Abstract
A survey on chromosome counts of different sections belonging to the genus Scutellaria L. (Lamiaceae) throughout the world is presented and the relationships between chromosome data of its sections and their biogeography are also discussed here. In addition, meiotic chromosome numbers of 20 populations belonging to eight species growing in Iran, namely S. tomentosa (2n = 2x = 22), S. theobromina (2n = 2x = 22), S. araxensis (2n = 2x = 22), S. platystegia (2n = 2x = 22), S. nepetifolia (2n = 2x = 22), S. farsistanica (2n = 2x = 22), S. persica (2n = 2x = 22) and S. pinnatifida (2n = 2x = 22) were determined. With exception of S. pinatifida, all chromosome counts are reported for the first time, and are consistent with proposed base number of x = 11.
Introduction
The family Lamiaceae comprises about 3500 species in 258 genera worldwide. Many of its species have great importance due to their economic value (Duarte and Lopes Citation2007). Many species of the family are aromatic plants and often used in folk medicine, as herb spices or fragrance (Werker et al. Citation1985). Scutellaria L. is a genus belonging to the subfamily Scutellarioideae and grows both in Old and New Worlds (Cantino et al. Citation1992). With 425 currently recognized species, it is one of the large genera within Lamaiceae, but when the possible synonyms are considered the actual number of species is closer to 360 (Paton Citation1990a). Scutellaria and its active principles possess a wide range of pharmacological actions, such as antitumor, anti-angiogenesis, hepatoprotective, antioxidant, anticonvulsant, antibacterial and antiviral activities (Shang et al. Citation2010). All members of the genus are recognized as a monophyletic clade, so their genetic distances from Stachys, Lamium and Marubium account for 2.7%, suggesting that this group should be classified as a tribe, e.g. Scutellarieae (Wink and Kaufmann 1995).
The Irano-Turanian region, particularly Central Asia and Afghanistan, is the centre of maximum diversity for the genus. However, Eastern Mediterranean and the Andes are the second centers of its speciation (Paton Citation1990a). In Flora Iranica the genus is represented by 40 species, of which only 22 species grow in Iran and 10 species are endemic (Rechinger Citation1982). They are distributed all over the country, mainly in mountainous to sub-mountainous areas, but with a few in wet places and also in forests. Scutellaria species are typically characterized by the shape of calyx with two undivided lips and the presence of a scutellum on the upper lip. The calyx shows variable characters in different species, and the scutellum may be absent or the calyx may be inflated in the upper lip.
The latest global taxonomic review and infrageneric classification of Scutellaria was presented by Paton (Citation1990a). He comprehensively studied Scutellaria and its allied genera and showed that the features related to inflorescence, calyx, corolla and nutlets are the most important and taxonomically reliable characters in distinguishing species (Paton Citation1990a, Citation1990b, Citation1992). He also divided Scutellaria into two subgenera, namely Scutellaria (Neveski ex Juz.) Juz. emend. Paton, which is characterized by one-sided or rarely spiral inflorescence and opposite flowers not subtended by leaves or by leaf-like bracts; and Apelthanthus (Neveski ex Juz.) Juz. emend Paton, characterized by 4-sided inflorescence, opposite and decussate flowers subtended by cucullate bracts. S. subgen. Scutellaria was divided into five sections, namely Scutellaria (Rech.) Paton, Anaspis (Rech.) Paton, Salazaria (Torrey) Paton, Perilomia (Kunth) Epling emend. Paton and Salviifoliae (Boiss.) Edmondson. Subgenus Apelthanthus was divided into two sections, namely Apeltanthus Nevski ex Juz. and Lupulinaria Hamilton. Based on this classification, the Iranian Scutellaria species will be recognized in S. sect. Scutellaria and S. sect. Anaspis of S. subgen. Scutellaria, and S. sect. Lupulinaria of S. subgen. Apeltanthus.
