandez ML, Rossello JA, Benedi VJ 1999. Methods for detecting additions of guar gum to locust bean gum. Unpublished.
- El Ferchichi , Ouarda H , Naghmouchi , S , Walker , DJ , Correal , E , Boussaid , M and Larbi , Khouja M . 2008 . Variability in the pod and seed parameters and nuclear DNA content of Tunisian populations of Ceratonia siliqua L . Agroforest Syst. , 74 : 73 – 81 .
- Feierabend J, Berberich T 1991. Heat-induced ribosome deficiency of plastids – mechanism and applications. In: Mache R, Stutz E, Subramanian AR, editors. The translational apparatus of photosynthetic organelles. Berlin – Heidelberg: Springer-Verlag. p. 215–227.
- Fluhr , R and Cséplo , A . 1986 . Induction and selection of chloroplast-code mutations in Nicotiana . Method Enzymol. , 118 : 611 – 623 .
- Frediani , M , Sassoli , O and Cremonini , R . 1992 . Nuclear DNA characterization of two species of Vicia: V. bithynica L and V. narbonensis L . Biol Plantarum. , 34 : 335 – 344 .
- Furukawa , H , Ito , K , Haruna , M and Jinno , Y . 1976 . Studies on the Erythrina alkaloids. XI. Alkaloids of Erythrina crysta-galli Linn. Structure of new alkaloids, crystamidine . Chem Pharm Bull. , 24 : 52 – 55 .
- Gettys , LA and Wofford , D . 2007 . Genetic control of albinism in pickerelweed (Pontederia cordata L.) . J Hered. , 98 : 356 – 359 .
- Ghosal , S , Majumdar , SK and Chakrabotti , A . 1971 . Erythrina alkaloids. Occurence of (+) N-norprotosinomenine and other alkaloids in Erythrina lithosperma . Aust J Chem. , 24 : 2733 – 2735 .
- Guglielminetti , L , Perata , P and Alpi , A . 1995 . Effect of anoxia on carbohydrate metabolism in rice seedlings . Plant Physiology. , 10 : 735 – 741 .
- Guglielminetti , L , Wu , Y , Boschi , E , Yamaguchi , J , Favati , A , Vergara , M , Perata , P and Alpi , A . 1997 . Effects of anoxia on sucrose degrading enzymes in cereal seeds . J Plant Physiol. , 150 : 251 – 258 .
- Hall TA 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid S. 41:95–98.
- Hu , JM , Lavin , M , Wojciechowski , MF and Sanderson , MJ . 2002 . Phylogenetic analysis of nuclear ribosomal ITS/5.8S sequences in the tribe Millettieae (Fabaceae): Poelilanthe-Cyclolobium, the core Millettieae, and the Calleryas group . Syst Bot. , 27 : 722 – 733 .
- Kim , JM , Liu , H , Taraki , M , Nagata , M and Aoki , F . 2003 . Changes in histone acetylation during mouse oocyte meiosis . J Cell Biol. , 162 : 37 – 48 .
- Klekowski , EJ Jr and Godfrey , PJ . 1989 . Ageing and mutation in plants . Nature. , 340 : 389 – 391 .
- Kotseruba , VV , Venora , G , Blangiforti , S , Ruffini Castiglione , M and Cremonini , R . 2000 . Cytology of Vicia species. IX. Nuclear DNA amount, chromatin organization and computer aided karyotyping of a Russian accession of Vicia faba L . Caryologia. , 53 : 195 – 204 .
- Kumari , M , Heather , JC , Small , I and Siddique , KH . 2009 . Albinism in plants: a major bottleneck in wide hybridization, androgenesis and double haploid culture . Crit Rev Plant Sci. , 28 : 393 – 409 .
- Lavin , M , Thulin , M , Labat , JN and Pennington , RT . 2000 . Africa, the odd man out: molecular biogeofraphy of dalbergioid legumes (Fabaceae) suggest otherwise . Syst Bot. , 25 : 449 – 467 .
- Lichtenthaler , HK and Wellburn , AR . 1983 . Determination of total carotenoids and chlorophylls a and b in leaf extract in different solvents . Biochem Soc Trans. , 11 : 591 – 592 .
- Mckee , TC , Bokesch , HR , McCormick , JL , Rashid , MA , Spiel-vogel , D , Gustafson , KR , Alavanja , MM , Cardellina , JH and Boyd , MR . 1997 . Isolation and characterization of new anti-HIV and cytotoxic leads from plants, marine, and microbial organisms . J Nat Prod. , 60 : 431 – 438 .
- Mitscher , LA , Drake , S , Gollapudi , SR and Okwute , SK . 1987 . A modern look at folkloric use of anti-infective agents . J Nat Prod. , 50 : 1025 – 1040 .
- Mitscher , LA , Okwute , SK , Gollapudi , SR , Drake , S. and Avona , E . 1988 . Antimicrobial Pterocarpans of Nigerian Erythrina mildbraedii . Phytochem. , 27 : 3449 – 3452 .
- Mitscher , LA , Okwute , SK , Gollapudi , SR and Keshavarz-Shokri , A . 1988 . Antimicrobial agents from higher plants. The isolation and structural characterization of two additional pterocarpan antimicrobial agents from Nigerian Erythrina mildbraedii . Heterocycles. , 27 : 2517 – 2522 .
- Neuffer MG, Coe EH, Wessler SR 1997. Mutants of maize. Plainville – New York: Cold Spring Harbor Laboratory Press.