Most cytological studies of the genus Scutellaria have concerned chromosome counts, but there is some report on the relationships between chromosomal criteria and biogeography. The first cytological observation of the genus was the chromosome number count of 2n = 26 in S. insignis reported by Lee (Citation1967). At least 14 different chromosome numbers have been found for the genus: 2n = 14 (S. sibthorpii), 2n = 16 (e.g. S. chodja-kasiani), 2n = 18 (e.g. S. intermedia), 2n = 20 (e.g. S. repens), 2n = 22 (e.g. S. grandiflora), 2n = 24 (e.g. S. discolor), 2n = 26 (e.g. S. indica), 2n = 28 (e.g. S. alpina), 2n = 30 (e.g. S. altissima), 2n = 32 (e.g. S. hastifolia), 2n = 34 (e.g. S. hirta), 2n = 44 (S. lateriflora), 2n = 60 (e.g. S. churchilliana) and 2n = 88 (S. lateriflora) (Sharma Citation1970; Vij and Kashyap Citation1975, Citation1976 Rostovtseva Citation1977; Gill and Morton Citation1978; Murin Citation1978; Astanova Citation1981; Montmollin Citation1982, Citation1986; Singh Citation1984; Yildiz and Gücel Citation2006). Due to the large chromosomal variation in the genus, the present work aimed at increasing the knowledge about the cytogenetics and biogeography of the species, and at comparing the base chromosome numbers and polyploidy levels between different sections, which are distributed throughout the world. Such findings would help us to promote our understanding about the relationships between chromosomal criteria and taxonomic delimitation.
This article follows previous studies conducted on cytological researches in Iran (Ranjbar et al. Citation2009, Citation2010a, b, c, Citation2011a, b, 2012, Sheidai et al. Citation2010).
Materials and methods
Description of database
Some chromosome records from online databases (http://www.tropics.org/Project/IPCN) and the literature are presented in Table . Each record in the database includes the following data: name of taxon as published in the original source, the standardized name (authorship of the name is corrected, as well as possible typing errors, and the currently accepted name); data on chromosomes includes mitotic or meiotic chromosome number, ploidy level, the name of person who counted chromosomes and the locality where plant materials have been collected.
Table 1. Scutellaria species analyzed in the present study.
Cytogenetics
The meiotic chromosome numbers were studied in five populations of S. pinnatifida, five populations of S. platystegia, three populations of S. araxensis, three populations of S. theobromina and only one population from each of S. tomentosa, S. farsistanica, S. persica and S. nepetifolia. Voucher specimens are deposited in BASU, Hamedan, Iran. Randomly selected flowers in the ideal stage for meiotic studies were collected and fixed in 96% ethanol, chloroform and propionic acid (6:3:2) for 24 h at room temperature, and then washed and preserved in 70% ethanol at 4°C until used. Microsporocytes were prepared by squashing and stained with 2% acetocarmine. Chromosome numbers were determined in five individuals of each population during diakinesis. The meiotic chromosome association was evaluated in at least 20 diakinesis cells. Meiotic stages were photographed by a BX-51 Olympus (Nagano, Japan) microscope equipped with a 3030 digital camera.