- Njamen , D , Mbafor , JT , Fomum , ZT , Kamanyi , A , Mbanya , JC , Recio , MC , Giner , RM , Máñez , S and Rios , JL . 2004 . Anti-inflammatory activities of two flavanones, sigmoidin A and sigmoidin B, from Erythrina sigmoidea . Planta Med. , 70 : 104 – 107 .
- Njamen , D , Talla , E , Mbafor , J , Fomum , ZT , Kamanyi , A , Mbanya , JC , Cerdá-Nicolás , M , Giner , RM , Recio , MC and Rios , JL . 2003 . Anti-inflammatory activity of erycristagallin, a pterocarpene from Erythrina mildbraedii . Eur J Pharm. , 468 : 67 – 74 .
- Ohnishi , O . 1982 . Population genetics of cultivated common buckwheat, Fagopyrum esculentum Moench. I. Frequency of chlorophyll-deficient mutants in Japanese population . Jap J Genet. , 57 : 623 – 639 .
- Ohri , D , Bhargava , A and Chatteriee , A . 2004 . Nuclear DNA amount in 112 species of tropical hardwoods – new estimates . Plant Biol. , 6 : 555 – 561 .
- Pillay , CCN , Jager , AK , Mulholland , DA and van Staden , J . 2001 . Cyclooxygenase inhibiting and anti-bacterial activities of South African Erythrina species . J Ethnopharm. , 74 : 231 – 237 .
- R Development Core Team, 2011. R 2.13.2: a Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
- Rukachaisirikul , T , Innok , P , Aroonrerk , N , Boonamnuaylap , W , Limrangsun , S , Boonyon , C , Umpawan , W and Suksamrarn , A . 2007 . Antibacterial pterocarpans from Erythrina subumbrans . J Ethnopharm. , 110 : 171 – 175 .
- Sato , M , Tanaka , H , Fujiwara , S , Hirata , M , Yamaguchi , R , Etoh , H and Tokuda , C . 2002 . Antibacterial property of isoflavonoids isolated from Erythrina variegata against cariogenic oral bacteria . Phytomedicine. , 9 : 427 – 433 .
- Shreekumari , MT and Abraham , K . 2005 . Occurrence of albino seedlings in elephant foot yam . J Root Crop. , 31 : 61 – 62 .
- Spreitzer , RJ and Salvucci , ME . 2002 . RuBisCO: structure, regulatory interactions, possibilities for better enzyme . Annu Rev Plant Biol. , 53 : 449 – 475 .
- Sukrong S, Khanthapok P, Pongsamart S 2008. DNA fingerprint and chemical assessment of selected tamarind cultivars with high laxative activity. Unpublished (EMBL Database AB 378735).
- Svab , Z and Maliga , P . 1986 . Nicotiana tabacum mutants with chloroplast encoded streptomycin resistance and pigment deficiency . Theor Appl Genet. , 72 : 637 – 643 .
- Tanaka , H , Sato , M , Fujiwara , S , Hirata , M , Etoh , H and Takeuchi , H . 2002 . Antibacterial activity of isoflavonoids isolated from Erythrina variegata against methicillin-resistant Staphylococcus aureus . Lett Appl Microbiol. , 35 : 494 – 498 .
- Tanaka , H , Sato , M , Oh-Uchi , T , Yamaguchi , R , Etoh , H , Shimizu , H , Sako , M and Takeuchi , H . 2004 . Antibacterial properties of a new isoflavonoid from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus . Phytomedicine. , 11 : 331 – 337 .
- Thompson , JD , Higgins , DG and Gibson , TJ . 1994 . CLUSTAL W: improving the sensitivity of progressive multiple sequences alignment through sequence weighting, position-specific gap penalties and weight matrix choice . Nucl Acid Res. , 22 : 4673 – 4680 .
- Tobias , RB , Boyer , CD and Shannon , JC . 1992 . Alterations in carbohydrate intermediates in the endosperm of starch-deficient maize (Zea mays L.) genotypes . Plant Physiol. , 99 : 146 – 152 .
- Venora , G , Blangiforti , S , Ruffini Castiglione , M , Pignone , D , Losavio , F and Cremonini , R . 2002 . Chromatin organization and computer aided karyotyping of Triticum durum Desf. cv . Timilia. Caryologia. , 55 : 91 – 98 .
- Wellburn , AR . 1994 . The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution . J Plant Physiol. , 144 : 307 – 313 .
- White , TJ , Bruns , T , Lee , S and Taylor , JW . 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR Protocols: A Guide to Methods and Applications , Edited by: Innis , MA , Gelfand , DH , Sninsky , JJ and White , TJ . 315 – 322 . New York (NY) : Academic Press .
- Xia , JC , Wang , YP , Ma , BT , Yin , ZQ , Hao , M , Kong , DW and Li , SG . 2006 . Ultrastructure and gene mapping of the albino mutant al12 in rice (Oryza sativa L.) . Acta Genet Sin. , 33 : 1112 – 1119 .
- Yenesew , A , Derese , S , Midiwo , JO , Bii , CC and Heydenreich , MG . 2005 . Antimicrobial flavonoids from the stem bark of Erythrina burttii . Fitoterapia. , 76 : 469 – 472 .
- Zubko , MK and Day , A . 1998 . Stable albinism induced without mutagenesis: a model for ribosome-free plastid inheritance . Plant J. , 15 : 265 – 271 .