Results and discussion
Chromosome data analysis
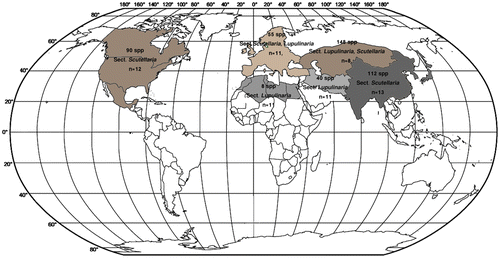
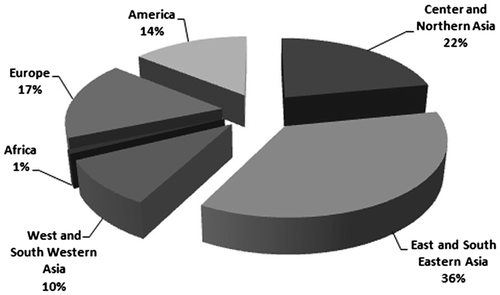
The current databases represent the reports for only 86 species (93 taxa including subspecies) from total of 425 species that are estimated for the genus Scutellaria throughout the world (Paton Citation1990a). There are records for approximately 21% of species with known chromosome numbers. The abundance of the species is not consistent with their geographical distributions (Figure ). Asian taxa are more intensively studied than the taxa growing in other geographical areas. About 300 species of the genus occur in Asia (Boissier Citation1879; Juzepczuk Citation1954; Edmondson Citation1982; Rechinger Citation1982; Tatsu-Nami-Sō Citation1984; Huang Citation1994), and as much as 68% of the chromosome number records originate from this continent. Even within Asia the data are not equally distributed: 36% of the Asian records originate from E and SE Asia, 22% in central and northern Asia and 10% in W and SW Asia (Brummitt Citation2001). This is somewhat proportional to the number of species: 112 species occur in E and SE Asia, 148 in the center and N; and 40 in W and SW Asia. Of about 90 taxa in North America, 55 taxa occur in Europe and eight taxa in Africa (Rionchardson Citation1972; Paton Citation1992; Joshee et al. Citation2010). that represented only 14.2%, 17.14% and 0.95% chromosome counts, respectively. Thus, an unequal spread of the records throughout the distribution area of the genus is apparent. However, the hypothesis is needed to be confirmed by deeper karyogeographical explorations. As the genus Scutellaria is a subcosmopolitan genus (Paton Citation1992), our estimated statistics represent the same pattern as expected by Kučera et al. (Citation2005) for the genus Cardamine.
Figure 1 Geographical distribution of chromosome number records in the genus Scutellaria (the geographical division of the world Brummitt Citation2001).
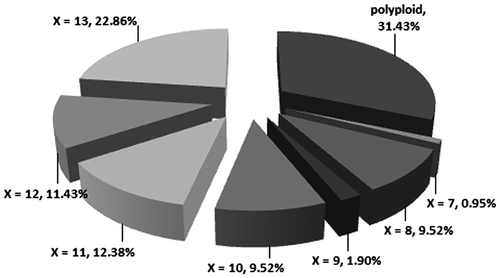
Another comparison can be made of the percentages of taxa that are entirely diploid, those that are both diploid and polyploid, and those that are entirely polyploid. For the genus Scutellaria, we have found 67.03% diploid, 7.69% diploid and polyploid, and 24.27% entirely polyploid taxa. Karyological data for entire diploidy (basic chromosome numbers) and polyploidy percentages are represented as follows (Figure ): x = 7, 0.95% (one taxon); x = 8, 9.52% (10 taxa), x = 9, 1.90% (two taxa), x = 10, 9.52% (10 taxa), x = 11, 12.38% (13 taxa), x = 12, 11.43% (11 taxa) and x = 13, 22.86% (24 taxa). The highest frequent chromosome number is 2n = 88, reported for S. lateriflora L. (Gill and Morton Citation1978) from N America (Canada).
Cytogenetics
Chromosome numbers of S. sect. Scutellaria, S. sect. Anaspis and S. sect. Salazaria of S. subgen. Scutellaria and S. sect. Lupulinaria of S. subgen. Apelthanthus have been previously reported (Paton Citation1990a). Results from analyses of chromosome data, summarized in Table , represent about 85.42% of the studied species belonging to S. sect. Scutellaria, 12.50% to S. sect. Lupulinaria and the remaining taxa (about 2%) to S. sect. Anaspis and S. sect. Salazaria based on Paton’s classification (Paton Citation1990a).
Biogeography
Geographical distribution of the taxa belonging to the genus Scutellaria was obtained from online databases (http://ww2.bgbm.org/euroPlusMed/query.asp) and atlases mainly based on their localities taken from Floras and literatures and mapped here. For readability the localities, species numbers and prevailing chromosome counts of the sections are presented (Figure ).
Scutellaria sect. Scutellaria (Rech.) Paton
Members of S. sect. Scutellaria are characterized by opposite or spiral flowers that are inserted in the leaf axis or leaf-like bracts, forming a one-sided inflorescence or rarely radiating out from the inflorescence axis in all directions. The section is probably the most primitive infrageneric taxon within the genus Scutellaria. The other sections of S. subgen. Scutellaria are probably evolved from S. sect. Scutellaria in response to various selection pressures in different regions of the world. It is the most widespread taxon, with about 240 species throughout the natural range of Scutellaria with unequal numbers in the Old and New Worlds (Paton Citation1990a). As Scutellaria is a subcosmopolitan section, most cytogenetic studies have been done on this section. Our results confirmed that there are different chromosome numbers in S. sect. Scutellaria (2n = 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 44, 60, 80 and 88). (Probatov and Sokolovskaya 1990) These numbers probably represent aneuploid members that establish communities surrounding the diploid and polyploid species. The diploid numbers 2n = 24 and 2n = 26 are known in most of the taxa belonging to the section. Therefore, x = 2 and x = 13 are the prevailing basic numbers in the section. The basic chromosome number x = 13 is dominant in E and SE Asia and x = 12 in America. Meanwhile, chromosome numbers 2n = 44, 80 and 88 are rare and reported only for S. lateriflora. The other sections of S. subgen. Scutellaria are not widespread and there are few chromosome reports on these sections. S. sect. Salazaria is distributed in S Utah, S Arizona, New Mexico, Texas, California, Baja California and Chihuahua. The diploid chromosome number 2n = 50 is reported for S. mexicana of S. sect. Salazaria (Paton Citation1990a). About 15 species of S. sect. anaspis are found in S Iran, Afghanistan, C Asia and Xizang (Tibet), of which S. petiolata shows a diploid number of 2n = 16. (Aryavand 1977; Khatoon and Ali 1993) About 11 species of S. sect. Perilomia are found in the Andes from Colombia to Chile and no counts have been reported on this section (Paton Citation1990a). Chromosome numbers 2n = 24–34 have been reported for S. subgen. Scutellaria by Paton (Citation1990a). This study is based on the published counts represent the known chromosome numbers 2n = 16–88.
Scutellaria sect. Lupulinaria A. Hamilton
S. sect. Lupulinaria are prostrate or erect suffruticose herbs and characterized by petiolate lower leaves, similar in form to the upper leaves, and dense or loose inflorescence. About 130 species of the section are found in the mountainous and upland regions in N Africa and Eurasia, usually over 1000 m elevation. The section was divided into two subsections, namely S. subsect. Lupulinaria (Hamilton) Paton and S. subsect. Cystaspis. (Juz.) Paton. Most studies on the section related to S. subsect. Lupulinaria (Paton Citation1990a). There are different chromosome numbers for the taxa belonging to the section (2n = 16, 18, 20 and 22). Probably most of them are euploids or aneuploids. The chromosome number 2n = 22 is known for the most of taxa in diploid level. Therefore, x = 11 is the prevailing basic chromosome number in the section and x = 8, x = 9 and x = 10 are less frequent. (Morton 1973; Aryavand 1977; Krogulevich 1978; Cherian and Kuriachan 1981; Montmollin Citation1982; Sekovski and Jovanovska 1983; Papanicolaou 1984; Vembu 1984; Nishikawa 1985; Saggoo and Bir 1986; Sokolovskaya et al. 1986; Galland 1988; Ghaffari 1988; Probatova et al. 1989; Probatova and Sokolovskaya 1990; Probatova et al. 1991; Galland 1991; Xu et al. 1992 ; Zakirova and Nafanailova 1992; Zhao 1996; Dempsey et al. 1994; Probatova et al. 1998; Vembu and Sampathkumar 1999; Shatalova 2000; Dmitrieva 2000; Huang et al. 2003; Naruhashi et al. 2004; Castro 2005; Cheng 2010; Sudarmono and Conn 2010).
We report here the chromosome numbers of 20 populations of eight species belong to S. sect. Lupulinaria, which were collected from different localities in Iran (Table ). Our results showed that all populations were diploid and possessed 2n = 2x = 22 chromosome number, consistent with the proposed base number of x = 11 (Figures). On the basis of published counts for S. sect. Lupulinaria together with the reports presented here, we have verified that x = 11 is the most common number for this section. The chromosome numbers 2n = 16–24 have been reported for S. subgen. Apelthanthus by Paton (Citation1990a). The chromosome numbers 2n = 16–22 for the subgenus result from the published counts and the present study.
Table 2. Scutellaria sect. Lupulinaria species analyzed in this study with their respective chromosome numbers, locations, and voucher specimens.
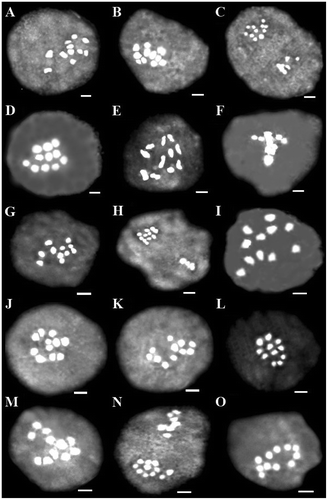
Figure 4 Representative meiotic cells of three Scutellaria species. (A–D) S. araxensis: (A) diakinesis (26100); (B) telophase I (26100); (C) diakinesis (26214); (D) asynchronous nuclei (26822). (E–M) S. platystegia: (E) diakinesis (25760); (F) telophase I with forward chromosome (25760); (G) diakinesis (26180); (H) cytomixis (26180); (I) diakinesis (26224); (J) asynchronous nuclei (26224); (K) diakinesis (26370); (L) asynchronous nuclei (26370); (M) asynchronous nuclei (26890). (N, O) S. farsistanica: (N) diakinesis (24693); (O) anaphase I with laggard chromosome (24693). Scale bars: 2 μm.
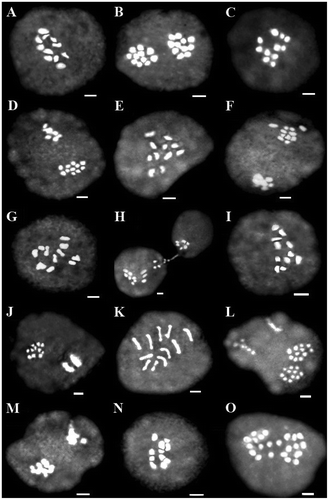
Figure 5 Representative meiotic cells of four Scutellaria species. (A) S. tomentosa: diakinesis (24420). (B, C) S. persica: (B) diakinesis (25500); (C) asynchronous nuclei (25500). (D–H) S. theobromina: (D) diakinesis (26554); (E) diakinesis (25838); (F) metaphase I with precocious chromosome migration to the pole (25838); (G) diakinesis (25824); (H) asynchronous nuclei (25824). (I) S. nepetifolia: diakinesis (27446). (J–O) S. pinnatifida: (J) diakinesis (25428); (K) diakinesis (26704); (L) diakinesis (25742); (M) diakinesis (28517); (N) asynchronous nuclei (28517); (O) diakinesis (26111). Scale bars: 2 μm.
Acknowledgment
The fieldwork in Iran was supported by grants from the Bu-Ali Sina University.
References
- Andreev VN. 1982. In IOPB chromosome number reports LXXVI. Taxon. 31 : 575–576.
- Arohonka T. 1982. Chromosome counts of vascular plants of the island Seili in Nauvo, southwestern Finland. Turun Yliopiston Julkaisuja, Sar. A 2. Biol Geog. 3 : 1–12.
- Astanova SB. 1981. Novye dannye o khromosomnikh chislakh nekotorykh vidov gubocvetnykh Tadzhikistana. Izvestiia Akademii Nauk Tadzhikskoi SSR: Otdelenie Biologicheskikh Nauk. 1 (82): 10–15.
- Astanova SB. 1984. Chromosome numbers in the species of the families Alliaceae, Asteraceae, Caryophyllaceae, Ebenaceae, Linaceae, Oleaceae. Lamiaceae from Tadjikistan. Bot Z. 69 (11): 1563–1564.
- Baltisberger M. 1988. Numeri cromosomici per la flora Italiana: 1167–1184. Inform Bot Ital. 20 : 627–636.
- Baltisberger M. 1991. IOPB chromosome data 3. Int Org Plant Biosyst Newsl. 17 : 5–7.
- Baltisberger M, Mullaj A, Tartari V. 1993. Mediterranean chromosome number reports 3. Flora Med. 3 : 348–353.
- Bir SS, Saggoo MIS. 1984. Cytological studies on the family Labiatae from Gharwal Himalayas. In: Paliwal GS, ed. The Vegetational Wealth of the Himalayas. New Delhi: Puja, 471–482.
- Bir SS, Saggoo MIS. 1985. Cytological studies on members of family Labiatae from Kodaikanal and adjoining areas (South India). Proc Indian Acad Sci. 94 : 619–626.
- Boissier E. 1879. Scutellaria L. In: Boissier E, ed. Flora Orientalis. Geneva: Basilease and Lugundi, 681–691.
- Bothmer RV. 1985. Differentiation patterns in the Scutellaria albida group (Lamiaceae) in the Aegean area. Nord J Bot. 5 : 421–439.
- Brummitt RK. 2001. World geographical scheme for recording plant distributions. Ed. II. Pittsburgh: Hunt Institute for Botanical Documentation Carnegie Mellon University 137.
- Buttler KP. 1989. Chromosomenzahlen von Gefässpflanzen aus Hessen 4. Hessische Floristische Briefe. 38 : 11–14.
- Cantino PD, Harley RM, Wagstaff SJ. 1992. Genera of Labiatae: Status and classification. In: Harley RM, Reynolds T, eds. Advances in Labiatae science. Kew: Royal Botanical Garden, 511–522.
- Duarte MR, Lopes JF. 2007. Stem and leaf anatomy of Plectranthus neochilus Schltr. Lamiaceae. Rev Brasil Farm. 17 : 549–556.
- Edmondson JR. 1982. Scutellaria L. In: Davis PH, ed. Flora of Turkey and the east Aegean islands. Edinburgh: Edinburgh University Press, 78–100.
- Fernandes A, Leitão MT. 1984. Contribution à l’étude cytotaxinomique des Spermatophyta du Portugal XVIII Lamiaceae. Mem Soc Broter. 27 : 27–75.
- Ghaffari SM. 2008. Chromosome records for some plant species from Iran. Iran J Bot. 14 : 39–46.
- Gill LS. 1974. B-chromosomes in the family Labiatae. Sci. and Cult. 40 : 118–119.
- Gill LS. 1980. Cytotaxonomy of the genus Scutellaria L. (Labiatae) in Canada. Caryologia. 33 : 339–346.
- Gill LS. 1981. Chromosomal evolution and incidence of polyploidy in the Canadian Labiatae. Rev Cytol Biol Vég Bot. 4 : 331–339.
- Gill LS. 1984. The incidence of polyploidy in the West-Himalayan Labiatae. Rev Cytol Biol Vég Bot. 7 : 5–16.
- Gill LS, Morton JK. 1978. Scutellaria churchilliana – hybrid or species. Syst Bot. 3 : 342–348.
- Hsieh Th, Huang TC. 1995. Notes on the flora of Taiwan (20) Scutellaria (Lamiaceae) in Taiwan. Taiwania. 40 : 35–56.
- Hsieh TH, Huang TC. 1997. Notes on the Flora of Taiwan (29) Scutellaria austrotaiwanensis Hsieh and Huang sp. nov. (Lamiaceae) from Taiwan. Taiwania. 42 : 109–116.
- Huang QS. 1994. Scutellaria L. In: Li XW, Hedge IC, editors. Flora of China, vol. 17. Missouri Botanical Garden Press. p. 75–103.
- Joshee N, Parajuli P, Medina-Bolivar F, Rimando AM, Yadav A. 2010. Scutellaria Biotechnology: Achievements and Future Prospects. Bulletin UASVM, Horticulture. 67 (1): 24–32.
- Juzepczuk SV. 1954. Scutellaria L. In: Shishkin BK, Juzepczuk SV, editors. Flora of the USSR, vol. 20. Moskva-Leningrad. p. 50–150.
- Krasnoborov IM, Rostovtseva TS, Ligus SA. 1980. Chromosome numbers of some plant species of South Siberia and the Far East. Bot Z. 65 : 659–668.
- Krasnoborov IM, Rostovtseva TS. 1975. Chromosome numbers of some plant species from the south of Siberia. Bot Z. 60 (6): 853–860.
- Krishnappa DG, Basavaraj I. 1982. In IOPB chromosome number reports LXXV. Taxon. 31 : 361–362.
- Kučera J, Valko I, Marhold K. 2005. On-line database of the chromosome numbers of the genus Cardamine (Brassicaceae). Biologia, Bratislava. 60 (4): 473–476.
- Lee YN. 1967. Chromosome numbers of flowering plants in Korea. J Korean Res Inst Ewha Woman Univ. 11 : 455–478.
- Love A, Love D. 1982. IOPB chromosome number reports LXXV. Taxon. 31 : 344–360.
- Lövkvist B, Hultgård UM. 1999. Chromosome numbers in south Swedish vascular plants. Opera Bot. 137 : 1–42.
- Markova M, Goranova V. 1995. Mediterranean chromosome number reports. Flora Med. 5 : 289–317.
- Montmollin, BD. 1982. Étude cytotaxonomique de la flore endémique de la Crète I. Note préliminaire. Bulletin de la Société neuchậteloise des sciences naturelles. 105: 65–77.
- Montmollin BD. 1986. Etude cytotaxonomique de la flore de la Crète. III. Nombres chromosomiques. Candollea. 41 : 431–439.
- Murin A. 1978. Index of chromosome numbers of Slovakian flora. Part 6. Acta Facultatis Rerum Naturalium Universitatis Comenianae. Botanica. 26 : 1–42.
- Olmstead RG. 1990. Systematics of the Scutellaria angustifolia complex (Labiatae). Contrib Univ MI Herb. 17 : 223–265.
- Paton A. 1990a. A global taxonomic investigation of Scutellaria L. Kew Bull. 45 : 399–450.
- Paton A. 1990b. The phylogeography of Scutellaria L. Notes R Bot Gard Edinb. 46 : 345–359.
- Paton A. 1992. The adaptive significance of calyx and nutlet morphology in Scutellaria. Royal Botanic Gardens, Kew 203–210.
- Pittman AB. 1987. Systematic studies in Scutellaria section Mixtae (Labiatae). Assoc Southeast Biol Bull. 34 : 72.
- Pogan E, Wcislow H, Jankun A. 1980. Further studies in chromosome numbers of Polish Angiosperms Part XlIl. Acta Biol Cracov, Ser Bot. 22 : 37–69.
- Probatova NS. 2006. Chromosome numbers of vascular plants from nature reserves of the Primorsky Territory and the Amur River basin. Bot Z. 91 : 1117–1134.
- Ranjbar M, Hajmoradi F, Karamian R. 2010a. Mitotic study of some species of Onobrychis sect. Hymenobrychis DC. in Iran. Iran J. Plant Biol. 1 (1–2): 47–54.
- Ranjbar M, Hajmoradi F, Karamain R. 2011a. Meiotic chromosome number and behaviour of Onobrychis alborzensis (Fabaceae): a new species from northern Iran. Feddes Repert. 122 (7–8): 1–12.
- Ranjbar M, Hajmoradi F, Karamian R. 2012. An overview on cytogenetics of the genus Onobrychis (Fabaceae) with special reference to O. sect. Hymenobrychis from Iran. Caryologia. 65 : 187–198.
- Ranjbar M, Karamian R, Hadadi A. 2009. Biosystematic study of Onobrychis vicifolia Scop. and Onobrychis altissima Grossh. (Fabaceae) in Iran. Iran J Bot. 15(1):85–95.
- Ranjbar M, Karamian R, Hadadi A. 2010b. Cytosystematics of three Onobrychis species (Fabaceae) in Iran. Caryologia. 63 : 237–249.
- Ranjbar M, Karamian R, Hajmoradi F. 2010c. Chromosome number and meiotic behaviour of two populations of Onobrychis chorassanica Bunge (O. sect. Hymenobrychis) in Iran. J Cell Mol Res. 2(1):49–55.
- Ranjbar M, Karamian R, Nouri S. 2011b. Impact of cytomixis on meiosis in Astragalus cyclophyllos Beck (Fabaceae) from Iran. Caryologia. 64 : 256–264.
- Rechinger KH. 1982. Scutellaria L. In: Rechinger KH, ed. Flora Iranica. Graz: Akademische Druck-u.-Verlagsanstalt, 44–84.
- Rionchardson IBK. 1972. Scutellaria L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine SM, Webb DA, eds. Flora Europaea. Cambridge: Cambridge University Press, 135–137.
- Rostovtseva TS. 1977. Chromosome numbers of some plant species from the south of Siberia. II. Bot Z. 62 : 1034–1042.
- Saggoo MI. 1983. Cytomorphological studies on plants of economic importance of Bicarpellatae from India Publication. p. 259.
- Saggoo MIS, Bir SS. 1981. Chromosome number in certain Acanthaceae and Labiatae III. In chromosome number reports LXXI. Taxon. 30 : 515.
- Saggoo MI, Bir SS. 1982. Chromosome number in certain Acanthaceae and Labiatae IV. In IOPB chromosome number reports LXXVI. Taxon. 31 : 593–595.
- Sawanomukai T, Iwatsubo Y, Naruhashi N. 2003. Chromosome numbers of Japanese Scutellaria (Lamiaceae). J Phytogeog Taxon. 51 : 131–136.
- Shang X, He X, He X, Li M, Zhang R, Fan P, Zhang Q, Jia Z. 2010. The genus Scutellaria: an ethnopharmacological and phytochemical review. J Ethnopharmacol. 128 : 279–313.
- Sharma AK. 1970. Annual report, 1967–1968. Research Bulletin [Cytogenetics Laboratory, Department of Botany, University of Calcutta]. 2:1–50.
- Shatokhina AV. 2006. Chromosome numbers of some plants of the Amur Region flora. Bot Z. 91 : 487–490.
- Sheidai M, Alijanpoo B, Khayyami M. 2010. Contribution to cytology of genus Salvia L. (Lamiaceae) in Iran. Caryologia. 63 : 405–410.
- Singh TP. 1979. Chromosome studies in Scutellaria. Sci. Culture. 45 : 336.
- Singh TP. 1984. Chromosome studies in some genera of Labiatae. Cell Chromosome Res. 7 : 53–55.
- Tatsu-Nami-Sō Z. 1984. Scutellaria L. In: Ohwi J, ed. Flora of Japan. Washington, DC: Smithsonian Institution, 770–772.
- Vachova M, Ferakova V. 1980. In chromosome number reports LXIX. Taxon. 29 : 722–723.
- Van Loon JC, Setten AKV. 1982. In IOPB plant chromosome number reports. Taxon. 31 : 589–592.
- Vij SP, Kashyap SK. 1975. In IOPB chromosome number reports XLVIII. Taxon. 24 : 367–372.
- Vij SP, Kashyap SK. 1976. Cytological studies in some north Indian Labiatae. Cytologia. 41 : 713–719.
- Werker E, Ravid U, Putievsky E. 1985. Structure of glandular hairs and identification of the main components of their secreted material in some species of the Labiatae. Israel J Bot. 34 : 31–45.
- Wink N, Kaufmann M. 1996. Phylogenetic relationships between some members of the subfamily Lamioideae (family Labiatae) inferred from nucleotide sequences of the rbcL gene. Bot Acta. 109 : 139–148.
- Yildiz K, Gücel S. 2006. Chromosome numbers of 16 endemic plant taxa from northern Cyprus. Türk Bot Derg. 30 : 181–192.